Influence of Glucocorticoids on Cellular Senescence Hallmarks in Osteoarthritic Fibroblast-like Synoviocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Immunohistochemistry (IHC)
2.3. Cell Culture
2.4. BrdU Cell Proliferation Assay
2.5. Cell Viability MTS Assay
2.6. Senescence β-Galactosidase Staining
2.7. Gene Expression Analysis by Real-Time Quantitative PCR
2.8. Protein Expression Analysis by Western Blotting
2.9. Protein Expression Analysis by Immunofluorescence
2.10. Statistical Analysis
3. Results
3.1. Senescent p16INK4A-Positive Cells in OA Synovial Membrane Obtained from Biopsy
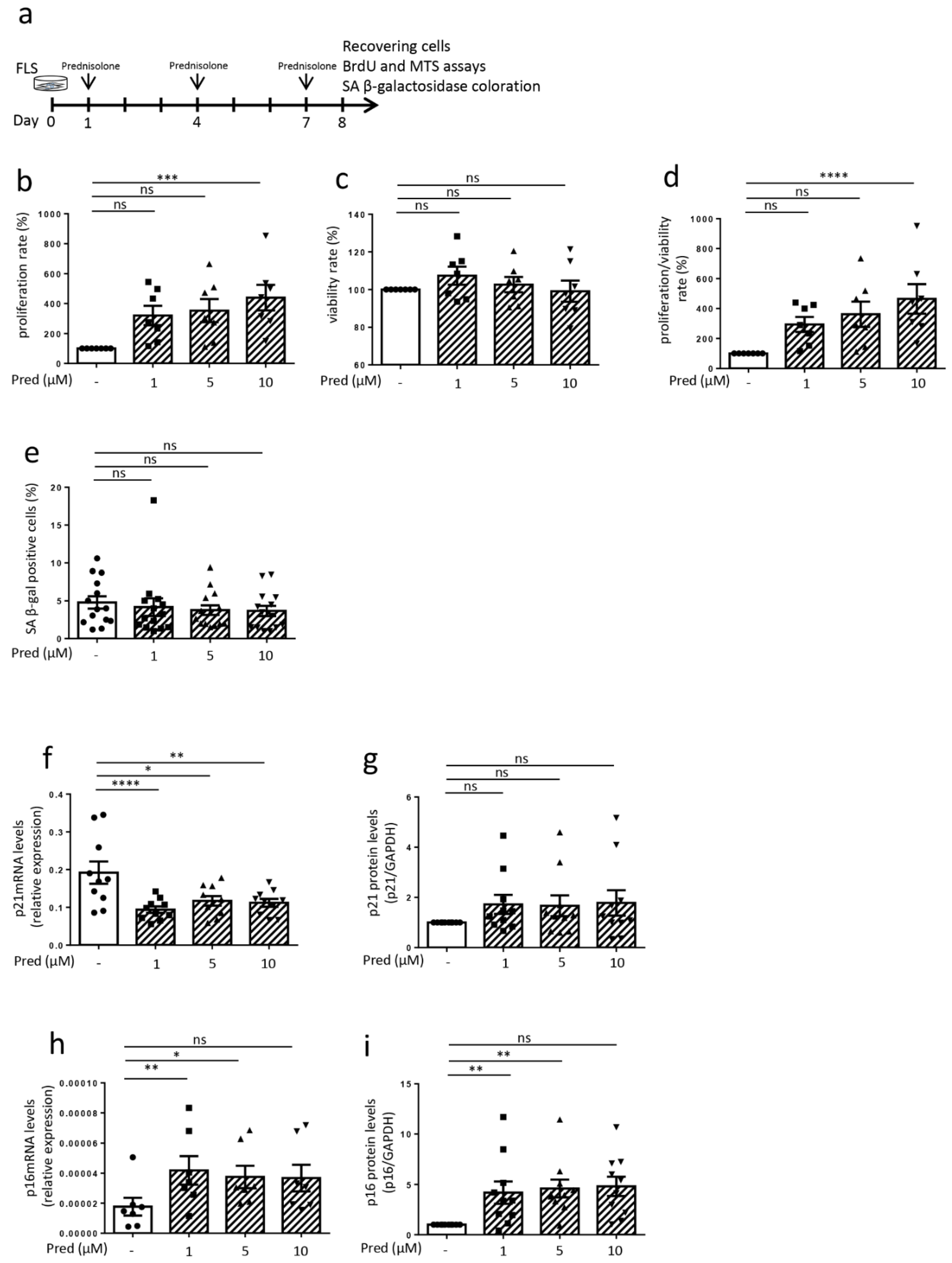
3.2. Influence of Prednisolone on Senescence Hallmarks in Osteoarthritic Fibroblast-like Synoviocytes (Cells Recovering after 8 Days)
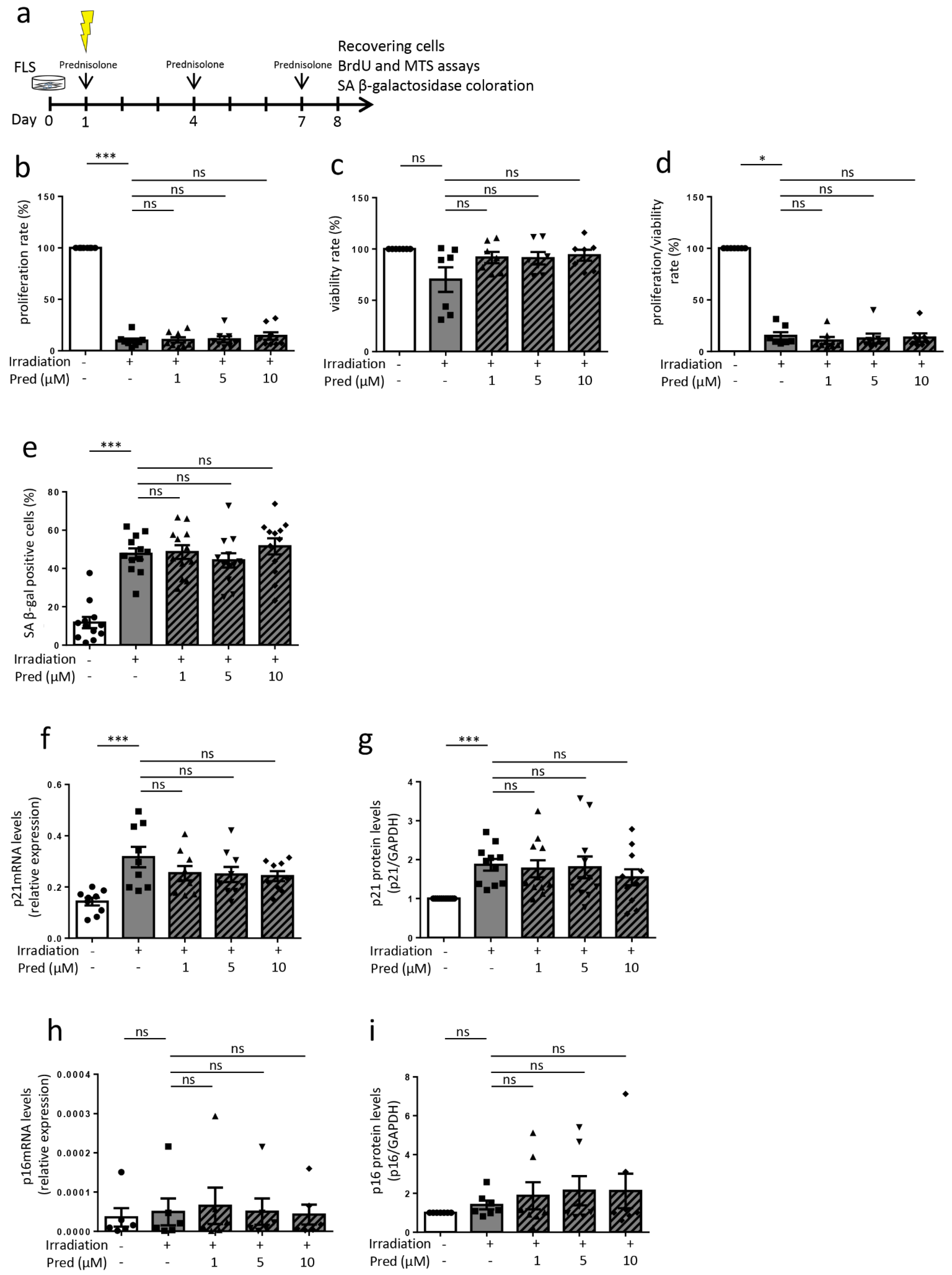
3.3. Influence of Prednisolone on Senescence Induction in Osteoarthritic Fibroblast-like Synoviocytes (Cells Recovering after 8 Days)
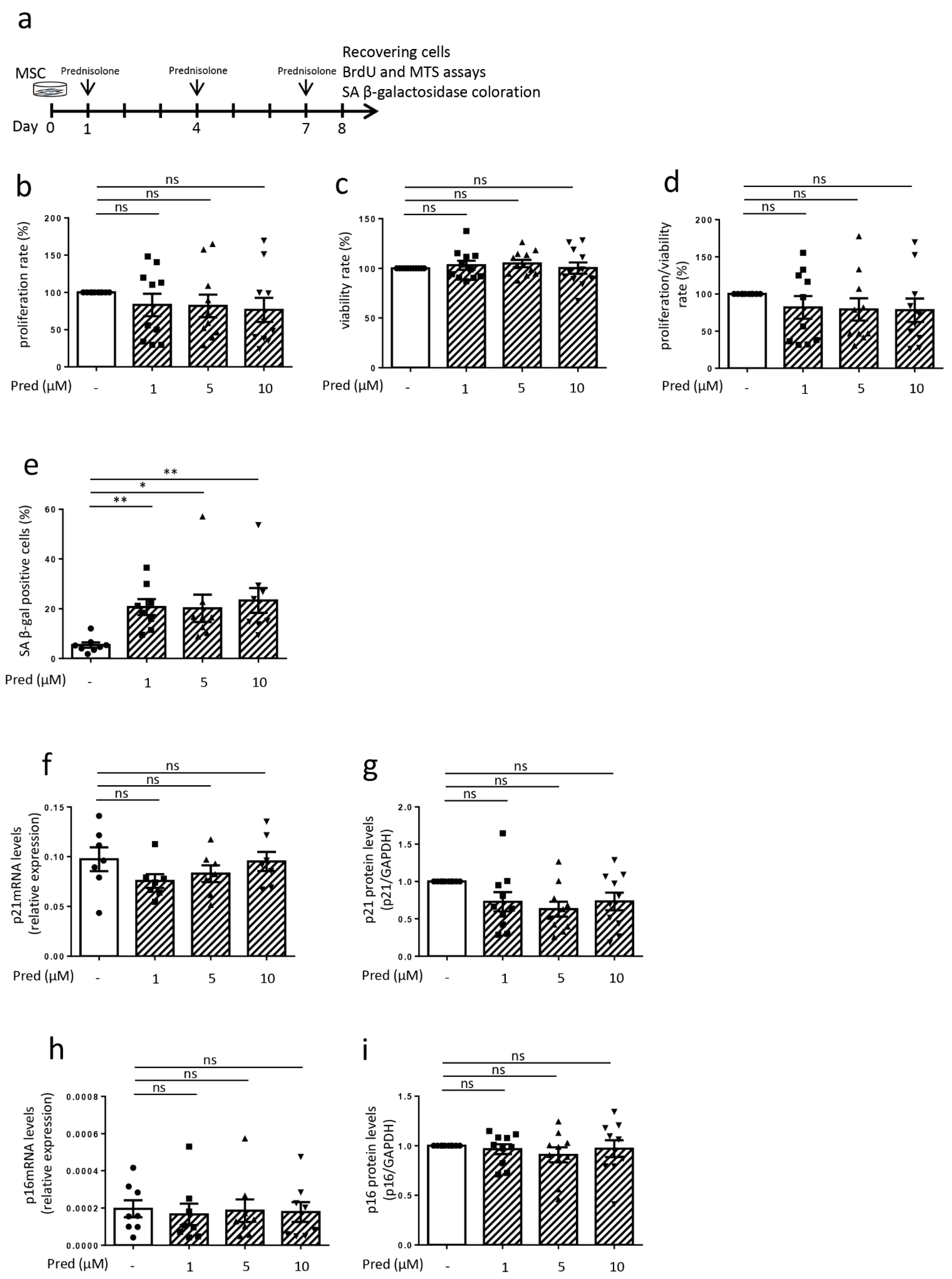
3.4. Influence of Prednisolone on Senescence Hallmarks in Mesenchymal Stem Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Tachikart, Y.; Malaise, O.; Mumme, M.; Jorgensen, C.; Brondello, J.M. Seno-suppressive molecules as new therapeutic perspectives in rheumatic diseases. Biochem. Pharmacol. 2019, 165, 126–133. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; de Jong, T.V.; Melov, S.; Guryev, V.; Campisi, J.; Demaria, M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr. Biol. 2017, 27, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.A.; Conaghan, P.; Le Bars, M.; Baron, G.; Grassi, W.; Martin-Mola, E.; Wakefield, R.; Brasseur, J.L.; So, A.; Backhaus, M.; et al. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: Prevalence of inflammation in osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1703–1709. [Google Scholar] [CrossRef]
