Immunohistochemistry for Thymidine Kinase-1 (TK1): A Potential Tool for the Prognostic Stratification of Breast Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. TMA Construction
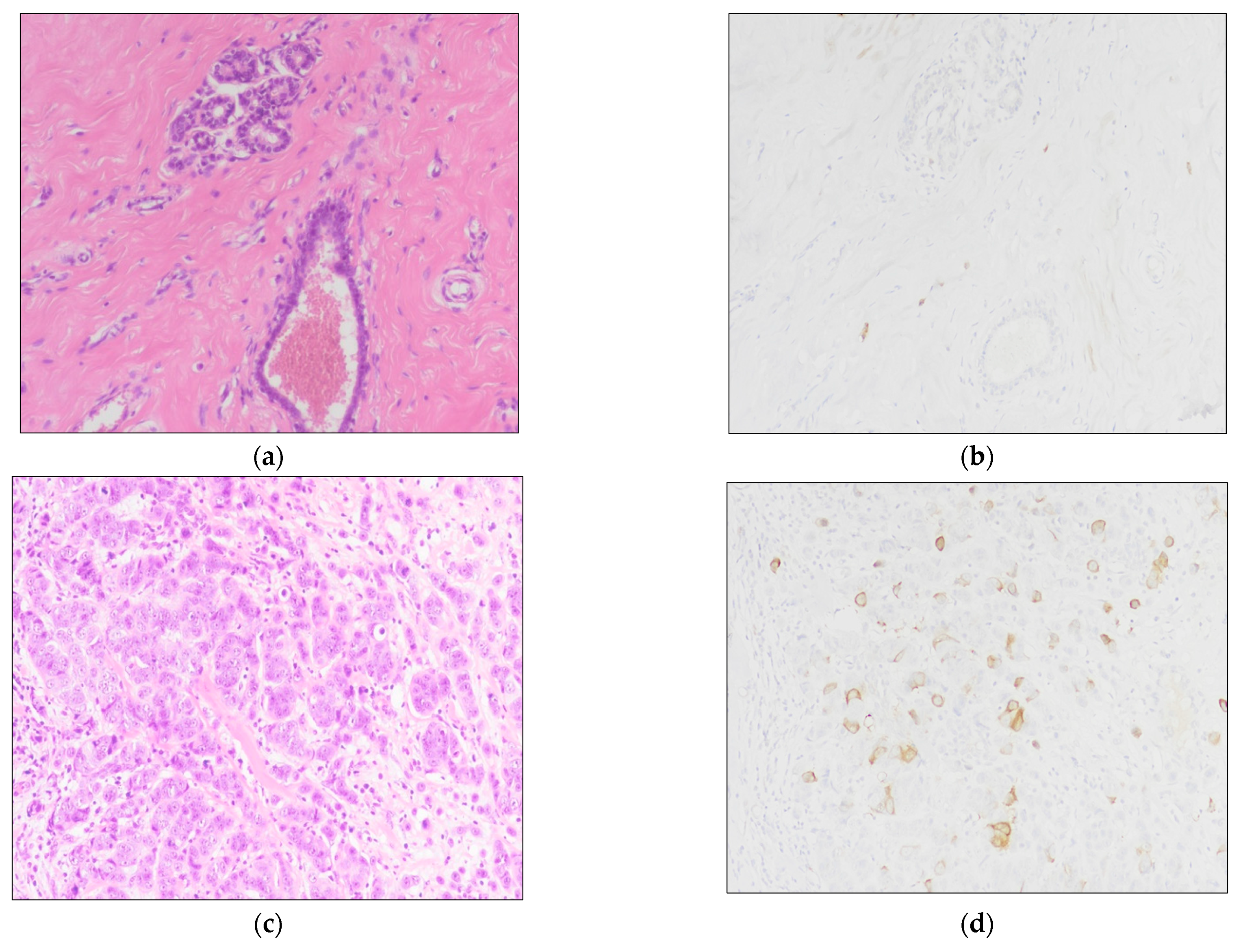
2.3. Immunohistochemistry (IHC)
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Findings
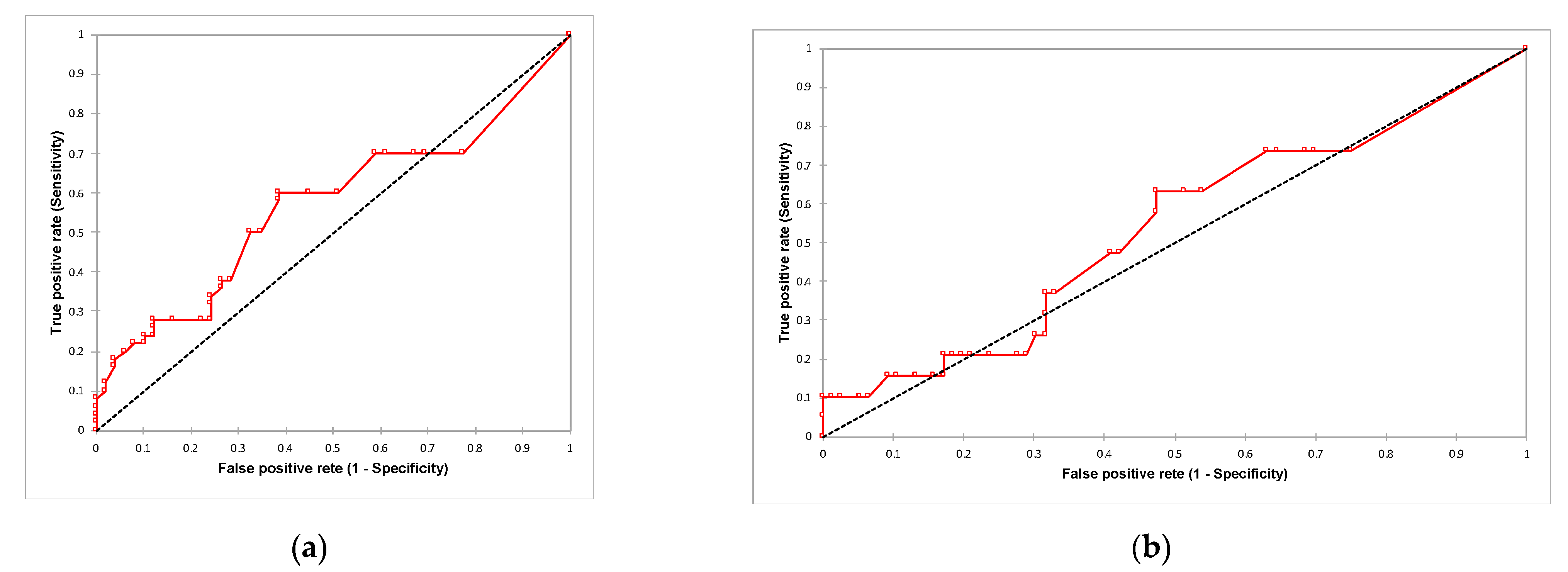
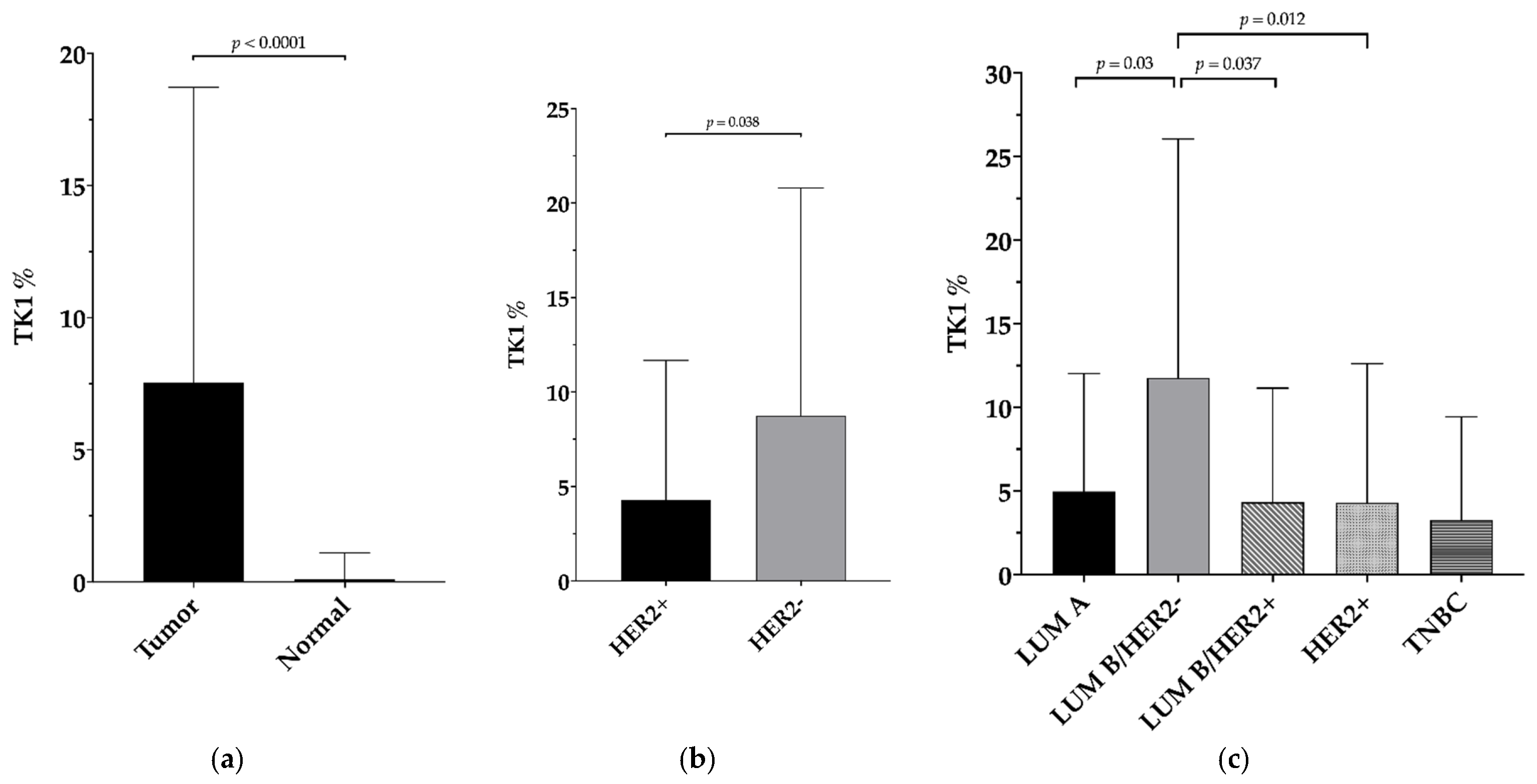
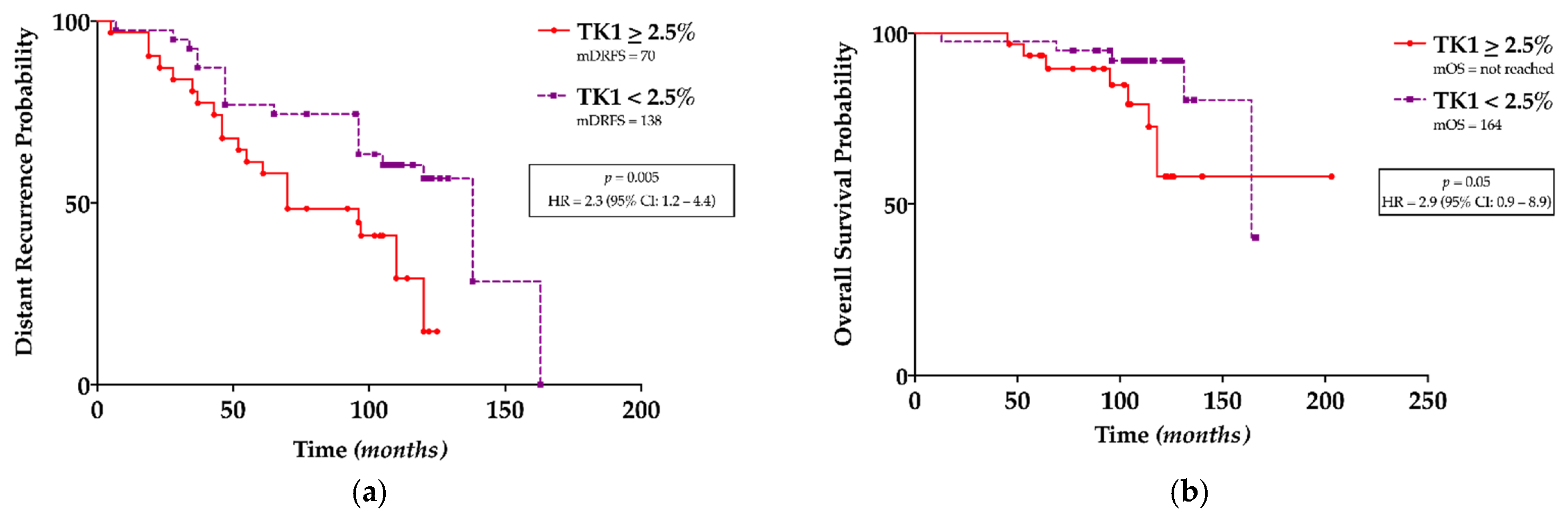
3.2. TK1 Expression and Its Association with Clinicopathological Findings and Survival Outcomes
4. Discussion
5. Potential Pitfalls
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kim, Y.; Yoo, K.Y.; Goodman, M.T. Differences in incidence, mortality and survival of breast cancer by regions and countries in asia and contributing factors. Asian Pac. J. Cancer Prev. 2015, 16, 2857–2870. [Google Scholar] [CrossRef] [Green Version]
- Johansson, I.; Killander, F.; Linderholm, B.; Hedenfalk, I. Molecular profiling of male breast cancer—Lost in translation? Int. J. Biochem. Cell Biol. 2014, 53, 526–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanelli, G.N.; Naccarato, A.G.; Scatena, C. Recent advances in cancer plasticity: Cellular mechanisms, surveillance strategies, and therapeutic optimization. Front. Oncol. 2020, 10, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Mechelen, M.; Van Herck, A.; Punie, K.; Nevelsteen, I.; Smeets, A.; Neven, P.; Weltens, C.; Han, S.; Vanderstichele, A.; Floris, G.; et al. Behavior of metastatic breast cancer according to subtype. Breast Cancer Res. Treat. 2020, 181, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Eriksson, S.; Chang, Z.F. Regulation and functional contribution of thymidine kinase 1 in repair of DNA damage. J. Biol. Chem. 2010, 285, 27327–27335. [Google Scholar] [CrossRef] [Green Version]
