Wearable Cardioverter-Defibrillator Used as a Telemonitoring System in a Real-Life Heart Failure Unit Setting
Abstract
:1. Introduction
2. Materials and Methods
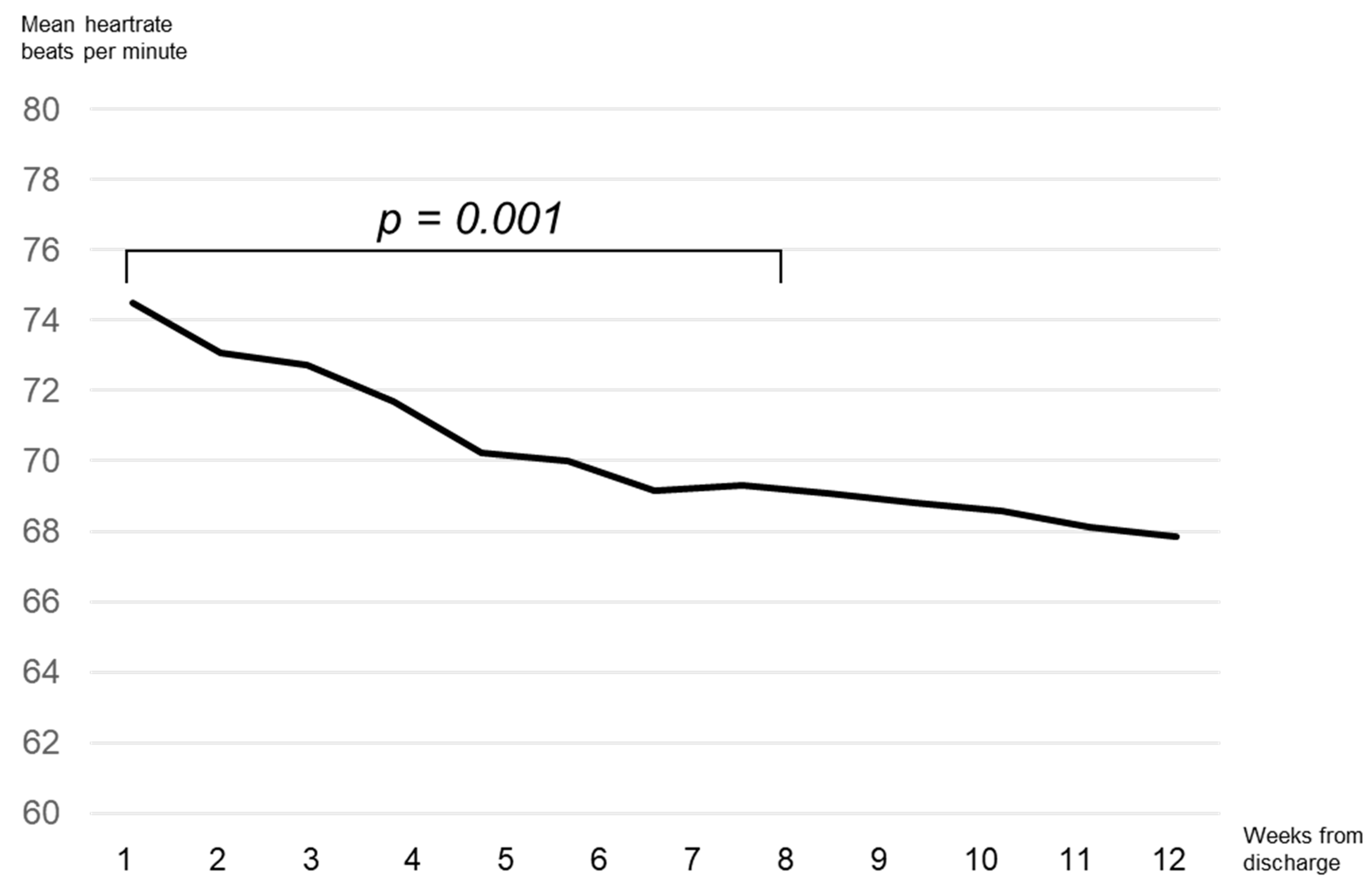
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huikuri, H.V.; Castellanos, A.; Myerburg, R.J. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 2001, 345, 1473–1482. [Google Scholar] [CrossRef]
- Solomon, S.D.; Zelenkofske, S.; McMurray, J.J.; Finn, P.V.; Velazquez, E.; Ertl, G.; Harsanyi, A.; Rouleau, J.L.; Maggioni, A.; Kober, L.; et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N. Engl. J. Med. 2005, 352, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Zipes, D.P.; Wellens, H.J. Sudden cardiac death. Circulation 1998, 98, 2334–2351. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Helms, T.M.; Muller, A.; Schwab, J.O.; Bansch, D.; Karle, C.; Klingenheben, T.; Zugck, C.; Perings, C. The Wearable Cardioverter-Defibrillator (WCD). Herzschrittmacherther Elektrophysiol. 2015, 26, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomstrom-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, R.; Combes, N.; Defaye, P.; Narayanan, K.; Guedon-Moreau, L.; Boveda, S.; Blangy, H.; Bouet, J.; Briand, F.; Chevalier, P.; et al. Wearable cardioverter-defibrillator in patients with a transient risk of sudden cardiac death: The WEARIT-France cohort study. Europace 2021, 23, 73–81. [Google Scholar] [CrossRef]
- Kutyifa, V.; Moss, A.J.; Klein, H.; Biton, Y.; McNitt, S.; MacKecknie, B.; Zareba, W.; Goldenberg, I. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: Data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry). Circulation 2015, 132, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Veltmann, C.; Winter, S.; Duncker, D.; Jungbauer, C.G.; Wassnig, N.K.; Geller, J.C.; Erath, J.W.; Goeing, O.; Perings, C.; Ulbrich, M.; et al. Protected risk stratification with the wearable cardioverter-defibrillator: Results from the WEARIT-II-EUROPE registry. Clin. Res. Cardiol. 2021, 110, 102–113. [Google Scholar] [CrossRef]