- Atukorala, I.; Kwoh, C.K.; Guermazi, A.; Roemer, F.W.; Boudreau, R.M.; Hannon, M.J.; Hunter, D.J. Synovitis in knee osteoarthritis: A precursor of disease? Ann. Rheum. Dis. 2016, 75, 390–395. [Google Scholar] [CrossRef]
- De Seny, D.; Bianchi, E.; Baiwir, D.; Cobraiville, G.; Collin, C.; Deliège, M.; Kaiser, M.J.; Mazzucchelli, G.; Hauzeur, J.P.; Delvenne, P.; et al. Proteins involved in the endoplasmic reticulum stress are modulated in synovitis of osteoarthritis, chronic pyrophosphate arthropathy and rheumatoid arthritis, and correlate with the histological inflammatory score. Sci. Rep. 2020, 10, 14149. [Google Scholar] [CrossRef]
- Chou, C.H.; Jain, V.; Gibson, J.; Attarian, D.E.; Haraden, C.A.; Yohn, C.B.; Laberge, R.M.; Gregory, S.; Kraus, V.B. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 2020, 10, 10868. [Google Scholar] [CrossRef]
- Malaise, O.; Tachikart, Y.; Constantinides, M.; Mumme, M.; Ferreira-Lopez, R.; Noack, S.; Krettek, C.; Noël, D.; Wang, J.; Jorgensen, C.; et al. Mesenchymal stem cell senescence alleviates their intrinsic and senosuppressive paracrine properties contributing to osteoarthritis development. Aging 2019, 11, 9128–9146. [Google Scholar] [CrossRef]
- Diekman, B.O.; Sessions, G.A.; Collins, J.A.; Knecht, A.K.; Strum, S.L.; Mitin, N.K.; Carlson, C.S.; Loeser, R.F.; Sharpless, N.E. Expression of p16INK4a is a biomarker of chondrocyte aging but does not cause osteoarthritis. Aging Cell 2018, 17, e12771. [Google Scholar] [CrossRef] [PubMed]
- Koenig, K.M.; Ong, K.L.; Lau, E.C.; Vail, T.P.; Berry, D.J.; Rubash, H.E.; Kurtz, S.; Bozic, K.J. The Use of Hyaluronic Acid and Corticosteroid Injections Among Medicare Patients With Knee Osteoarthritis. J. Arthroplast. 2016, 31, 351–355. [Google Scholar] [CrossRef]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis a randomized clinical trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef]
