Use of Coronary CT Angiography to Predict Obstructive Lesions in Patients with Chest Pain without Enzyme and ST-Segment Elevation
Abstract
1. Introduction
2. Materials and Methods
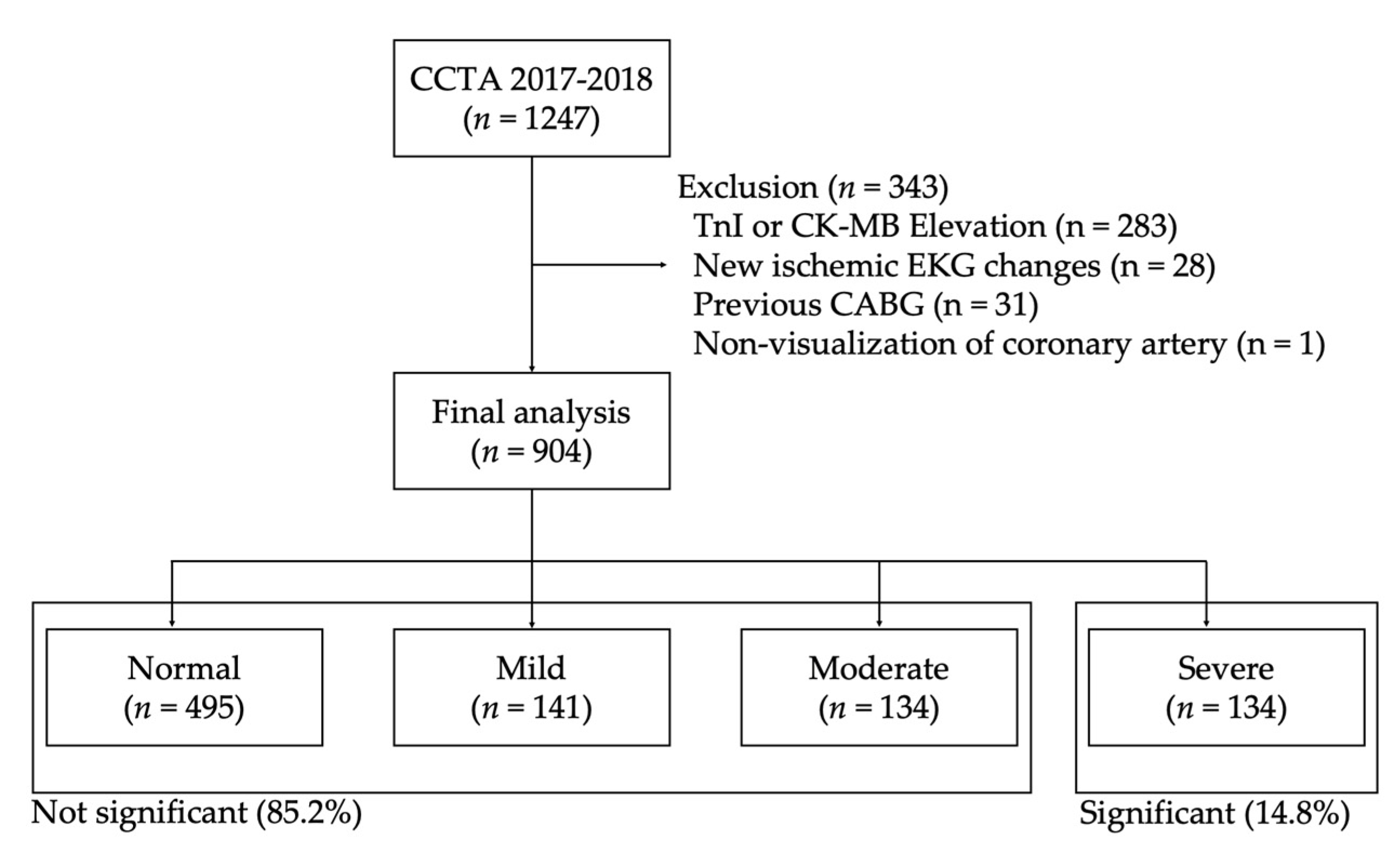
3. Results
3.1. Baseline Characteristics of the Study Population
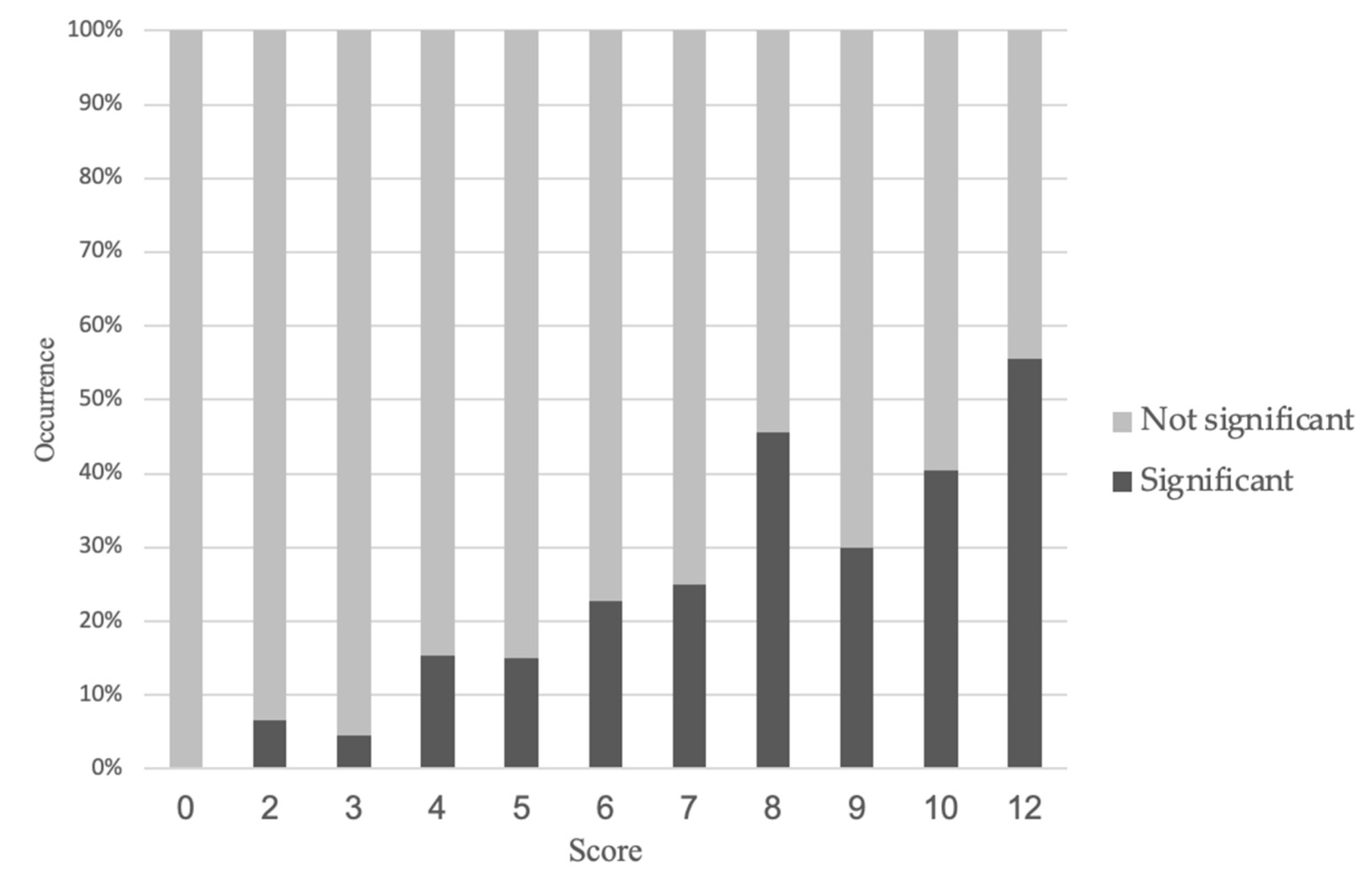
3.2. New Prediction Model for the Occurrence of Significant Stenosis on CCTA
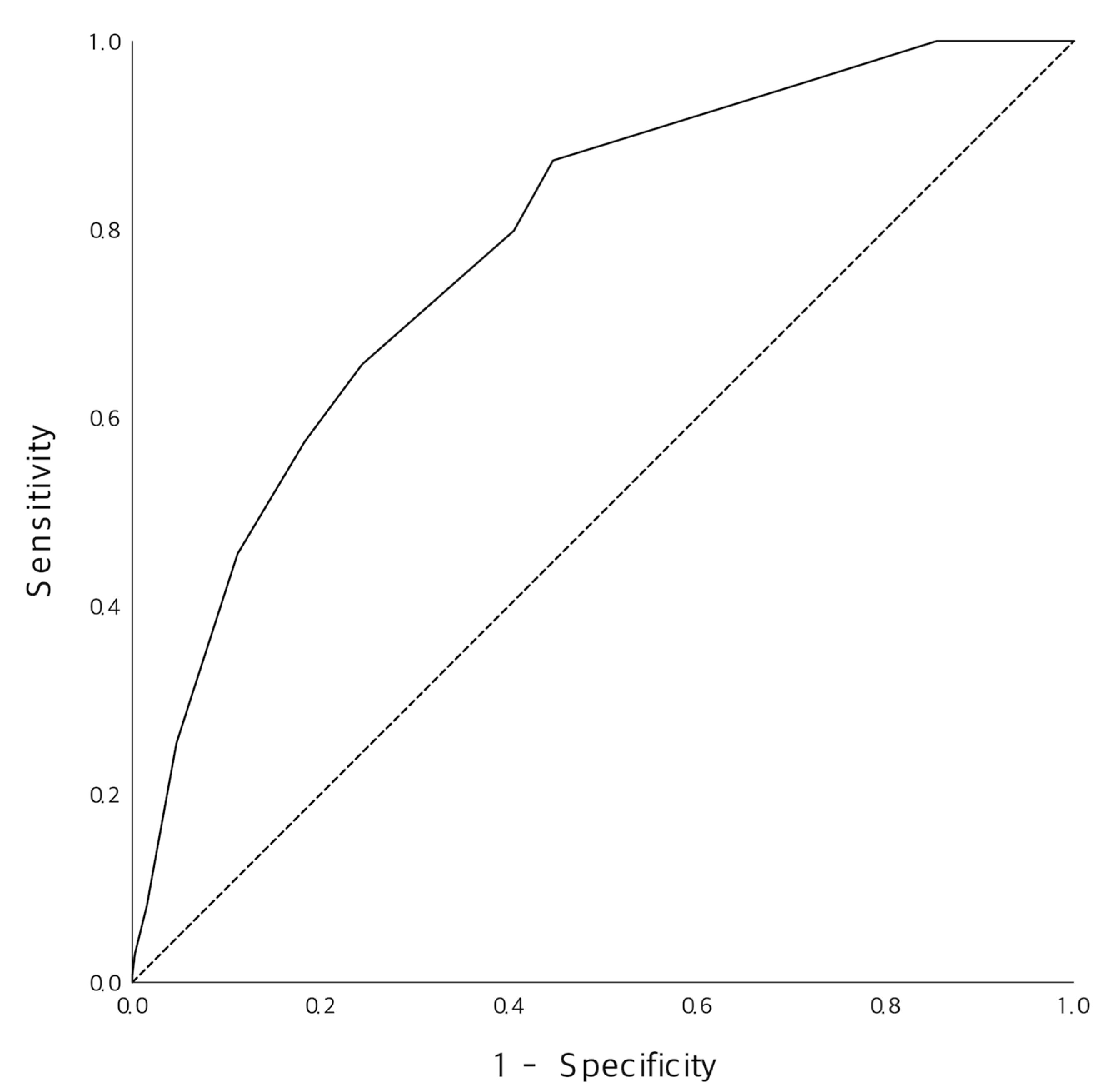
3.3. Diagnostic Performance of the New Prediction Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hulten, E.; Pickett, C.; Bittencourt, M.S.; Villines, T.C.; Petrillo, S.; Di Carli, M.F.; Blankstein, R. Outcomes after coronary computed tomography angiography in the emergency department. J. Am. Coll. Cardiol. 2013, 61, 880–892. [Google Scholar] [CrossRef]
- Levin, D.C.; Parker, L.; Halpern, E.J.; Rao, V.M. Coronary CT angiography: Use in patients with chest pain presenting to emergency departments. Am. J. Roentgenol. 2018, 210, 816–820. [Google Scholar] [CrossRef]
- Kim, R.B.; Hwang, J.Y.; Park, H.W.; Her, A.-Y.; Lee, J.H.; Kim, M.H.; Yoon, C.H.; Cho, J.Y.; Woo, S.-I.; Kim, Y.; et al. Contemporary status of acute myocardial infarction in Korean patients: Korean registry of acute myocardial infarction for regional cardiocerebrovascular centers. J. Clin. Med. 2021, 10, 498. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Omland, T.; Pfeffer, M.A.; Solomon, S.D.; Lemos, J.A.; Rosjo, H.; Benth, J.S.; Maggioni, A.; Domanski, M.J.; Rouleau, J.L.; Sabatine, M.S.; et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 2013, 61, 1240–1249. [Google Scholar] [CrossRef]
