Expert Consensus on the Contraindications and Cautions of Foam Rolling—An International Delphi Study
Abstract
:1. Background
2. Methods
2.1. Ethics and General Approach
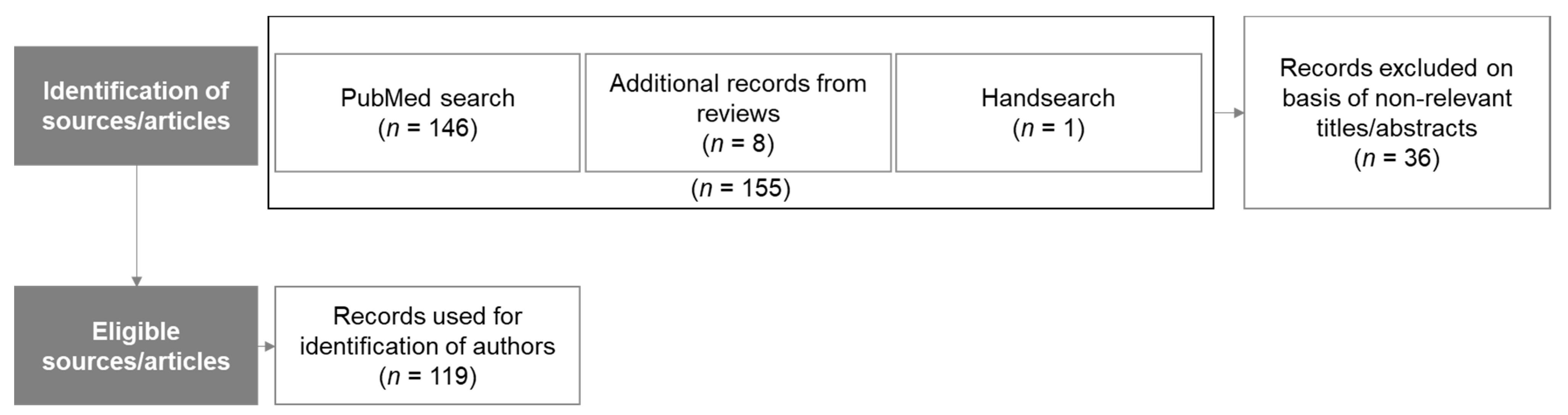
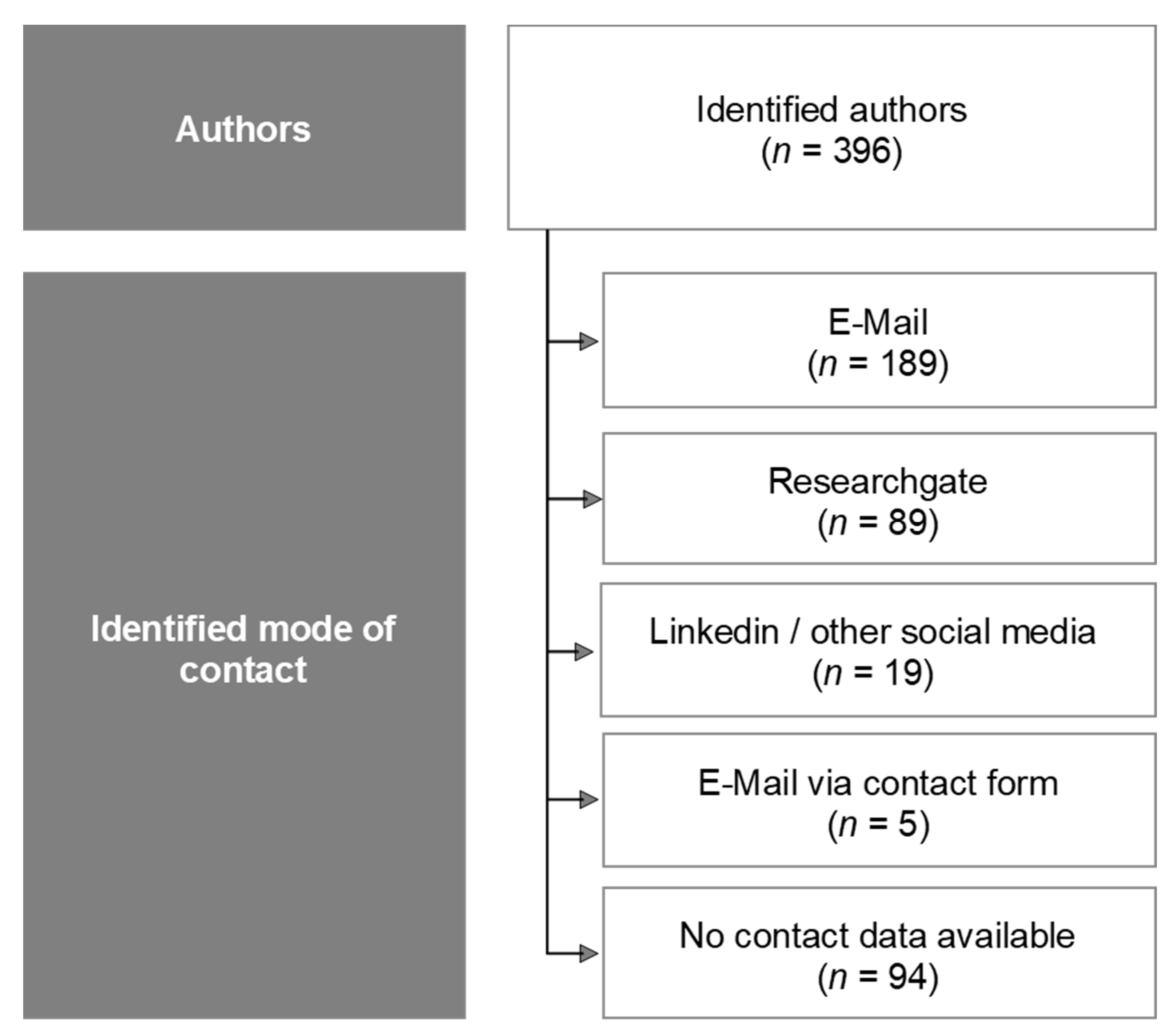
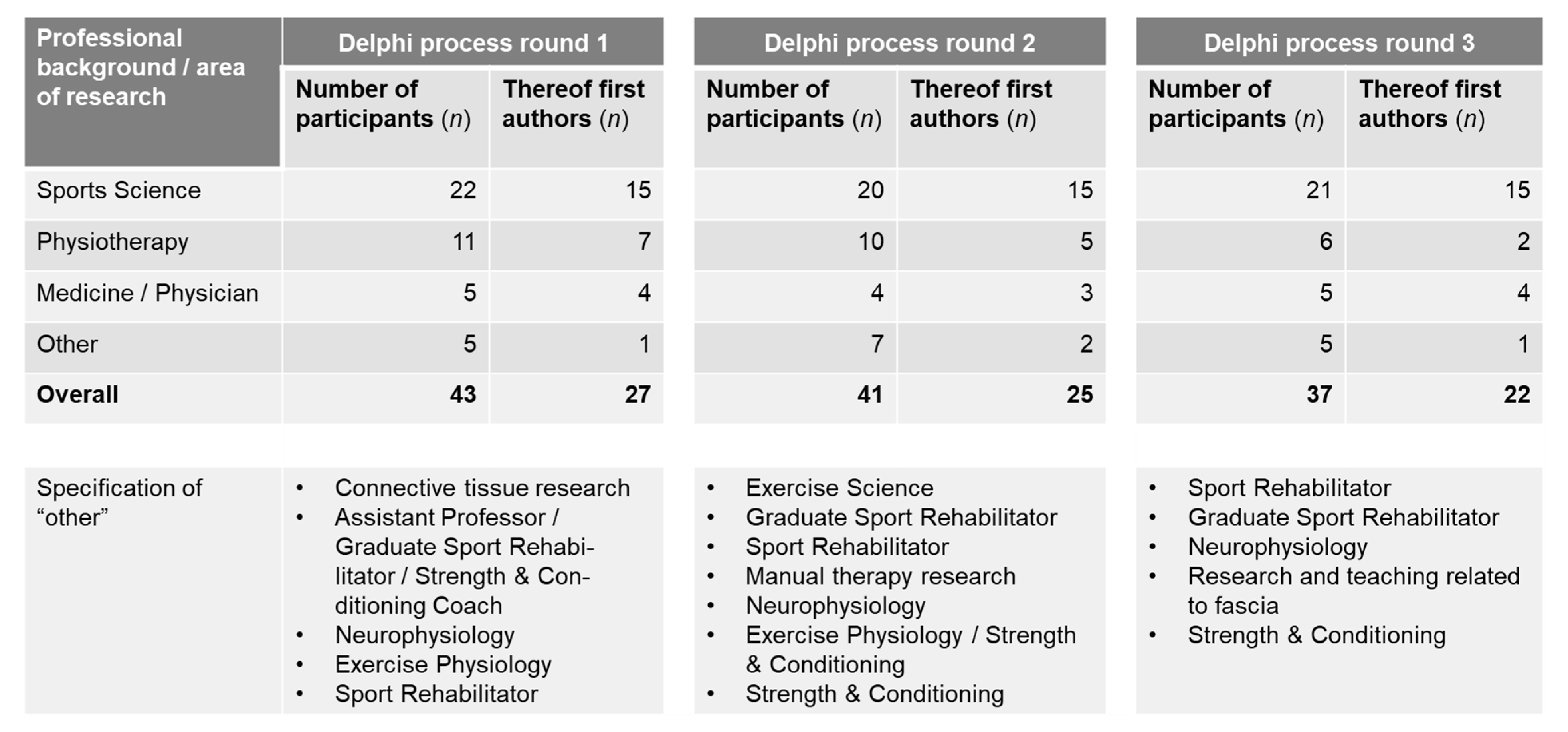
2.2. Recruitment of Panel Members
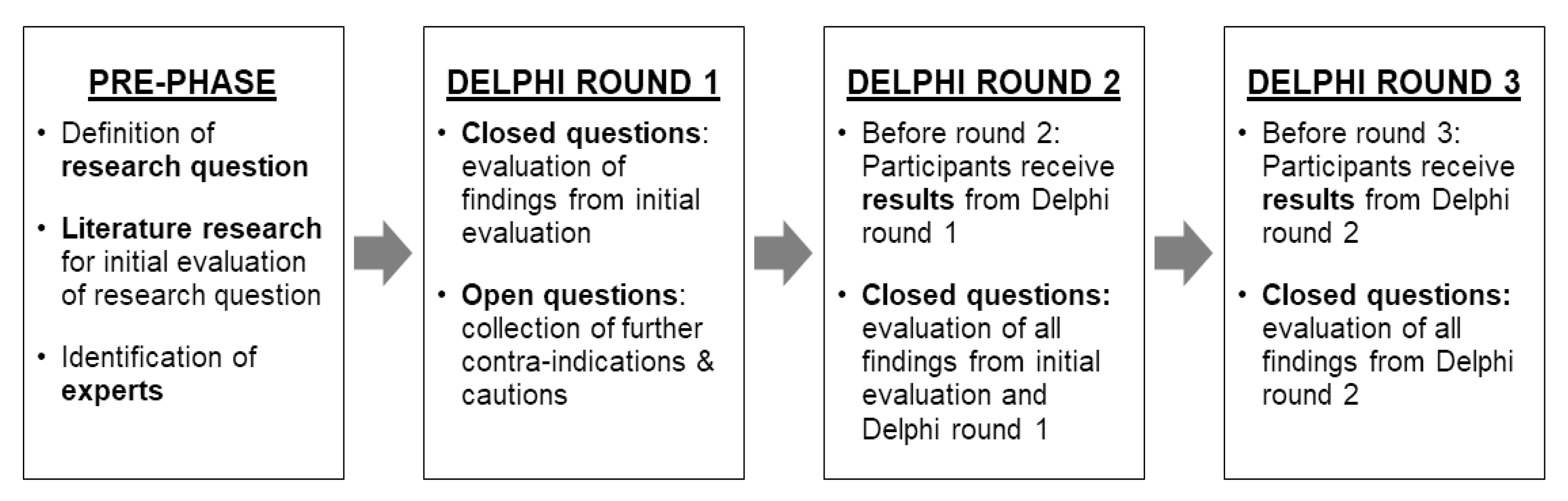
2.2.1. Delphi Process Rounds and Agreement
2.2.2. Preparatory Phase
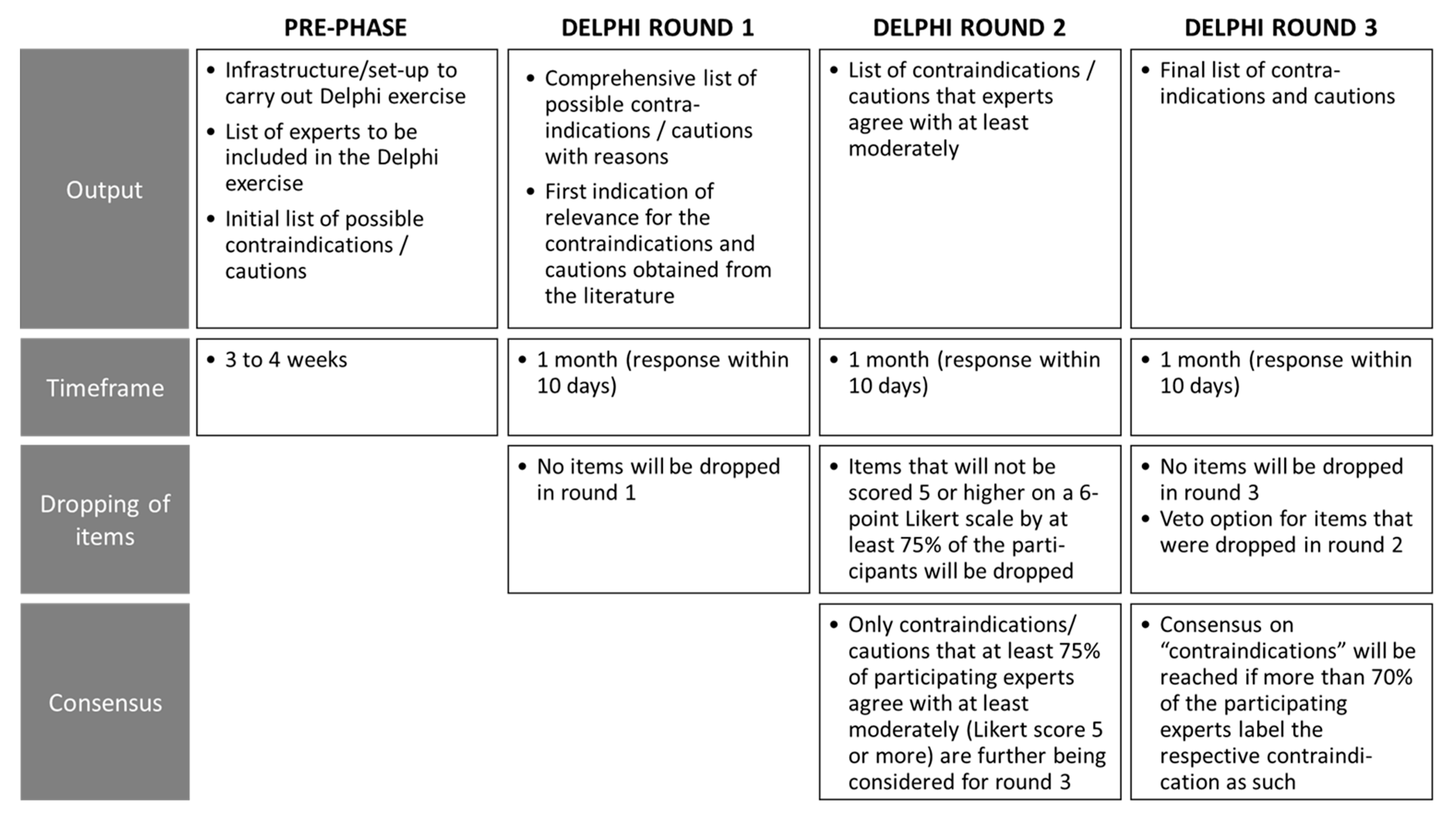
2.2.3. Delphi Process Round 1
2.2.4. Delphi Process Round 2
2.2.5. Delphi Process Round 3
2.2.6. Data Processing
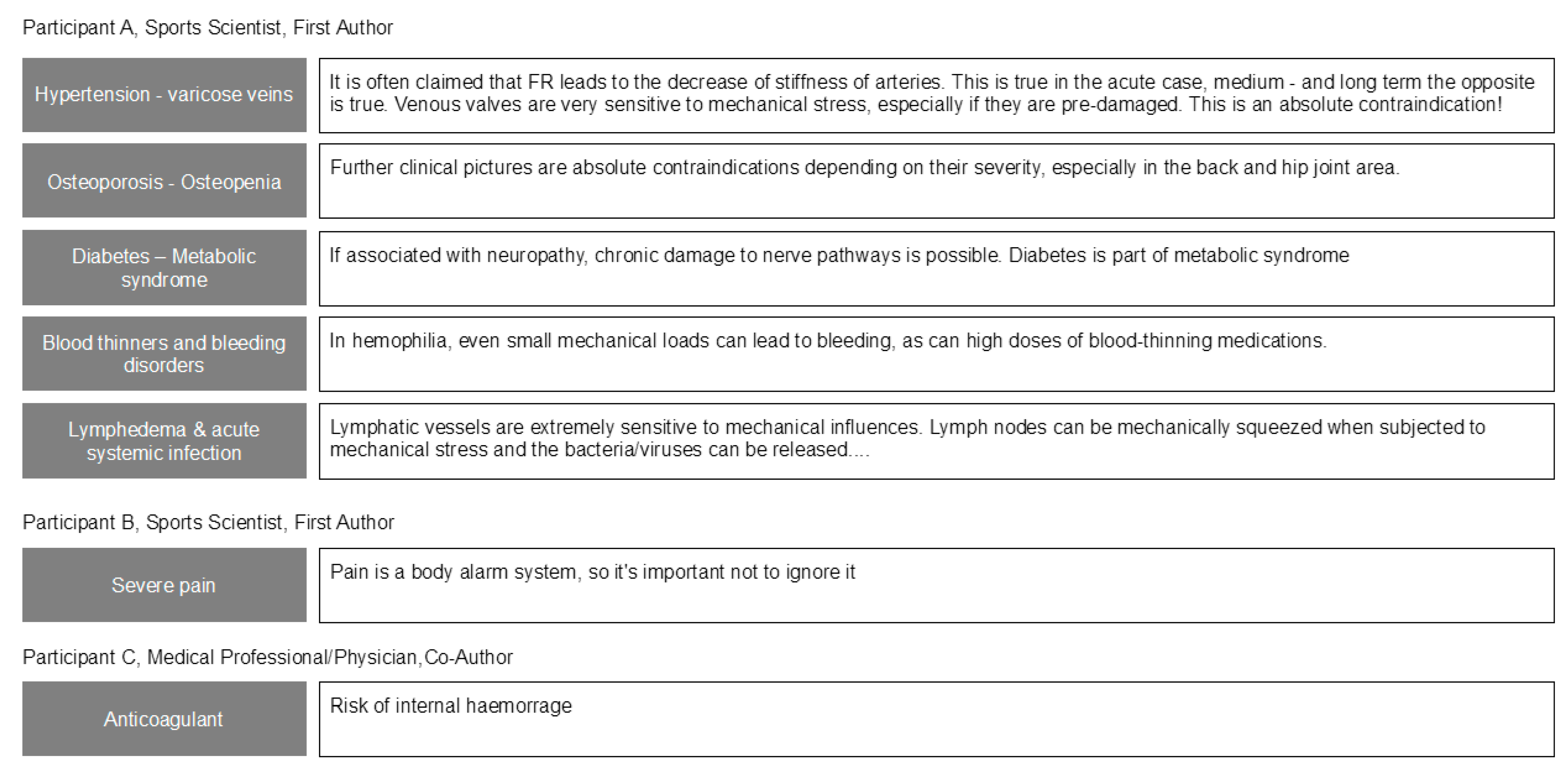
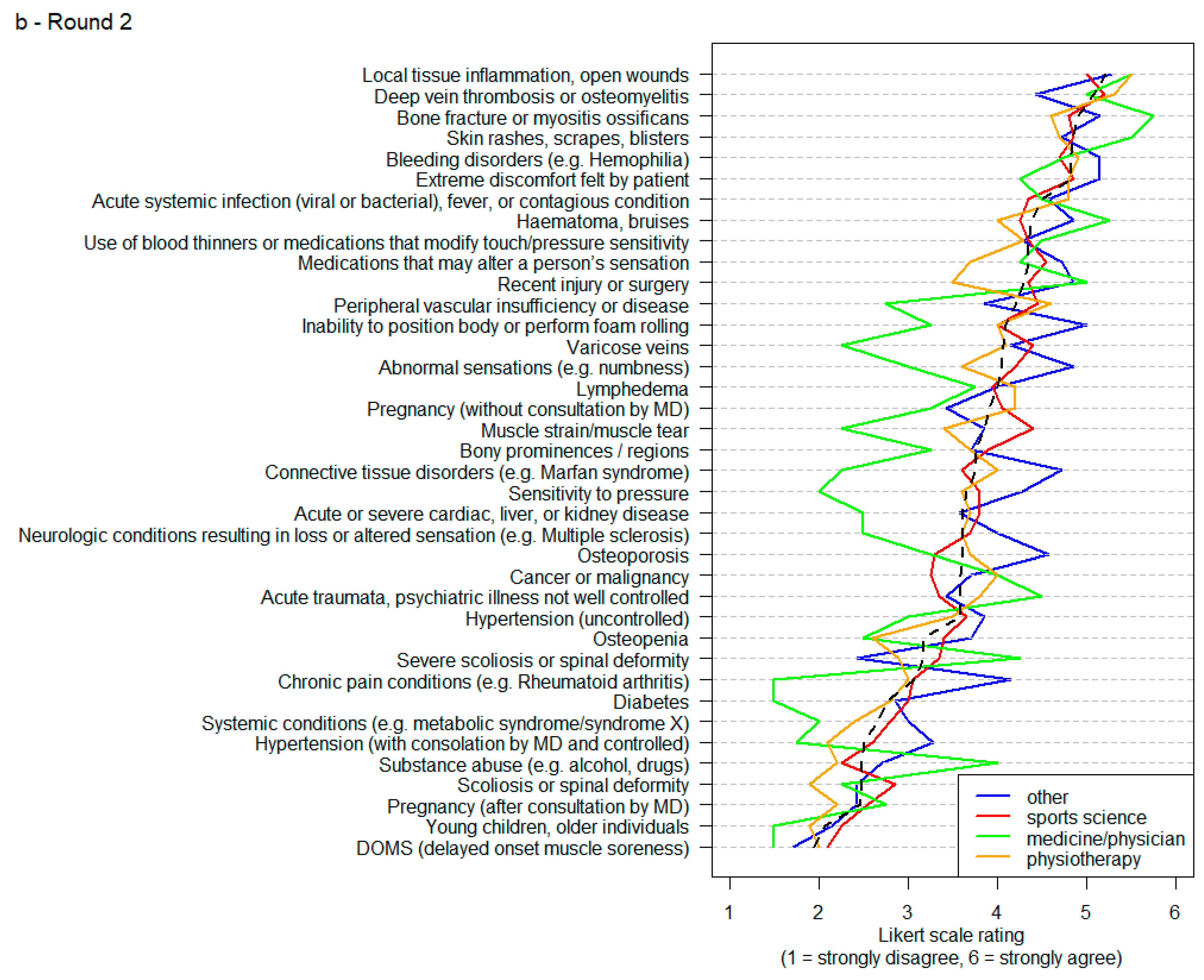
3. Results
4. Discussion
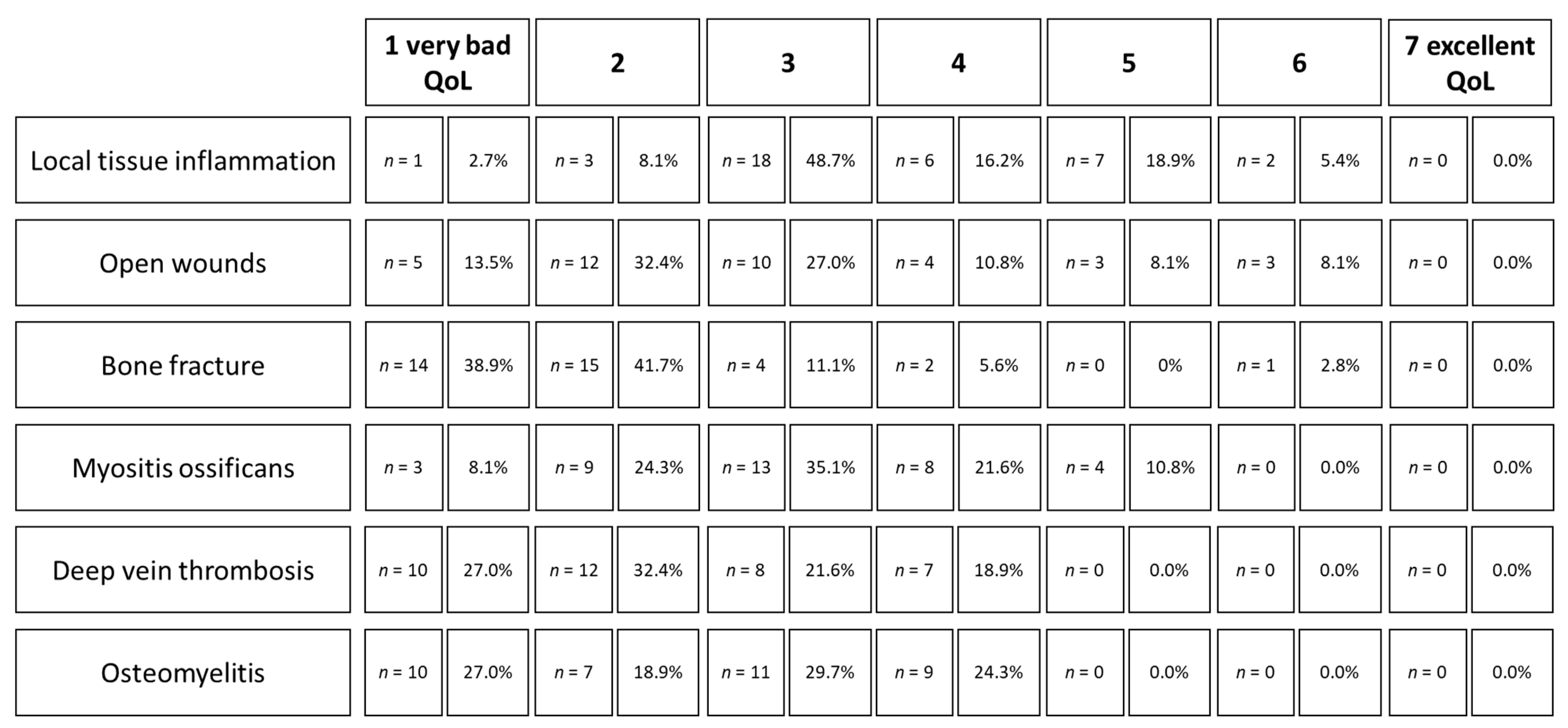
4.1. Open Wounds and Local Tissue Inflammation
4.2. Bone Fractures
4.3. Deep Vein Thrombosis
4.4. Osteomyelitis
4.5. Myositis Ossificans
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beardsley, C.; Škarabot, J. Effects of self-myofascial release: A systematic review. J. Bodyw. Mov. Ther. 2015, 19, 747–758. [Google Scholar] [CrossRef]
- Cheatham, S.W.; Kolber, M.J.; Cain, M.; Lee, M. The Effects of Self-Myofascial Release using a Foam Roll or Roller Massager on Joint Range of Motion, Muscle Recovery, and Performance: A Systematic Review. Int. J. Sports Phys. Ther. 2015, 10, 827–838. [Google Scholar] [PubMed]
- Schroeder, A.N.; Best, T.M. Is self myofascial release an effective preexercise and recovery strategy? A literature review. Curr. Sports Med. Rep. 2015, 14, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Wiewelhove, T.; Doweling, A.; Schneider, C.; Hottenrott, L.; Meyer, T.; Kellmann, M.; Pfeiffer, M.; Ferrauti, A. A Meta-Analysis of the Effects of Foam Rolling on Performance and Recovery. Front. Physiol. 2019, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Altarriba-Bartes, A.; Peña, J.; Vicens-Bordas, J.; Casals, M.; Peirau, X.; Calleja-González, J. The use of recovery strategies by Spanish first division soccer teams: A cross-sectional survey. Physician Sportsmed. 2021, 49, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Querido, S.M.; Brito, J.; Figueiredo, P.; Carnide, F.; Vaz, J.R.; Freitas, S.R. Post-Match Recovery Practices in Professional Football: Design, Validity, and Reliability of a New Questionnaire. Front. Sports Act Living 2021, 3, 680799. [Google Scholar] [CrossRef]
- Freiwald, J.; Baumgart, C.; Kühnemann, M.; Hoppe, M.W. Foam-Rolling in sport and therapy—Potential benefits and risks: Part 1—Definitions, anatomy, physiology, and biomechanics. Sports Orthop. Traumatol. 2016, 32, 258–266. [Google Scholar] [CrossRef]