- Fubini, S.L.; Todhunter, R.J.; Burton-Wurster, N.; Vernier-Singer, M.; MacLeod, J.N. Corticosteroids alter the differentiated phenotype of articular chondrocytes. J. Orthop. Res. 2001, 19, 688–695. [Google Scholar] [CrossRef]
- Black, R.; Grodzinsky, A.J. Dexamethasone: Chondroprotective corticosteroid or catabolic killer? Eur. Cells Mater. 2019, 38, 246–263. [Google Scholar]
- Xue, E.; Zhang, Y.; Song, B.; Xiao, J.; Shi, Z. Effect of autophagy induced by dexamethasone on senescence in chondrocytes. Mol. Med. Rep. 2016, 14, 3037–3044. [Google Scholar] [CrossRef]
- Poulsen, R.C.; Watts, A.C.; Murphy, R.J.; Snelling, S.J.; Carr, A.J.; Hulley, P.A. Glucocorticoids induce senescence in primary human tenocytes by inhibition of sirtuin 1 and activation of the p53/p21 pathway: In vivo and in vitro evidence. Ann. Rheum. Dis. 2014, 73, 1405–1413. [Google Scholar] [PubMed]
- Lechanteur, C.; Briquet, A.; Giet, O.; Delloye, O.; Baudoux, E.; Beguin, Y. Clinical-scale expansion of mesenchymal stromal cells: A large banking experience. J. Transl. Med. 2016, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Relić, B.; Benoit, V.; Franchimont, N.; Ribbens, C.; Kaiser, M.J.; Gillet, P.; Merville, M.P.; Bours, V.; Malaise, M.G. 15-Deoxy-Δ12,14-prostaglandin J2 inhibits bay 11-7085-induced sustained extracellular signal-regulated kinase phosphorylation and apoptosis in human articular chondrocytes and synovial fibroblasts. J. Biol. Chem. 2004, 279, 22399–22403. [Google Scholar] [CrossRef]
- Deroyer, C.; Charlier, E.; Neuville, S.; Malaise, O.; Gillet, P.; Kurth, W.; Chariot, A.; Malaise, M.; de Seny, D. CEMIP (KIAA1199) induces a fibrosis-like process in osteoarthritic chondrocytes. Cell Death Dis. 2019, 10, 103. [Google Scholar] [CrossRef]