- Reichlin, T.; Hochholzer, W.; Bassetti, S.; Steuer, S.; Stelzig, C.; Hartwiger, S.; Biedert, S.; Schaub, N.; Buerge, C.; Potocki, M.; et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N. Engl. J. Med. 2009, 361, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorse, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2017, 39, 119–177. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2019, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Meijboom, W.B.; Meijs, M.F.L.; Schuijf, J.D.; Cramer, M.J.; Mollet, N.R.; van Mieghem, C.A.G.; Nieman, K.; van Werkhoven, J.M.; Pundziute, G.; Weustink, A.C.; et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: A prospective, multicenter, multivendor study. J. Am. Coll. Cardiol. 2008, 52, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Chinnaiyan, K.M.; Aiden Abidov, M.D.P.; Achenbach, S.; Berman, D.S.; Hayes, S.W.; Hoffmann, U.; Lesser, J.R.; Mikati, I.A.; O’Neil, B.J.; et al. The CT-STAT (Coronary computed tomographic angiography for systematic triage of acute chest pain patients to treatment) Trial. J. Am. Coll. Cardiol. 2011, 58, 1414–1422. [Google Scholar] [CrossRef]
- Hoffmann, U.; Truong, Q.A.; Schoenfeld, D.A.; Chou, E.T.; Woodard, P.K.; Nagurney, J.T.; Pope, J.H.; Hauser, T.H.; White, C.S.; Weiner, S.G.; et al. Coronary CT angiography versus standard evaluation in acute chest pain. N. Engl. J. Med. 2012, 367, 299–308. [Google Scholar] [CrossRef]
- Fesmire, F.M.; Martin, E.J.; Cao, Y.; Heath, G.W. Improving risk stratification in patients with chest pain: The Erlanger HEARTS3 score. Am. J. Emerg. Med. 2012, 30, 1829–1837. [Google Scholar] [CrossRef]