- Bitter, E.E.; Townsend, M.H.; Erickson, R.; Allen, C.; O’Neill, K.L. Thymidine kinase 1 through the ages: A comprehensive review. Cell Biosci. 2020, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, B.; Shi, Q.L.; Huang, P.L.; Zhou, X.J.; Ma, H.H.; Lu, Z.F.; Bo, Y.; Eriksson, S.; He, E.; et al. Thymidine kinase 1 is a better prognostic marker than ki-67 for pt1 adenocarcinoma of the lung. Int. J. Clin. Exp. Med. 2014, 7, 2120–2128. [Google Scholar]
- O’Neill, K.L.; Buckwalter, M.R.; Murray, B.K. Thymidine kinase: Diagnostic and prognostic potential. Expert Rev. Mol. Diagn. 2001, 1, 428–433. [Google Scholar] [CrossRef]
- Topolcan, O.; Holubec, L., Jr. The role of thymidine kinase in cancer diseases. Expert Opin. Med. Diagn. 2008, 2, 129–141. [Google Scholar] [CrossRef]
- Zhou, J.; He, E.; Skog, S. The proliferation marker thymidine kinase 1 in clinical use. Mol. Clin. Oncol. 2013, 1, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alegre, M.M.; Robison, R.A.; O’Neill, K.L. Thymidine kinase 1 upregulation is an early event in breast tumor formation. J. Oncol. 2012, 2012, 575647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, H.; Sun, Y.; Zan, Q.; Xu, M.; Li, Y.; Zhou, J.; He, E.; Eriksson, S.; Wen, W.; Skog, S. Thymidine kinase 1 expression in atypical ductal hyperplasia significantly differs from usual ductal hyperplasia and ductal carcinoma in situ: A useful tool in tumor therapy management. Mol. Med. Rep. 2009, 2, 923–929. [Google Scholar]
- Nisman, B.; Allweis, T.; Kaduri, L.; Maly, B.; Gronowitz, S.; Hamburger, T.; Peretz, T. Serum thymidine kinase 1 activity in breast cancer. Cancer Biomark. 2010, 7, 65–72. [Google Scholar] [CrossRef] [PubMed]