- Mizuno, M.; Katano, H.; Mabuchi, Y.; Ogata, Y.; Ichinose, S.; Fujii, S.; Otabe, K.; Komori, K.; Ozeki, N.; Koga, H.; et al. Specific markers and properties of synovial mesenchymal stem cells in the surface, stromal, and perivascular regions. Stem Cell Res. Ther. 2018, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Manferdini, C.; Toupet, K.; Peyrafitte, J.A.; Ferreira, R.; Facchini, A.; Gabusi, E.; Bourin, P.; Jorgensen, C.; Lisignoli, G.; et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013, 11, 834–844. [Google Scholar] [CrossRef]
- Malaise, O.; Relic, B.; Quesada-Calvo, F.; Charlier, E.; Zeddou, M.; Neuville, S.; Gillet, P.; Louis, E.; De Seny, D.; Malaise, M.G. Selective glucocorticoid receptor modulator compound A, in contrast to prednisolone, does not induce leptin or the leptin receptor in human osteoarthritis synovial fibroblasts. Rheumatology 2015, 54, 1087–1092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Relic, B.; Zeddou, M.; Desoroux, A.; Beguin, Y.; De Seny, D.; Malaise, M.G. Genistein induces adipogenesis but inhibits leptin induction in human synovial fibroblasts. Lab. Investig. 2009, 89, 811–822. [Google Scholar] [CrossRef]
- Malaise, O.; Relic, B.; Charlier, E.; Zeddou, M.; Neuville, S.; Deroyer, C.; Gillet, P.; Louis, E.; Malaise, M.G.; de Seny, D. Glucocorticoid-induced leucine zipper (GILZ) is involved in glucocorticoid-induced and mineralocorticoid-induced leptin production by osteoarthritis synovial fibroblasts. Arthritis Res. Ther. 2016, 18, 219. [Google Scholar] [CrossRef]
- De Seny, D.; Cobraiville, G.; Charlier, E.; Neuville, S.; Esser, N.; Malaise, D.; Malaise, O.; Calvo, F.Q.; Relic, B.; Malaise, M.G. Acute-Phase Serum Amyloid A in Osteoarthritis: Regulatory Mechanism and Proinflammatory Properties. PLoS ONE 2013, 8, e66769. [Google Scholar]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Res. Ther. 2020, 11, 381. [Google Scholar] [CrossRef]