- Hess, E.P.; Agarwal, D.; Chandra, S.; Murad, M.H.; Erwin, P.J.; Hollander, J.E.; Montori, V.M.; Stiell, I.G. Diagnostic accuracy of the TIMI risk score in patients with chest pain in the emergency department: A meta-analysis. CMAJ 2010, 182, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, A.; Petritsch, B.; Weng, A.M.; Bley, T.A.; Gassenmaier, T. Radiation dose of coronary CT angiography with a third-generation dual-source CT in a “real-world” patient population. Eur. Radiol. 2019, 29, 4341–4348. [Google Scholar] [CrossRef]
- Yang, C.C.; Law, W.Y.; Lu, K.M.; Wu, T.H. Relationship between heart rate and optimal reconstruction phase in coronary CT angiography performed on a 256-slice multidetector CT. Br. J. Radiol. 2019, 92, 20180945. [Google Scholar] [CrossRef]
- Douglas, P.S.; Hoffmann, U.; Patel, M.R.; Mark, D.B.; Al-Khalidi, H.R.; Cavanaugh, B.; Cole, J.; Doler, R.J.; Fordyce, C.B.; Huang, M.; et al. Outcomes of anatomical versus functional testing for coronary artery disease. N. Engl. J. Med. 2015, 372, 1291–1300. [Google Scholar] [CrossRef]
- Diamond, G.A.; Forrester, J.S. Analysis of probability in the clinical diagnosis of coronary-artery disease. N. Engl. J. Med. 1979, 300, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Smith, S.W.; Pearce, L.A.; Ler, R.; Murakami, M.M. Use of the Centaur TnI-Ultra Assay for detection of myocardial infarction and adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin. Chem. 2008, 54, 723–728. [Google Scholar] [CrossRef]
- Stepinska, J.; Lettino, M.; Ahrens, I.; Bueno, H.; Garcia-Castrillo, L.; Khoury, A.; Lancellotti, P.; Mueller, C.; Muenzel, T.; Oleksiak, A.; et al. Diagnosis and risk stratification of chest pain patients in the emergency department: Focus on acute coronary syndromes. A position paper of the Acute Cardiovascular Care Association. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Thelin, J.; Melander, O.; Öhlin, B. Early rule-out of acute coronary syndrome using undetectable levels of high sensitivity troponin T. Eur. Heart J. Acute Cardiovasc. Care 2014, 4, 403–409. [Google Scholar] [CrossRef]
- Scheuermeyer, F.X.; Grunau, B.; Raju, R.; Choy, S.; Naoum, C.; Blanke, P.; Hague, C.; Heibron, B.; Taylor, C.; Kalla, D.; et al. Safety and efficiency of outpatient versus emergency department-based coronary CT angiography for evaluation of patients with potential ischemic chest pain. J. Cardiovasc. Comput. Tomogr. 2015, 9, 534–537. [Google Scholar] [CrossRef]
- Cho, M.S.; Rho, J.-H.; Park, H.; Cho, S.C.; Kang, D.-Y.; Lee, P.H.; Ahn, J.-M.; Koo, H.J.; Yang, D.H.; Kang, J.-W.; et al. Practice pattern, diagnostic yield, and long-term prognostic impact of ccoronary computed tomographic angiography. J. Am. Heart Assoc. 2020, 9, e016620. [Google Scholar] [CrossRef] [PubMed]
- Nasis, A.; Meredith, I.T.; Sud, P.S.; Cameron, J.D.; Troupis, J.M.; Seneviratne, S.K. Long-term outcome after CT angiography in patients with possible acute coronary syndrome. Radiology 2014, 272, 674–682. [Google Scholar] [CrossRef][Green Version]
- Liu, N.; Ng, J.C.J.; Ting, C.E.; Sakamoto, J.T.; Ho, A.F.W.; Koh, Z.X.; Pek, P.P.; Lim, S.H.; Ong, M.E.H. Clinical scores for risk stratification of chest pain patients in the emergency department: An updated systematic review. J. Emerg. Crit. Care Med. 2018, 2, 16–26. [Google Scholar] [CrossRef]