- He, E.; Xu, X.H.; Guan, H.; Chen, Y.; Chen, Z.H.; Pan, Z.L.; Tang, L.L.; Hu, G.Z.; Li, Y.; Zhang, M.; et al. Thymidine kinase 1 is a potential marker for prognosis and monitoring the response to treatment of patients with breast, lung, and esophageal cancer and non-hodgkin’s lymphoma. Nucleosides Nucleotides Nucleic Acids 2010, 29, 352–358. [Google Scholar] [CrossRef]
- World Health Organization; International Agency for Research on Cancer. Breast Tumours; IARC: Lyon, France, 2019. [Google Scholar]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2017. [Google Scholar]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and progesterone receptor testing in breast cancer: American society of clinical oncology/college of american pathologists guideline update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline focused update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of ki67 in breast cancer: Updated recommendations from the international ki67 in breast cancer working group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in breast cancer survival by molecular subtypes in the united states. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, M.A.; Arslan, U.Y.; Isikdogan, A.; Dane, F.; Oksuzoglu, B.; Inanc, M.; Akman, T.; Kucukoner, M.; Cinkir, H.Y.; Rzazade, R.; et al. Biological subtypes and distant relapse pattern in breast cancer patients after curative surgery (study of anatolian society of medical oncology). Breast Care 2016, 11, 248–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, E.; Metzger-Filho, O.; Winer, E.P. The natural history of hormone receptor-positive breast cancer. Oncology 2012, 26, 688–694, 696. [Google Scholar]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Medeiros Alencar, V.H.; Badran, A.; Bonfill, X.; et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: Atlas, a randomised trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Fornander, T.; Johansson, H.; Johansson, U.; Hu, G.Z.; Rutqvist, L.E.; Skog, S. Thymidine kinase 1 in serum predicts increased risk of distant or loco-regional recurrence following surgery in patients with early breast cancer. Anticancer Res. 2006, 26, 4753–4759. [Google Scholar] [PubMed]
- Nisman, B.; Allweis, T.; Kadouri, L.; Mali, B.; Hamburger, T.; Baras, M.; Gronowitz, S.; Peretz, T. Comparison of diagnostic and prognostic performance of two assays measuring thymidine kinase 1 activity in serum of breast cancer patients. Clin. Chem. Lab. Med. 2013, 51, 439–447. [Google Scholar] [CrossRef]
- Bjohle, J.; Bergqvist, J.; Gronowitz, J.S.; Johansson, H.; Carlsson, L.; Einbeigi, Z.; Linderholm, B.; Loman, N.; Malmberg, M.; Soderberg, M.; et al. Serum thymidine kinase activity compared with ca 15-3 in locally advanced and metastatic breast cancer within a randomized trial. Breast Cancer Res. Treat. 2013, 139, 751–758. [Google Scholar] [CrossRef]
- Del Re, M.; Bertolini, I.; Crucitta, S.; Fontanelli, L.; Rofi, E.; De Angelis, C.; Diodati, L.; Cavallero, D.; Gianfilippo, G.; Salvadori, B.; et al. Overexpression of tk1 and cdk9 in plasma-derived exosomes is associated with clinical resistance to cdk4/6 inhibitors in metastatic breast cancer patients. Breast Cancer Res. Treat. 2019, 178, 57–62. [Google Scholar] [CrossRef]
- Welin, M.; Kosinska, U.; Mikkelsen, N.E.; Carnrot, C.; Zhu, C.; Wang, L.; Eriksson, S.; Munch-Petersen, B.; Eklund, H. Structures of thymidine kinase 1 of human and mycoplasmic origin. Proc. Natl. Acad. Sci. USA 2004, 101, 17970–17975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagegni, N.; Thomas, S.; Liu, N.; Luo, J.; Hoog, J.; Northfelt, D.W.; Goetz, M.P.; Forero, A.; Bergqvist, M.; Karen, J.; et al. Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib. Breast Cancer Res. 2017, 19, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvi, P.; Janostiak, R.; Nagarajan, A.; Cai, G.; Wajapeyee, N. Loss of thymidine kinase 1 inhibits lung cancer growth and metastatic attributes by reducing gdf15 expression. PLoS Genet. 2019, 15, e1008439. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Mao, Y.; Wu, J.; Decker, C.; Merza, M.; Wang, N.; Eriksson, S.; Castro, J.; Skog, S. Cytosolic thymidine kinase is a specific histopathologic tumour marker for breast carcinomas. Int. J. Oncol. 2004, 25, 945–953. [Google Scholar]
- McCartney, A.; Bonechi, M.; De Luca, F.; Biagioni, C.; Curigliano, G.; Moretti, E.; Minisini, A.M.; Bergqvist, M.; Benelli, M.; Migliaccio, I.; et al. Plasma thymidine kinase activity as a biomarker in patients with luminal metastatic breast cancer treated with palbociclib within the trend trial. Clin. Cancer Res. 2020, 26, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Bello, L.J. Regulation of thymidine kinase synthesis in human cells. Exp. Cell. Res. 1974, 89, 263–274. [Google Scholar] [CrossRef]
- Sharma, P.; Connolly, R.M.; Roussos Torres, E.T.; Thompson, A. Best foot forward: Neoadjuvant systemic therapy as standard of care in triple-negative and HER2-positive breast cancer. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e1–e16. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Avila, J.; Rolfo, C.; Ruiz-Patino, A.; Russo, A.; Ricaurte, L.; Ordonez-Reyes, C.; Arrieta, O.; Zatarain-Barron, Z.L.; Recondo, G.; et al. When tissue is an issue the liquid biopsy is nonissue: A review. Oncol. Ther. 2021, 9, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Bedard, P.L. Luminal-b breast cancer and novel therapeutic targets. Breast Cancer Res. 2011, 13, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, C.; Zeng, Q.; Arbaretaz, F.; Devevre, E.; Calderaro, J.; Lomenie, N.; Maiuri, M.C. Artificial intelligence for solid tumor diagnosis in digital pathology. Br. J. Pharmacol. 