- Behm, D.G.; Wilke, J. Do Self-Myofascial Release Devices Release Myofascia? Rolling Mechanisms: A Narrative Review. Sports Med. 2019, 49, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, S.; Hill, H.; Hollander, S.D.; Lombard, W.; Parker, R. Effects of foam rolling on performance and recovery: A systematic review of the literature to guide practitioners on the use of foam rolling. J. Bodyw. Mov. Ther. 2020, 24, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Skinner, B.; Moss, R.; Hammond, L. A systematic review and meta-analysis of the effects of foam rolling on range of motion, recovery and markers of athletic performance. J. Bodyw. Mov. Ther. 2020, 24, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Muller, A.L.; Giesche, F.; Power, G.; Ahmedi, H.; Behm, D.G. Acute Effects of Foam Rolling on Range of Motion in Healthy Adults: A Systematic Review with Multilevel Meta-analysis. Sports Med. 2020, 50, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Freiwald, J.; Baumgart, C.; Kühnemann, M.; Hoppe, M.W. Foam-Rolling in sport and therapy—Potential benefits and risks: Part 2—Positive and adverse effects on athletic performance. Sports Orthop. Traumatol. 2016, 32, 267–275. [Google Scholar] [CrossRef]
- Cheatham, S.W.; Stull, K.R. Roller Massage: A Commentary on Clinical Standards and Survey of Physical Therapy Professionals—Part 1. Int. J. Sports Phys. Ther. 2018, 13, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Henschke, N.; Maher, C.G.; Ostelo, R.W.; de Vet, H.C.; Macaskill, P.; Irwig, L. Red flags to screen for malignancy in patients with low-back pain. Cochrane Database Syst. Rev. 2013, 2, CD008686. [Google Scholar] [CrossRef]
- Verhagen, A.P.; Downie, A.; Popal, N.; Maher, C.; Koes, B.W. Red flags presented in current low back pain guidelines: A review. Eur. Spine J. 2016, 25, 2788–2802. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wang, Z.; Zhou, Y.; Onoda, K.; Maruyama, H.; Hu, C.; Liu, Z. Summary of respiratory rehabilitation and physical therapy guidelines for patients with COVID-19 based on recommendations of World Confederation for Physical Therapy and National Association of Physical Therapy. J. Phys. Ther. Sci. 2020, 32, 545–549. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM′s Health-Related Physical Fitness Assessment Manual; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; pp. 135–158. [Google Scholar]
- Ernst, E. The safety of massage therapy. Rheumatology 2003, 42, 1101–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batavia, M. Contraindications for therapeutic massage: Do sources agree? J. Bodyw. Mov. Ther. 2004, 8, 48–57. [Google Scholar] [CrossRef]
- Nielsen, A.; Kligler, B.; Koll, B.S. Safety protocols for gua sha (press-stroking) and baguan (cupping). Complement. Ther. Med. 2012, 20, 340–344. [Google Scholar] [CrossRef]