- Ellenbogen, K.A.; Koneru, J.N.; Sharma, P.S.; Deshpande, S.; Wan, C.; Szymkiewicz, S.J. Benefit of the wearable cardioverter-defibrillator in protecting patients after implantable-cardioverter defibrillator explant: Results from the National Registry. JACC Clin. Electrophysiol. 2017, 3, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Wranicz, J.; Malik, R.; Morin, D.P.; Zweibel, S.; Buxton, A.E.; Elayi, C.S.; Chung, E.H.; et al. Wearable cardioverter-defibrillator after myocardial infarction. N. Engl. J. Med. 2018, 379, 1205–1215. [Google Scholar] [CrossRef]
- Wassnig, N.K.; Gunther, M.; Quick, S.; Pfluecke, C.; Rottstadt, F.; Szymkiewicz, S.J.; Ringquist, S.; Strasser, R.H.; Speiser, U. Experience with the wearable cardioverter-defibrillator in patients at high risk for sudden cardiac death. Circulation 2016, 134, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Daimee, U.A.; Vermilye, K.; Moss, A.J.; Goldenberg, I.; Klein, H.U.; McNitt, S.; Zareba, W.; Kutyifa, V. Experience with the wearable cardioverter-defibrillator in older patients: Results from the prospective registry of patients using the wearable cardioverter-defibrillator. Heart Rhythm 2018, 15, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Olgin, J.E.; Lee, B.K. Wearable cardioverter-defibrillators: A review of evidence and indications. Trends Cardiovasc. Med. 2021, 31, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Komajda, M.; Bohm, M.; Borer, J.; Robertson, M.; Tavazzi, L.; Ford, I.; Investigators, S. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: Is there an influence of beta-blocker dose? Findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine trial) study. J. Am. Coll. Cardiol. 2012, 59, 1938–1945. [Google Scholar] [CrossRef] [Green Version]
- Swedberg, K.; Komajda, M.; Bohm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L.; Investigators, S. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010, 376, 875–885. [Google Scholar] [CrossRef]
- Hansen, C.; Loges, C.; Seidl, K.; Eberhardt, F.; Troster, H.; Petrov, K.; Gronefeld, G.; Bramlage, P.; Birkenhauer, F.; Weiss, C. Investigation on routine follow-up in congestive heart failure patients with remotely monitored implanted cardioverter defibrillators systems (InContact). BMC Cardiovasc. Disord. 2018, 18, 131. [Google Scholar] [CrossRef]