- Shin, Y.S.; Ahn, S.; Kim, Y.J.; Ryoo, S.M.; Sohn, C.H.; Kim, W.Y. Risk stratification of patients with chest pain or anginal equivalents in the emergency department. Intern. Emerg. Med. 2019, 15, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Six, A.J.; Backus, B.E.; Kelder, J.C. Chest pain in the emergency room: Value of the HEART score. Neth. Heart J. 2008, 16, 191–196. [Google Scholar] [CrossRef]
- Mushtaq, S.; Conte, E.; Melotti, E.; Andreini, D. Coronary CT angiography in challenging patients: High heart rate and atrial fibrillation. Acad. Radiol. 2019, 26, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Bing, R.; Singh, T.; Dweck, M.R.; Mills, N.L.; Williams, M.C.; Adamson, P.D.; Newby, D.E. Validation of European Society of Cardiology pre-test probabilities for obstructive coronary artery disease in suspected stable angina. Eur. Heart J. Qual. Care Clin. Outcomes 2020, 6, 293–300. [Google Scholar] [CrossRef]
| Variables | Total (n = 904) | Not Significant (n = 770) | Significant (n = 134) | p-Value |
|---|---|---|---|---|
| Age, years | 61.0 (54.0–70.0) | 61.0 (52.0–70.0) | 66.0 (58.0–80.0) | <0.001 |
| Male | 543 (60.1) | 436 (56.6) | 107 (79.9) | <0.001 |
| Vital signs 1 | ||||
| SBP, mmHg | 141.0 (128.0–155.0) | 141.0 (128.0–155.0) | 142.0 (126.5–157.0) | 0.852 |
| DBP, mmHg | 85.0 (76.0–94.0) | 85.0 (77.0–94.0) | 84.0 (73.5–92.5) | 0.129 |
| Pulse rate | 78.0 (68.0–89.0) | 78.0 (68.0–90.0) | 76.0 (67.0–87.0) | 0.202 |
| Respiratory rate | 18.0 (18.0–20.0) | 18.0 (18.0–20.0) | 18.0 (18.0–20.0) | 0.992 |
| Underlying disease | ||||
| Hypertension | 383 (42.4) | 313 (40.6) | 70 (52.2) | 0.012 |
| Diabetes | 160 (17.7) | 122 (15.8) | 38 (28.4) | <0.001 |
| Hyperlipidemia | 157 (17.4) | 136 (17.7) | 21 (15.7) | 0.575 |