- McKenney, K.; Elder, A.S.; Elder, C.; Hutchins, A. Myofascial release as a treatment for orthopaedic conditions: A systematic review. J. Athl. Train. 2013, 48, 522–527. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.; Gao, N.; Wu, J.; Litscher, G.; Xu, S. Adverse events of massage therapy in pain-related conditions: A systematic review. Evid. Based Complement. Altern. Med. 2014, 2014, 480956. [Google Scholar] [CrossRef] [PubMed]
- Westman, K.F.; Blaisdell, C. Many Benefits, Little Risk: The Use of Massage in Nursing Practice. Am. J. Nurs. 2016, 116, 34–39, quiz 40–31. [Google Scholar] [CrossRef]
- Laimi, K.; Makila, A.; Barlund, E.; Katajapuu, N.; Oksanen, A.; Seikkula, V.; Karppinen, J.; Saltychev, M. Effectiveness of myofascial release in treatment of chronic musculoskeletal pain: A systematic review. Clin. Rehabil. 2018, 32, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Cantrill, J.A.; Sibbald, B.; Buetow, S. The Delphi and nominal group techniques in health services research. Int. J. Pharm. Pract. 1996, 4, 67–74. [Google Scholar] [CrossRef]
- Wells, C.; Kolt, G.S.; Marshall, P.; Bialocerkowski, A. Indications, benefits, and risks of Pilates exercise for people with chronic low back pain: A Delphi survey of Pilates-trained physical therapists. Phys. Ther. 2014, 94, 806–817. [Google Scholar] [CrossRef] [Green Version]
- Alexiades, N.G.; Shao, B.; Braga, B.P.; Bonfield, C.M.; Brockmeyer, D.L.; Browd, S.R.; DiLuna, M.; Groves, M.L.; Hankinson, T.C.; Jea, A.; et al. Development of best practices in the utilization and implementation of pediatric cervical spine traction: A modified Delphi study. J. Neurosurg. Pediatrics 2021, 27, 649-600. [Google Scholar] [CrossRef] [PubMed]
- Robben, E.; Kempeneers, K.; De Groef, A.; Depreitere, B.; Peers, K. Guidelines for Rehabilitation and Return to Play After Cervical Surgery in a General Athletic Population: A Delphi Analysis. Clin. J. Sport Med. 2021, 31, 145–150. [Google Scholar] [CrossRef]
- Fish, L.; Busby, D. The Delphi method. In Research Methods in Family Therapy; The Guilford Press: New York, NY, USA, 1996; pp. 469–482. [Google Scholar]
- Boulkedid, R.; Abdoul, H.; Loustau, M.; Sibony, O.; Alberti, C. Using and reporting the Delphi method for selecting healthcare quality indicators: A systematic review. PLoS ONE 2011, 6, e20476. [Google Scholar] [CrossRef] [PubMed]
- Trevelyan, E.G.; Robinson, P.N. Delphi methodology in health research: How to do it? Eur. J. Integr. Med. 2015, 7, 423–428. [Google Scholar] [CrossRef]
- Diamond, I.R.; Grant, R.C.; Feldman, B.M.; Pencharz, P.B.; Ling, S.C.; Moore, A.M.; Wales, P.W. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J. Clin. Epidemiol. 2014, 67, 401–409. [Google Scholar] [CrossRef]
- Dirks, M.; Niessen, L.W.; Koudstaal, P.J.; Franke, C.L.; van Oostenbrugge, R.J.; Dippel, D.W. Intravenous thrombolysis in acute ischaemic stroke: From trial exclusion criteria to clinical contraindications. An international Delphi study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 685–689. [Google Scholar] [CrossRef] [Green Version]