- Singhal, A.; Cowie, M.R. The role of wearables in heart failure. Curr. Heart Fail. Rep. 2020, 17, 125–132. [Google Scholar] [CrossRef]
- Schmitt, J.; Abaci, G.; Johnson, V.; Erkapic, D.; Gemein, C.; Chasan, R.; Weipert, K.; Hamm, C.W.; Klein, H.U. Safety of the Wearable Cardioverter Defibrillator (WCD) in patients with implanted pacemakers. Pacing Clin. Electrophysiol. 2017, 40, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Tscholl, V.; Wielander, D.; Kelch, F.; Stroux, A.; Attanasio, P.; Tschope, C.; Landmesser, U.; Roser, M.; Huemer, M.; Heidecker, B.; et al. Benefit of a wearable cardioverter defibrillator for detection and therapy of arrhythmias in patients with myocarditis. ESC Heart Fail. 2021, 8, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Rosenkaimer, S.L.; Hohneck, A.; Lang, S.; El-Battrawy, I.; Rudic, B.; Tulumen, E.; Stach, K.; Kuschyk, J.; Akin, I.; et al. Therapy optimization in patients with heart failure: The role of the wearable cardioverter-defibrillator in a real-world setting. BMC Cardiovasc. Disord. 2018, 18, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, I.; Erath, J.W.; Russo, A.M.; Burch, A.E.; Assmus, B.; Bonderman, D.; McNitt, S.; Kutyifa, V. Sex differences in arrhythmic burden with the wearable cardioverter-defibrillator. Heart Rhythm 2021, 18, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Zylla, M.M.; Hillmann, H.A.K.; Proctor, T.; Kieser, M.; Scholz, E.; Zitron, E.; Katus, H.A.; Thomas, D. Use of the wearable cardioverter-defibrillator (WCD) and WCD-based remote rhythm monitoring in a real-life patient cohort. Heart Vessel. 2018, 33, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Olgin, J.E.; Lee, B.K.; Vittinghoff, E.; Morin, D.P.; Zweibel, S.; Rashba, E.; Chung, E.H.; Borggrefe, M.; Hulley, S.; Lin, F.; et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: As-treated and per-protocol analyses. J. Cardiovasc. Electrophysiol. 2020, 31, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Batalik, L.; Dosbaba, F.; Hartman, M.; Batalikova, K.; Spinar, J. Benefits and effectiveness of using a wrist heart rate monitor as a telerehabilitation device in cardiac patients: A randomized controlled trial. Medicine 2020, 99, e19556. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, E.; Jasionowska, A.; Banaszak-Bednarczyk, M.; Gwilkowska, J.; Piotrowicz, R. ECG telemonitoring during home-based cardiac rehabilitation in heart failure patients. J. Telemed. Telecare. 2012, 18, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.I.; Mattera, J.A.; Curtis, J.P.; Spertus, J.A.; Herrin, J.; Lin, Z.; Phillips, C.O.; Hodshon, B.V.; Cooper, L.S.; Krumholz, H.M. Telemonitoring in patients with heart failure. N. Engl. J. Med. 2010, 363, 2301–2309. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Chen, S.H.; Edwards, I.; Jayasena, R.; Doecke, J.; Layland, J.; Yang, I.A.; Maiorana, A. Effects of different telemonitoring strategies on chronic heart failure care: Systematic review and subgroup meta-analysis. J. Med. Internet Res. 2020, 22, e20032. [Google Scholar] [CrossRef]
- Galinier, M.; Roubille, F.; Berdague, P.; Brierre, G.; Cantie, P.; Dary, P.; Ferradou, J.M.; Fondard, O.; Labarre, J.P.; Mansourati, J.; et al. Telemonitoring versus standard care in heart failure: A randomised multicentre trial. Eur. J. Heart Fail. 2020, 22, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Burch, A.E.; D’Souza, B.; Gimbel, J.R.; Rohrer, U.; Masuda, T.; Sears, S.; Scherr, D. Physical activity is reduced prior to ventricular arrhythmias in patients with a wearable cardioverter defibrillator. Clin. Cardiol. 2020, 43, 60–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alapati, V.; Tang, F.; Charlap, E.; Chan, P.S.; Heidenreich, P.A.; Jones, P.G.; Spertus, J.A.; Srinivas, V.; Kizer, J.R. Discharge heart rate after hospitalization for myocardial infarction and long-term mortality in 2 US registries. J. Am. Heart Assoc. 2019, 8, e010855. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Total n = 173 | Group 1 (n = 79; 45.66%) | Group 2 (n = 65; 37.57%) | Group 3 (n = 29; 16.76%) | |
|---|---|---|---|---|
| Sex (female) | 45 (26.01%) | 17 (21.52%) | 16 (24.62%) | 12 (41.38%) |