| Current smoking | 79 (8.7) | 63 (8.2) | 16 (11.9) | 0.155 |
| Obesity | 8 (0.9) | 4 (0.5) | 4 (3.0) | 0.005 |
| History of AMI | 222 (24.6) | 148 (19.2) | 74 (55.2) | <0.001 |
| History of stroke | 31 (3.4) | 25 (3.2) | 6 (4.5) | 0.470 |
| Family history of CAD | 25 (2.8) | 22 (2.9) | 3 (2.2) | 0.687 |
| Symptoms | ||||
| Typical pain 2 | 243 (26.9) | 178 (23.1) | 65 (48.5) | <0.001 |
| Atypical pain 3 | 438 (48.5) | 385 (50.0) | 53 (39.6) | 0.026 |
| Radiating | 199 (22.0) | 167 (21.7) | 32 (23.9) | 0.572 |
| Diaphoresis | 133 (14.7) | 106 (13.8) | 27 (20.1) | 0.054 |
| Laboratories | ||||
| Hb, mg/dL | 13.8 (12.6–14.8) | 13.9 (12.7–14.9) | 13.7 (12.6–14.8) | 0.292 |
| Platelet, ×103/µL | 231.0 (197.0–275.0) | 233.0 (199.0–278.0) | 218.0 (180.0–259.5) | 0.002 |
| PT, INR | 0.99 (0.96–1.04) | 0.99 (0.96–1.04) | 1.00 (0.96–1.06) | 0.357 |
| D-dimer, µg/mL | 0.29 (0.19–0.50) | 0.29 (0.19–0.49) | 0.33 (0.21–0.60) | 0.023 |
| CK-MB, ng/mL | 0.95 (0.50–1.80) | 0.90 (0.40–1.70) | 1.10 (0.60–2.00) | 0.067 |
| TnI, ng/mL | 0.006 (0.006–0.006) | 0.006 (0.006–0.006) | 0.006 (0.006–0.008) | 0.001 |
| Creatinine, mg/dL | 0.84 (0.71–0.97) | 0.83 (0.70–0.96) | 0.91 (0.77–1.05) | <0.001 |
| Variables | Multivariate Analysis | Points | ||
|---|---|---|---|---|
| Adjusted OR | 95% CI | p-Value | ||
| Age ≥ 65 years 1 | 1.914 | 1.265–2.897 | 0.002 | 2 |
| Male | 2.940 | 1.809–4.780 | <0.001 | 3 |
| Typical chest pain | 2.186 | 1.446–3.304 | <0.001 | 2 |
| Diabetes | 1.838 | 1.155–2.926 | 0.010 | 2 |
| History of AMI | 3.442 | 2.283–5.190 | <0.001 | 3 |
| Sum | 12 | |||
| Cutoff | Sensitivity | Specificity | PLR | NLR | PPV | NPV |
|---|---|---|---|---|---|---|