2021, 178, 4291–4315. [Google Scholar] [CrossRef] [PubMed]
| Patients | 80 |
| Age(range) | 52.9 (23–85) |
| pT | n. (%) |
| pT1b | 14 (17.5) |
| pT1c | 42 (52.5) |
| pT2 | 21 (26.25) |
| pT3 | 2 (2.5) |
| pT4 | 1 (1.25) |
| pN | |
| pN0 | 40 (50) |
| pN1mi | 3 (3.75) |
| pN1 | 21 (26.25) |
| pN2 | 9 (11.25) |
| pN3 | 7 (8.75) |
| M | |
| M0 | 77 (96.25) |
| M1 | 3 (3.75) |
| Stage(AJCC 2017) | |
| IA | 32 (40) |
| IB | 3 (3.75) |
| IIA | 18 (22.5) |
| IIB | 7 (8.75) |
| IIIA | 10 (12.5) |
| IIIB | 1 (1.25) |
| IIIC | 6 (7.5) |
| IV | 3 (3.75) |
| Multicentric/Multifocal | |
| Yes | 18 (22.5) |
| No | 62 (77.5) |
| Adjuvant Chemotherapy | |
| Yes | 52 (65) |
| No | 28 (35) |
| Distant Recurrence | |
| Yes | 40 (50) |
| No | 40 (50) |
| mDRFS (months—range) | 97.5 (5–165) |
| Cancer-Related Death | |
| Yes | 18 (22.5) |
| No | 62 (77.5) |
| mOS (months—range) | 107 (13–206) |
| Tumor Samples | 99 | TK1 Positivity | |
| Grade(Nottingham combined histologic grade) | n. (%) | Median (range) | |
| G2 | 24 (24.2) | 2.50 (0–25) | |
| G3 | 75 (75.8) | 3.30 (0–60) | |
| Histotype(WHO 2019) | |||
| NST | 93 (93.94) | 2.50 (0–60) | |
| Lobular | 5 (5.05) | 2.00 (0–28.33) | |
| Mucinous | 1 (1.01) | 16.67 (*) | |
| Hormone Receptor Status | |||
| ER | Positive | 83 (83.8) | 3.30 (0–60) |
| Negative | 16 (16.2) | 0.33 (0–25) | |
| PgR | Positive | 78 (78.8) | 3.30 (0–60) |
| Negative | 21 (21.2) | 0.46 (0–28.33) | |
| HER2 status | |||
| Amplified | 25 (25.3) | 0.67 (0–25) | |
| Not amplified | 74 (74.7) | 3.30 (0–60) | |
| Molecular subtype | |||