- Goluchowicz, K.; Blind, K. Identification of future fields of standardisation: An explorative application of the Delphi methodology. Technol. Forecast. Soc. Chang. 2011, 78, 1526–1541. [Google Scholar] [CrossRef]
- Rowe, G.; Wright, G. The Delphi technique: Past, present, and future prospects—Introduction to the special issue. Technol. Forecast. Soc. Chang. 2011, 78, 1487–1490. [Google Scholar] [CrossRef]
- Schmidt, R.; Lyytinen, K.; Keil, M.; Cule, P. Identifying Software Project Risks: An International Delphi Study. J. Manag. Inf. Syst. 2001, 17, 5–36. [Google Scholar] [CrossRef]
- Okoli, C.; Pawlowski, S.D. The Delphi method as a research tool: An example, design considerations and applications. Inf. Manag. 2004, 42, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Hasson, F.; Keeney, S. Enhancing rigour in the Delphi technique research. Technol. Forecast. Soc. Chang. 2011, 78, 1695–1704. [Google Scholar] [CrossRef]
- Heick, J.; Lazaro, R.T. Differential Diagnosis for Physical Therapists: Screening for Referral, 6th ed.; Elsevier: St. Louis, MO, USA, 2018. [Google Scholar]
- Haraldstad, K.; Wahl, A.; Andenaes, R.; Andersen, J.R.; Andersen, M.H.; Beisland, E.; Borge, C.R.; Engebretsen, E.; Eisemann, M.; Halvorsrud, L.; et al. A systematic review of quality of life research in medicine and health sciences. Qual. Life Res. 2019, 28, 2641–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, W.R.; Scott, A.; Loghmani, M.T.; Ward, S.R.; Warden, S.J. Understanding Mechanobiology: Physical Therapists as a Force in Mechanotherapy and Musculoskeletal Regenerative Rehabilitation. Phys. Ther. 2016, 96, 560–569. [Google Scholar] [CrossRef]
- Janis, J.E.; Harrison, B. Wound Healing: Part I. Basic Science. Plast. Reconstr. Surg. 2016, 138, 9S–17S. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Zein-Hammoud, M.; Standley, P.R. Optimized Modeled Myofascial Release Enhances Wound Healing in 3-Dimensional Bioengineered Tendons: Key Roles for Fibroblast Proliferation and Collagen Remodeling. J. Manip. Physiol. Ther. 2019, 42, 551–564. [Google Scholar] [CrossRef]
- Zein-Hammoud, M.; Standley, P.R. Modeled Osteopathic Manipulative Treatments: A Review of Their in Vitro Effects on Fibroblast Tissue Preparations. J. Am. Osteopath. Assoc. 2015, 115, 490–502. [Google Scholar] [CrossRef]