| ≥2 | 100.0 | 15.5 | 1.2 | 0.0 | 17.1 | 100.0 |
| ≥3 | 100.0 | 15.5 | 1.2 | 0.0 | 17.1 | 100.0 |
| ≥4 | 93.3 | 31.8 | 1.4 | 0.2 | 19.2 | 96.5 |
| ≥5 | 86.6 | 56.4 | 2.0 | 0.2 | 25.7 | 96.0 |
| ≥6 | 82.1 | 60.7 | 2.1 | 0.3 | 26.6 | 95.1 |
| ≥7 | 64.9 | 77.5 | 2.9 | 0.5 | 33.5 | 92.7 |
| ≥8 | 57.5 | 82.0 | 3.2 | 0.5 | 35.7 | 91.7 |
| ≥9 | 41.0 | 90.5 | 4.3 | 0.7 | 43.0 | 89.8 |
| ≥10 | 21.6 | 94.6 | 4.0 | 0.8 | 40.9 | 87.4 |
| ≥11 | 19.4 | 95.5 | 4.3 | 0.8 | 42.6 | 87.2 |
| ≥12 | 3.7 | 99.5 | 7.2 | 1.0 | 55.6 | 85.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-s.; Kim, Y.-J.; Shin, Y.S.; Ahn, S.; Kim, W.Y. Use of Coronary CT Angiography to Predict Obstructive Lesions in Patients with Chest Pain without Enzyme and ST-Segment Elevation. J. Clin. Med. 2021, 10, 5442. https://doi.org/10.3390/jcm10225442
Kim J-s, Kim Y-J, Shin YS, Ahn S, Kim WY. Use of Coronary CT Angiography to Predict Obstructive Lesions in Patients with Chest Pain without Enzyme and ST-Segment Elevation. Journal of Clinical Medicine. 2021; 10(22):5442. https://doi.org/10.3390/jcm10225442
Chicago/Turabian StyleKim, June-sung, Youn-Jung Kim, Yo Sep Shin, Shin Ahn, and Won Young Kim. 2021. "Use of Coronary CT Angiography to Predict Obstructive Lesions in Patients with Chest Pain without Enzyme and ST-Segment Elevation" Journal of Clinical Medicine 10, no. 22: 5442. https://doi.org/10.3390/jcm10225442
APA StyleKim, J.-s., Kim, Y.-J., Shin, Y. S., Ahn, S., & Kim, W. Y. (2021). Use of Coronary CT Angiography to Predict Obstructive Lesions in Patients with Chest Pain without Enzyme and ST-Segment Elevation. Journal of Clinical Medicine, 10(22), 5442. https://doi.org/10.3390/jcm10225442