- Liu, X.; Chai, Y.; Liu, G.; Su, W.; Guo, Q.; Lv, X.; Gao, P.; Yu, B.; Ferbeyre, G.; Cao, X.; et al. Osteoclasts protect bone blood vessels against senescence through the angiogenin/plexin-B2 axis. Nat. Commun. 2021, 12, 1832. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Liang, Z.; Wang, L.; Su, F.; Wang, X.; You, X.; He, C. Targeting cellular senescence prevents glucocorticoid-induced bone loss through modulation of the DPP4-GLP-1 axis. Signal Transduct. Target. Ther. 2021, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Kim, S.G.; Kim, J.R.; Choi, H.C. Prednisolone suppresses adriamycin-induced vascular smooth muscle cell senescence and inflammatory response via the SIRT1-AMPK signaling pathway. PLoS ONE 2020, 15, e0239976. [Google Scholar] [CrossRef] [PubMed]
- Bogarin, T.; Saraswathy, S.; Akiyama, G.; Xie, X.; Weinreb, R.N.; Zheng, J.; Huang, A.S. Cellular and cytoskeletal alterations of scleral fibroblasts in response to glucocorticoid steroids. Exp. Eye Res. 2019, 187, 107774. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.F.; Richardson, L.S.; Da Silva, M.G.; Sheller-Miller, S.; Menon, R. Dexamethasone induces primary amnion epithelial cell senescence through telomere-P21 associated pathway. Biol. Reprod. 2019, 100, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mawal-Dewan, M.; Cristofalo, V.J.; Sell, C. Enhanced proliferation of human fibroblasts, in the presence of dexamethasone, is accompanied by changes in p21(Waf1/Cip1/Sdi1) and the insulin-like growth factor type 1 receptor. J. Cell. Physiol. 1998, 177, 396–401. [Google Scholar] [CrossRef]
- Zannas, A.S.; Kosyk, O.; Leung, C.S. Prolonged glucocorticoid exposure does not accelerate telomere shortening in cultured human fibroblasts. Genes 2020, 11, 1425. [Google Scholar] [CrossRef]