- Anloague, A.; Mahoney, A.; Ogunbekun, O.; Hiland, T.A.; Thompson, W.R.; Larsen, B.; Loghmani, M.T.; Hum, J.M.; Lowery, J.W. Mechanical stimulation of human dermal fibroblasts regulates pro-inflammatory cytokines: Potential insight into soft tissue manual therapies. BMC Res. Notes 2020, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.V.; Hicks, M.R.; Campbell, D.; Standley, P.R. Dosed myofascial release in three-dimensional bioengineered tendons: Effects on human fibroblast hyperplasia, hypertrophy, and cytokine secretion. J. Manip. Physiol. Ther. 2013, 36, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Kugelmass, N.; Berg, R.N. Ossification: I. Callus Formation and Calcification. Am. J. Dis. Child. 1931, 41, 236–248. [Google Scholar] [CrossRef]
- Mavcic, B.; Antolic, V. Optimal mechanical environment of the healing bone fracture/osteotomy. Int. Orthop. 2012, 36, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Di Nisio, M.; van Es, N.; Büller, H.R. Deep vein thrombosis and pulmonary embolism. Lancet 2016, 388, 3060–3073. [Google Scholar] [CrossRef]
- Streiff, M.B.; Agnelli, G.; Connors, J.M.; Crowther, M.; Eichinger, S.; Lopes, R.; McBane, R.D.; Moll, S.; Ansell, J. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J. Thromb. Thrombolysis 2016, 41, 32–67. [Google Scholar] [CrossRef] [Green Version]
- Jabr, F.I. Massive pulmonary emboli after legs massage. Am. J. Phys. Med. Rehabil. 2007, 86, 691. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.C.; Jayanthi, H.K.; Money-Kyrle, A.; Ramrakha, P. Massaging the outcome: An unusual presentation of pulmonary embolism. BMJ Case Rep. 2009, 2009, bcr0120091505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behera, C.; Devassy, S.; Mridha, A.R.; Chauhan, M.; Gupta, S.K. Leg massage by mother resulting in fatal pulmonary thromboembolism. Med. Leg. J. 2018, 86, 146–150. [Google Scholar] [CrossRef]
- Sutham, K.; Na-Nan, S.; Paiboonsithiwong, S.; Chaksuwat, P.; Tongsong, T. Leg massage during pregnancy with unrecognized deep vein thrombosis could be life threatening: A case report. BMC Pregnancy Childbirth 2020, 20, 237. [Google Scholar] [CrossRef]
- Goldhaber, S.Z.; Bounameaux, H. Pulmonary embolism and deep vein thrombosis. Lancet 2012, 379, 1835–1846. [Google Scholar] [CrossRef] [Green Version]
- Wilbur, J.; Shian, B. Deep Venous Thrombosis and Pulmonary Embolism: Current Therapy. Am. Fam. Physician 2017, 95, 295–302. [Google Scholar] [PubMed]
- Lima, A.L.; Oliveira, P.R.; Carvalho, V.C.; Cimerman, S.; Savio, E. Diretrizes Panamericanas para el Tratamiento de las Osteomielitis e Infecciones de Tejidos Blandos, G. Recommendations for the treatment of osteomyelitis. Braz. J. Infect. Dis. 2014, 18, 526–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Yao, J.L.; Wu, Z.Q.; Zeng, D.L.; Zheng, L.Y.; Chen, D.; Guo, Z.D.; Peng, L. Current opinions on the mechanism, classification, imaging diagnosis and treatment of post-traumatic osteomyelitis. Chin. J. Traumatol. 2021. in Press. [Google Scholar] [CrossRef] [PubMed]