| Age (years) | 56.64 ± 14.46 58.0 (50.0−67.0) | 60.42 ± 11.28 60.0 (53.0−69.0) | 54.51 ± 14.17% 57.0 (43.0−66.0) | 51.1 ± 19.5 54.0 (38.0−64.0) |
| Initial LVEF (%) | 28.88 ± 10.45 28.0 (22.0−32.0) | 29.16 ± 7.8 30.0 (24.0−33.0) | 23.57 ± 5.58 23.0 (20.0−29.0) | 40.03 ± 15.68 35.0 (28.0−55.0) |
| BMI | 28.57 ± 7.10 27.4 (25.0−31.0) | 28.2 ± 5.1 28.0 (31.0−44.0) | 30.53 ± 8.9 27.9 (25.0−35.0) | 25.27 ± 6 26.0 (24.0−28.0) |
| NYHA class I NYHA class II NYHA class III NYHA class IV | 15 (8.67%) 51 (29.48%) 64 (36.99%) 43 (24.85%) | 6 (7.6%) 32 (40.5%) 5 (31.65%) 16 (20.25%) | 1(1.54%) 15 (23.97%) 30 (46.15%) 19 (29.23%) | 8 (27.59%) 4 (13.79%) 9 (31.04%) 8 (27.58%) |
| Coronary artery disease | 99 (57.23%) | 79 (100%) | 15 (23.07%) | 6 (20.69%) |
| Arterial hypertension | 100 (57.80%) | 60 (75.95%) | 30 (46.15%) | 10 (34.48%) |
| History of stroke | 19 (10.98%) | 8 (10.12%) | 8 (12.3%) | 3 (10.34%) |
| Diabetes mellitus II | 44 (25.43%) | 26 (32.91%) | 13 (20%) | 5 (17.24%) |
| Sleep apnea | 18 (10.40%) | 8 (10.13%) | 10 (15.38%) | 0 |
| COPD | 23 (13.29%) | 16 (20.25%) | 4 (6.15%) | 3 (10.34%) |
| AF | 51 (29.48%) | 20 (25.32%) | 23 (35.38%) | 8 (27.59%) |
| Creatinine at admission (mg/dL) | 1.06 ± 0.46 0.95 (0.80−1.16) | 1.13 ± 0.59 0.94 (0.81−1.30) | 1.04 ± 0.29 1.00 (0.82−1.18) | 0.87 ± 0.23 0.09 (0.80−1.00) |
| Pro-nt-BNP at admission (pg/mL) | 4830.39 ± 6657.95 3009 (1470−6198) | 5675.43 ± 8994.82 3174 (2046−5899) | 4357.09 ± 4102.8 3124 (1170−6274) | 3732.74 ± 3856.7 2183 (650−7189) |
| n = 173 | Group 1 (n = 79; 45.66%) | Group 2 (n = 65; 37.57%) | Group 3 (n = 29; 16.76%) | |
|---|---|---|---|---|
| Beta-blocker | 168 (97.11%) | 79 (100%) | 65 (100%) | 25 (86.2%) |
| ACE-I/AT or AT/neprilysin inhibitor | 163 (94.22%) | 78 (98.73%) | 65 (100%) | 23 (79.3%) |
| Aldosteron antagonist | 147 (84.97%) | 68 (86.07%) | 63 (96.92%) | 17 (58.62%) |
| Ivabradin | 16 (9.25%) | 5 (6.3%) | 7 (10.77%) | 4 (13.79%) |
| Digitalis | 16 (9.25%) | 5 (6.3%) | 8 (12.3%) | 3 (10.34%) |
| Anticoagulation | 64 (36.99%) | 30 (37.97%) | 25 (38.46%) | 10 (34.48%) |
| n = 173 | Group 1 (n = 79; 45.66%) | Group 2 (n = 65; 37.57%) | Group 3 (n = 29; 16.76%) | |
|---|---|---|---|---|