- Carvalho, C.; L’Hôte, V.; Courbeyrette, R.; Kratassiouk, G.; Pinna, G.; Cintrat, J.C.; Denby-Wilkes, C.; Derbois, C.; Olaso, R.; Deleuze, J.F.; et al. Glucocorticoids delay RAF-induced senescence promoted by EGR1. J. Cell Sci. 2019, 132, 230748. [Google Scholar] [CrossRef]
- Laberge, R.M.; Zhou, L.; Sarantos, M.R.; Rodier, F.; Freund, A.; de Keizer, P.L.J.; Liu, S.; Demaria, M.; Cong, Y.S.; Kapahi, P.; et al. Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell 2012, 11, 569–578. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaise, O.; Paulissen, G.; Deroyer, C.; Ciregia, F.; Poulet, C.; Neuville, S.; Plener, Z.; Daniel, C.; Gillet, P.; Lechanteur, C.; et al. Influence of Glucocorticoids on Cellular Senescence Hallmarks in Osteoarthritic Fibroblast-like Synoviocytes. J. Clin. Med. 2021, 10, 5331. https://doi.org/10.3390/jcm10225331
Malaise O, Paulissen G, Deroyer C, Ciregia F, Poulet C, Neuville S, Plener Z, Daniel C, Gillet P, Lechanteur C, et al. Influence of Glucocorticoids on Cellular Senescence Hallmarks in Osteoarthritic Fibroblast-like Synoviocytes. Journal of Clinical Medicine. 2021; 10(22):5331. https://doi.org/10.3390/jcm10225331
Chicago/Turabian StyleMalaise, Olivier, Geneviève Paulissen, Céline Deroyer, Federica Ciregia, Christophe Poulet, Sophie Neuville, Zelda Plener, Christophe Daniel, Philippe Gillet, Chantal Lechanteur, and et al. 2021. "Influence of Glucocorticoids on Cellular Senescence Hallmarks in Osteoarthritic Fibroblast-like Synoviocytes" Journal of Clinical Medicine 10, no. 22: 5331. https://doi.org/10.3390/jcm10225331
APA StyleMalaise, O., Paulissen, G., Deroyer, C., Ciregia, F., Poulet, C., Neuville, S., Plener, Z., Daniel, C., Gillet, P., Lechanteur, C., Brondello, J.-M., de Seny, D., & Malaise, M. (2021). Influence of Glucocorticoids on Cellular Senescence Hallmarks in Osteoarthritic Fibroblast-like Synoviocytes. Journal of Clinical Medicine, 10(22), 5331. https://doi.org/10.3390/jcm10225331