- Devilbiss, Z.; Hess, M.; Ho, G.W.K. Myositis Ossificans in Sport: A Review. Curr. Sports Med. Rep. 2018, 17, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, T.A.; Jarvinen, T.L.; Kaariainen, M.; Aarimaa, V.; Vaittinen, S.; Kalimo, H.; Jarvinen, M. Muscle injuries: Optimising recovery. Best Pract. Res. Clin. Rheumatol. 2007, 21, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Grabow, L.; Young, J.D.; Alcock, L.R.; Quigley, P.J.; Byrne, J.M.; Granacher, U.; Škarabot, J.; Behm, D.G. Higher Quadriceps Roller Massage Forces Do Not Amplify Range-of-Motion Increases nor Impair Strength and Jump Performance. J. Strength Cond. Res. 2018, 32, 3059–3069. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.A.; Ramer, L.M. Duration of Myofascial Rolling for Optimal Recovery, Range of Motion, and Performance: A Systematic Review of the Literature. Int. J. Sports Phys. Ther. 2019, 14, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Niemeyer, P.; Niederer, D.; Schleip, R.; Banzer, W. Influence of Foam Rolling Velocity on Knee Range of Motion and Tissue Stiffness: A Randomized, Controlled Crossover Trial. J. Sport Rehabil. 2019, 28, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, S.W.; Stull, K.R. Roller massage: Comparison of three different surface type pattern foam rollers on passive knee range of motion and pain perception. J. Bodyw. Mov. Ther. 2019, 23, 555–560. [Google Scholar] [CrossRef]
- Cheatham, S.W.; Stull, K.R. Comparison of Three Different Density Type Foam Rollers on Knee Range of Motion and Pressure Pain Threshold: A Randomized Controlled Trial. Int. J. Sports Phys. Ther. 2018, 13, 474–482. [Google Scholar] [CrossRef]
- Kim, Y.; Hong, Y.; Park, H.S. A soft massage tool is advantageous for compressing deep soft tissue with low muscle tension: Therapeutic evidence for self-myofascial release. Complement. Ther. Med. 2019, 43, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, S.W.; Stull, K.R.; Kolber, M.J. Comparison of a Vibration Roller and a Nonvibration Roller Intervention on Knee Range of Motion and Pressure Pain Threshold: A Randomized Controlled Trial. J. Sport Rehabil. 2019, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Park, C.B. The immediate effects of foam roller with vibration on hamstring flexibility and jump performance in healthy adults. J. Exerc. Rehabil. 2019, 15, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Moraleda, B.; González-García, J.; Cuéllar-Rayo, Á.; Balsalobre-Fernández, C.; Muñoz-García, D.; Morencos, E. Effects of Vibration and Non-Vibration Foam Rolling on Recovery after Exercise with Induced Muscle Damage. J. Sports Sci. Med. 2019, 18, 172–180. [Google Scholar] [PubMed]
- Available online: www.faszienforschung.de (accessed on 9 September 2021).
| Search Steps | Search Terms | Search Results |
|---|---|---|
| 1 | “foam roll”, “foam rolling”, or “foam roller” combined by Boolean logic (“OR”) | 146 |