| LUM-A | 29 (29.3) | 1.67 (0–28.33) | |
| LUM-B/HER2- | 41 (41.41) | 5.00 (0–60) | |
| LUM-B/HER2+ | 13 (13.13) | 2.00 (0–25) | |
| HER2-OE | 12 (12.12) | 0.33 (0–18.33) | |
| Triple-negative | 4 (4.04) | 0.33 (0–12.50) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanelli, G.N.; Scarpitta, R.; Cinacchi, P.; Fuochi, B.; Szumera-Ciećkiewicz, A.; De Ieso, K.; Ferrari, P.; Fontana, A.; Miccoli, M.; Naccarato, A.G.; et al. Immunohistochemistry for Thymidine Kinase-1 (TK1): A Potential Tool for the Prognostic Stratification of Breast Cancer Patients. J. Clin. Med. 2021, 10, 5416. https://doi.org/10.3390/jcm10225416
Fanelli GN, Scarpitta R, Cinacchi P, Fuochi B, Szumera-Ciećkiewicz A, De Ieso K, Ferrari P, Fontana A, Miccoli M, Naccarato AG, et al. Immunohistochemistry for Thymidine Kinase-1 (TK1): A Potential Tool for the Prognostic Stratification of Breast Cancer Patients. Journal of Clinical Medicine. 2021; 10(22):5416. https://doi.org/10.3390/jcm10225416
Chicago/Turabian StyleFanelli, Giuseppe Nicolò, Rosa Scarpitta, Paola Cinacchi, Beatrice Fuochi, Anna Szumera-Ciećkiewicz, Katia De Ieso, Paola Ferrari, Andrea Fontana, Mario Miccoli, Antonio Giuseppe Naccarato, and et al. 2021. "Immunohistochemistry for Thymidine Kinase-1 (TK1): A Potential Tool for the Prognostic Stratification of Breast Cancer Patients" Journal of Clinical Medicine 10, no. 22: 5416. https://doi.org/10.3390/jcm10225416
APA StyleFanelli, G. N., Scarpitta, R., Cinacchi, P., Fuochi, B., Szumera-Ciećkiewicz, A., De Ieso, K., Ferrari, P., Fontana, A., Miccoli, M., Naccarato, A. G., & Scatena, C. (2021). Immunohistochemistry for Thymidine Kinase-1 (TK1): A Potential Tool for the Prognostic Stratification of Breast Cancer Patients. Journal of Clinical Medicine, 10(22), 5416. https://doi.org/10.3390/jcm10225416