| LVEF after follow-up | 38.94 ± 10.64 38.0 (30.0−47.0) | 37.72 ± 9.68 37.0 (31.0−44.0) | 35.81 ± 8.46 36.0 (30.0−43) | 49.21 ± 11.59 50.0 (45.0−55.0) |
| ICD or CRT-D implantation | 46 (20.24%) | 21 (26.58%) | 18 (27.69%) | 6 (20.69%) |
| LVAD or HTX | 1 (0.58%) | 0 | 1 (1.54%) | 0 |
| VT + shock | 1 (0.58%) | 1 (1.26%) | 0 | 0 |
| VT ± Response Button, no shock | 5 (2.89%) | 0 | 1 (1.54%) | 4 (13.79%) |
| Non sustained VT > 10 s | 3 (1.73%) | 3 (3.79%) | 0 | 0 |
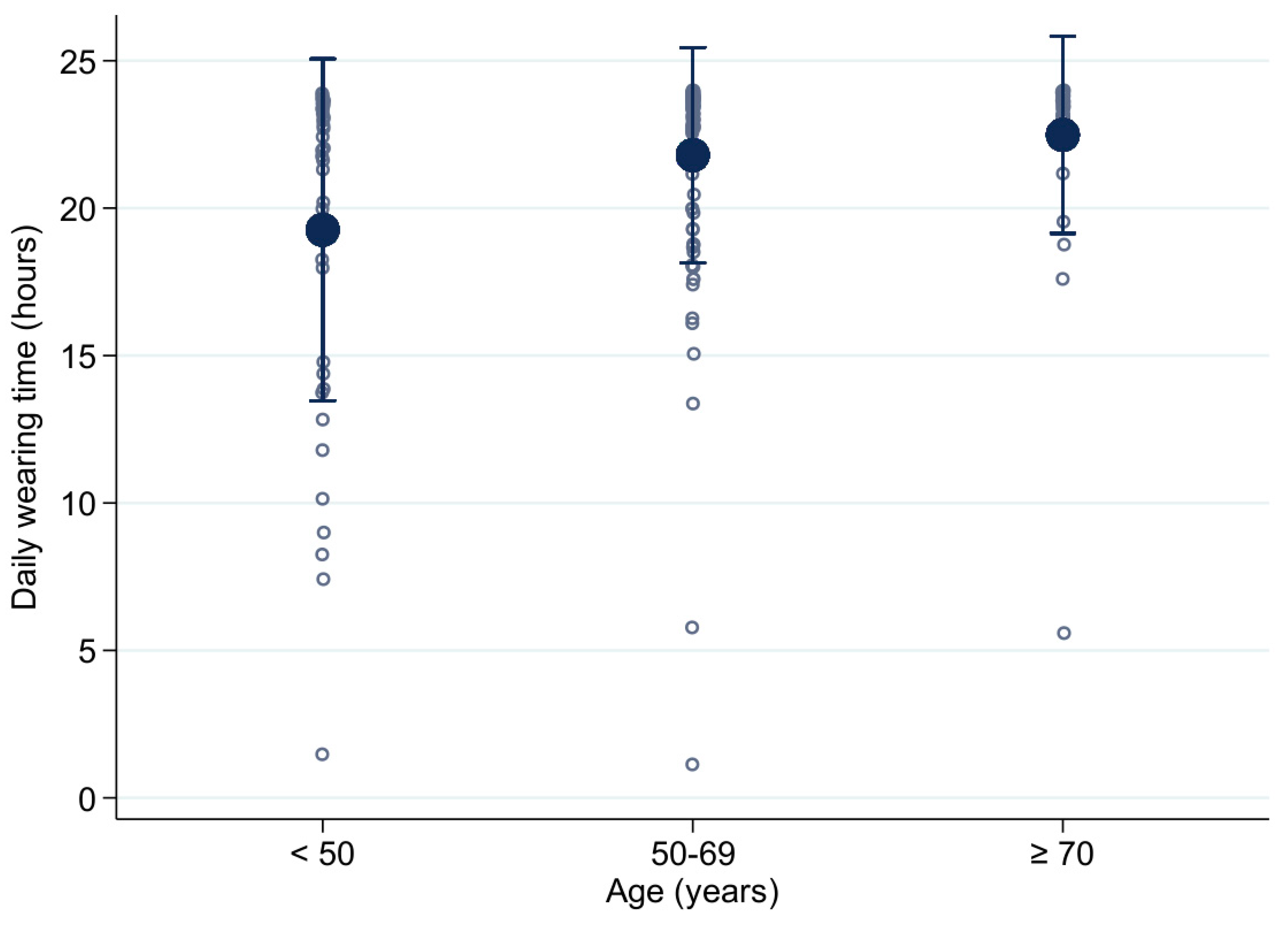
| Mean daily wearing time (h) | 21.19 ± 4.65 23.4 (20.5−23.7) | 21.95 ± 3.2 23.4 (21.8−23.8) | 20 ± 5.85 23.0 (18.7−23.7) | 21.81 ± 4.48 23.4 (22.6−23.7) |
| Mean WCD wearing time (days) | 59.75 ± 35.6 55.0 (34.0−85.0) | 54.57 ± 37.19 47.0 (29.0−71.0) | 63.08 ± 35.4 64.0 (38.0−89.0) | 66.14 ± 30.06 59.0 (48.0−90.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blockhaus, C.; List, S.; Waibler, H.-P.; Gülker, J.-E.; Klues, H.; Bufe, A.; Seyfarth, M.; Koektuerk, B.; Shin, D.-I. Wearable Cardioverter-Defibrillator Used as a Telemonitoring System in a Real-Life Heart Failure Unit Setting. J. Clin. Med. 2021, 10, 5435. https://doi.org/10.3390/jcm10225435
Blockhaus C, List S, Waibler H-P, Gülker J-E, Klues H, Bufe A, Seyfarth M, Koektuerk B, Shin D-I. Wearable Cardioverter-Defibrillator Used as a Telemonitoring System in a Real-Life Heart Failure Unit Setting. Journal of Clinical Medicine. 2021; 10(22):5435. https://doi.org/10.3390/jcm10225435
Chicago/Turabian StyleBlockhaus, Christian, Stephan List, Hans-Peter Waibler, Jan-Erik Gülker, Heinrich Klues, Alexander Bufe, Melchior Seyfarth, Buelent Koektuerk, and Dong-In Shin. 2021. "Wearable Cardioverter-Defibrillator Used as a Telemonitoring System in a Real-Life Heart Failure Unit Setting" Journal of Clinical Medicine 10, no. 22: 5435. https://doi.org/10.3390/jcm10225435
APA StyleBlockhaus, C., List, S., Waibler, H.-P., Gülker, J.-E., Klues, H., Bufe, A., Seyfarth, M., Koektuerk, B., & Shin, D.-I. (2021). Wearable Cardioverter-Defibrillator Used as a Telemonitoring System in a Real-Life Heart Failure Unit Setting. Journal of Clinical Medicine, 10(22), 5435. https://doi.org/10.3390/jcm10225435