| 2 | “contraindication*”, “caution*”, “precaution*”, “red flag*”, or “yellow flag*” combined by Boolean logic (“OR”) | 75.613 |
| 1 AND 2 | combined by Boolean logic (“AND”) | 1 |
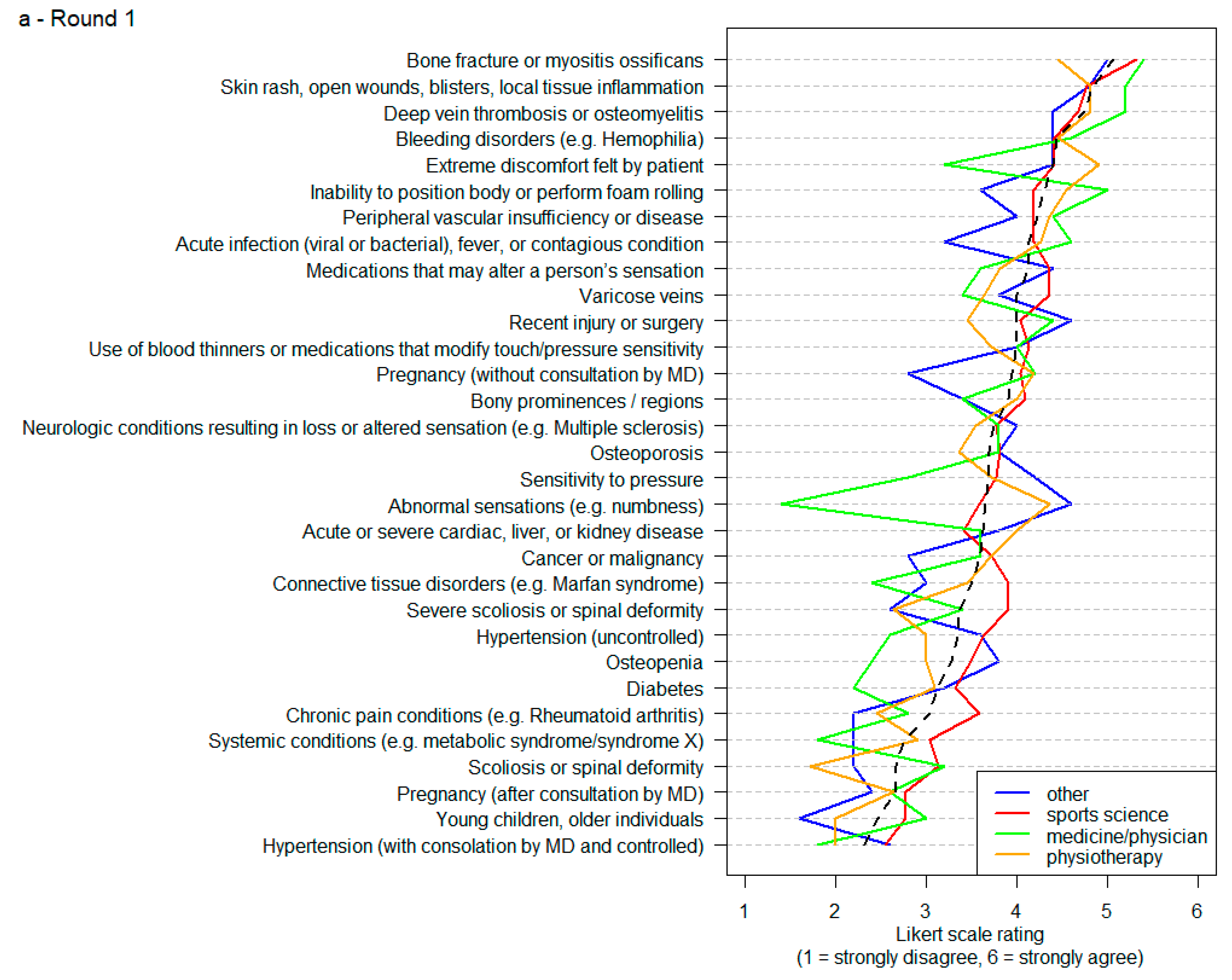
| Item | Round 1 | Round 2 | Round 3 |
|---|---|---|---|
| 1 | Abnormal sensations (e.g., numbness) | Abnormal sensations (e.g., numbness) | Local tissue inflammation |
| 2 | Hypertension (with consolation by MD and controlled) | Hypertension (with consolation by MD and controlled) | Open wounds |
| 3 | Hypertension (uncontrolled) | Hypertension (uncontrolled) | Bone fracture |
| 4 | Diabetes | Diabetes | Myositis ossificans |
| 5 | Systemic conditions (e.g., metabolic syndrome/syndrome X) | Systemic conditions (e.g., metabolic syndrome/syndrome X) | Deep vein thrombosis |
| 6 | Chronic pain conditions (e.g., Rheumatoid Arthritis) | Chronic pain conditions (e.g., Rheumatoid Arthritis) | Osteomyelitis |
| 7 | Connective tissue disorders (e.g., Marfan syndrome) | Connective tissue disorders (e.g., Marfan syndrome) | |
| 8 | Pregnancy (without consultation by MD) | Pregnancy (without consultation by MD) | |
| 9 | Pregnancy (after consultation by MD) | Pregnancy (after consultation by MD) | |
| 10 | Inability to position body or perform foam rolling | Inability to position body or perform foam rolling | |
| 11 | Sensitivity to pressure | Sensitivity to pressure | |
| 12 | Extreme discomfort felt by patient | Extreme discomfort felt by patient | |
| 13 | Medications that may alter a person’s sensation | Medications that may alter a person’s sensation | |
| 14 | Use of blood thinners or medications that modify touch/pressure sensitivity | Use of blood thinners or medications that modify touch/pressure sensitivity | |
| 15 | Bleeding disorders (e.g., Hemophilia) | Bleeding disorders (e.g., Hemophilia) | |
| 16 | Young children, older individuals | Young children, older individuals | |
| 17 | Skin rash, open wounds, blisters, local tissue inflammation | Hematoma, bruises | |
| 18 | Acute infection (viral or bacterial), fever, or contagious condition | Skin rashes, scrapes, blisters | |
| 19 | Bony prominences/regions | Local tissue inflammation, open wounds | |
| 20 | Bone fracture or myositis ossificans | Acute systemic infection (viral or bacterial), fever, or contagious condition | |
| 21 | Recent injury or surgery | Lymphedema | |
| 22 | Scoliosis or spinal deformity | Muscle strain/muscle tear | |
| 23 | Severe scoliosis or spinal deformity | DOMS (delayed onset muscle soreness) | |
| 24 | Osteoporosis | Bony prominences/regions | |
| 25 | Osteopenia | Bone fracture or myositis ossificans | |
| 26 | Neurologic conditions resulting in loss or altered sensation (e.g., Multiple Sclerosis) | Recent injury or surgery | |
| 27 | Acute or severe cardiac, liver or kidney disease | Scoliosis or spinal deformity | |
| 28 | Peripheral vascular insufficiency or disease | Severe scoliosis or spinal deformity | |
| 29 | Deep vein thrombosis or osteomyelitis | Osteoporosis | |
| 30 | Varicose Veins | Osteopenia | |
| 31 | Cancer or malignancy | Neurologic conditions resulting in loss or altered sensation (e.g., Multiple Sclerosis) | |
| 32 | Acute traumata, psychiatric illness not well controlled | ||
| 33 | Acute or severe cardiac, liver or kidney disease | ||
| 34 | Peripheral vascular insufficiency or disease | ||
| 35 | Deep vein thrombosis or osteomyelitis | ||
| 36 | Varicose Veins | ||
| 37 | Cancer or malignancy | ||
| 38 | Substance abuse (e.g., alcohol, drugs) |
| Contraindication | Caution | Neither Contraindication nor Caution | Contraindication + Caution | Result According to Valuation Logic | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | Sum | ||
| Local tissue inflammation | 15 | 41 | 21 | 57 | 1 | 3 | 36 | 97 | 37 | Caution |
| Open wounds | 27 | 73 | 8 | 22 | 2 | 5 | 35 | 95 | 37 | Contraindication |
| Bone fracture | 31 | 84 | 4 | 11 | 2 | 5 | 35 | 95 | 37 | Contraindication |
| Myositis ossificans | 20 | 54 | 14 | 38 | 3 | 8 | 34 | 92 | 37 | Caution |
| Deep vein thrombosis | 24 | 65 | 12 | 32 | 1 | 3 | 36 | 97 | 37 | Caution |
| Osteomyelitis | 22 | 61 | 12 | 33 | 2 | 6 | 34 | 94 | 36 | Caution |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartsch, K.M.; Baumgart, C.; Freiwald, J.; Wilke, J.; Slomka, G.; Turnhöfer, S.; Egner, C.; Hoppe, M.W.; Klingler, W.; Schleip, R. Expert Consensus on the Contraindications and Cautions of Foam Rolling—An International Delphi Study. J. Clin. Med. 2021, 10, 5360. https://doi.org/10.3390/jcm10225360
Bartsch KM, Baumgart C, Freiwald J, Wilke J, Slomka G, Turnhöfer S, Egner C, Hoppe MW, Klingler W, Schleip R. Expert Consensus on the Contraindications and Cautions of Foam Rolling—An International Delphi Study. Journal of Clinical Medicine. 2021; 10(22):5360. https://doi.org/10.3390/jcm10225360
Chicago/Turabian StyleBartsch, Katja Martina, Christian Baumgart, Jürgen Freiwald, Jan Wilke, Gunda Slomka, Sascha Turnhöfer, Christoph Egner, Matthias W. Hoppe, Werner Klingler, and Robert Schleip. 2021. "Expert Consensus on the Contraindications and Cautions of Foam Rolling—An International Delphi Study" Journal of Clinical Medicine 10, no. 22: 5360. https://doi.org/10.3390/jcm10225360
APA StyleBartsch, K. M., Baumgart, C., Freiwald, J., Wilke, J., Slomka, G., Turnhöfer, S., Egner, C., Hoppe, M. W., Klingler, W., & Schleip, R. (2021). Expert Consensus on the Contraindications and Cautions of Foam Rolling—An International Delphi Study. Journal of Clinical Medicine, 10(22), 5360. https://doi.org/10.3390/jcm10225360







