Targeting the Gut Microbiome in Prader-Willi Syndrome
Abstract
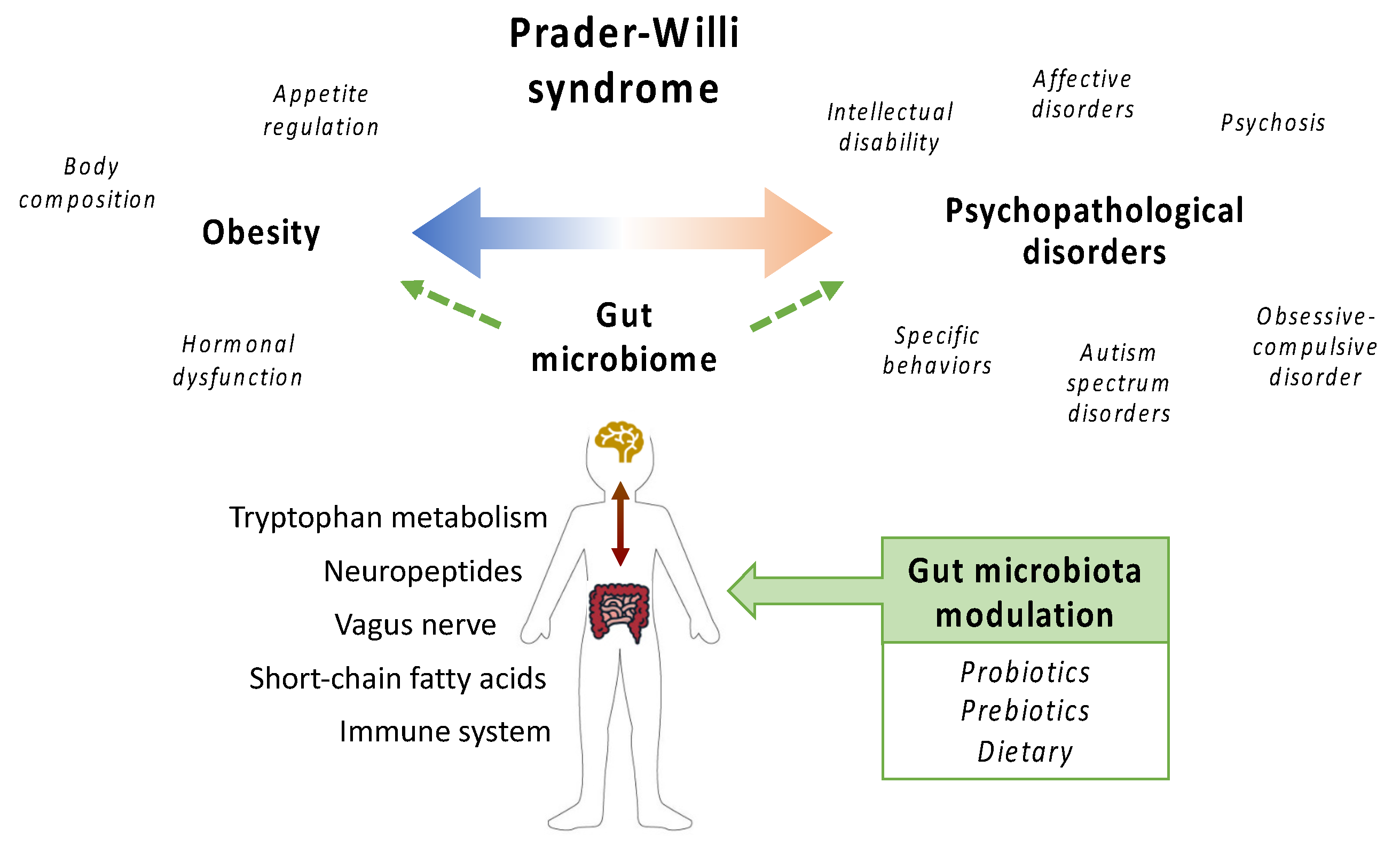
1. Introduction
1.1. Prader-Willi Syndrome
1.2. The Gut Microbiome in Health and Disease
2. Characterization of the Prader-Willi Syndrome-Associated Gut Microbiome
3. Targeting the Gut Microbiome as a Therapeutic Approach for Prader-Willi Syndrome
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Correction Statement
Abbreviations
References
- Whittington, J.E.; Holland, A.J.; Webb, T.; Butler, J.; Clarke, D.; Boer, H. Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J. Med. Genet. 2001, 38, 792–798. [Google Scholar] [CrossRef]
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef]
- Angulo, M.A.; Butler, M.G.; Cataletto, M.E. Prader-Willi syndrome: A review of clinical, genetic, and endocrine findings. J. Endocrinol. Investig. 2015, 38, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Lynn, C.H.; Driscoll, D.C.; Goldstone, A.P.; Gold, J.-A.; Kimonis, V.; Dykens, E.; Butler, M.G.; Shuster, J.J.; Driscoll, D.J. Nutritional phases in Prader–Willi syndrome. Am. J. Med Genet. Part. A 2011, 155, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Skokauskas, N.; Sweeny, E.; Meehan, J.; Gallagher, L. Mental health problems in children with prader-willi syndrome. Journal of the Canadian Academy of Child and Adolescent Psychiatry. J. De L’Academie Can. De Psychiatr. De L’enfant Et De L’Adolescent 2012, 21, 194–203. [Google Scholar]
- Dykens, E.M.; Lee, E.; Roof, E. Prader-Willi syndrome and autism spectrum disorders: An evolving story. J. Neurodev. Disord. 2011, 3, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.J.; Aman, L.C.S.; Whittington, J.E. Defining Mental and Behavioural Disorders in Genetically Determined Neurodevelopmental Syndromes with Particular Reference to Prader-Willi Syndrome. Genes 2019, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Triador, L.; Field, C.J.; Tun, H.M.; Han, J.C.; Müller, T.D.; Haqq, A.M. Current and emerging therapies for managing hyperphagia and obesity in Prader-Willi syndrome: A narrative review. Obes. Rev. 2020, 21, e12992. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota–gut–brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Lehtinen, M.J.; Meland, N.; Christensen, J.E.; Yeung, N.; Saarinen, M.T.; Courtney, M.; Burcelin, R.; Lähdeaho, M.L.; Linros, J.; et al. Probiotic with or without Fiber Controls Body Fat Mass, Associated with Serum Zonulin, in Overweight and Obese Adults-Randomized Controlled Trial. EBioMedicine 2016, 13, 190–200. [Google Scholar] [CrossRef]
- Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: A randomized controlled trial. Int J. Obes. 2019, 43, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, C.E.; Martin, J.A.; Manriquez, F.V.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Focus on the essentials: Tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin Pharm. 2019, 48, 137–145. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357. [Google Scholar] [CrossRef] [PubMed]
- Purton, T.; Staskova, L.; Lane, M.M.; Dawson, S.L.; West, M.; Firth, J.; Clarke, G.; Cryan, J.F.; Berk, M.; O’Neil, A.; et al. Prebiotic and probiotic supplementation and the tryptophan-kynurenine pathway: A systematic review and meta analysis. Neurosci. Biobehav. Rev. 2021, 123, 1–13. [Google Scholar] [CrossRef]
- Chen, L.M.; Bao, C.H.; Wu, Y.; Liang, S.H.; Wang, D.; Wu, L.Y.; Huang, Y.; Liu, H.R.; Wu, H.G. Tryptophan-kynurenine metabolism: A link between the gut and brain for depression in inflammatory bowel disease. J. Neuroinflammation 2021, 18, 135. [Google Scholar] [CrossRef]
- Olsson, L.M.; Poitou, C.; Tremaroli, V.; Coupaye, M.; Aron-Wisnewsky, J.; Bäckhed, F.; Clément, K.; Caesar, R. Gut microbiota of obese subjects with Prader-Willi syndrome is linked to metabolic health. Gut 2020, 69, 1229–1238. [Google Scholar] [CrossRef]
- Garcia-Ribera, S.; Amat-Bou, M.; Climent, E.; Llobet, M.; Chenoll, E.; Corripio, R.; Ibáñez, L.; Ramon-Krauel, M.; Lerin, C. Specific Dietary Components and Gut Microbiota Composition are Associated with Obesity in Children and Adolescents with Prader-Willi Syndrome. Nutrients 2020, 12, 1063. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tan, Q.; Afhami, S.; Deehan, E.C.; Liang, S.; Gantz, M.; Triador, L.; Madsen, K.L.; Walter, J.; Tun, H.M.; et al. The Gut Microbiota Profile in Children with Prader-Willi Syndrome. Genes 2020, 11, 904. [Google Scholar] [CrossRef]
- Dahl, W.J.; Auger, J.; Alyousif, Z.; Miller, J.L.; Tompkins, T.A. Adults with Prader-Willi syndrome exhibit a unique microbiota profile. BMC Res. Notes 2021, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Amat-Bou, M.; Garcia-Ribera, S.; Climent, E.; Piquer-Garcia, I.; Corripio, R.; Sanchez-Infantes, D.; Villalta, L.; Elias, M.; Jiménez-Chillarón, J.C.; Chenoll, E.; et al. Effects of Bifidobacterium animalis Subsp. lactis (BPL1) Supplementation in Children and Adolescents with Prader-Willi Syndrome: A Randomized Crossover Trial. Nutrients 2020, 12, 3123. [Google Scholar] [CrossRef] [PubMed]
- Alyousif, Z.; Miller, J.L.; Auger, J.; Sandoval, M.; Piano, A.; Tompkins, T.A.; Dahl, W.J. Microbiota profile and efficacy of probiotic supplementation on laxation in adults affected by Prader-Willi Syndrome: A randomized, double-blind, crossover trial. Mol. Genet. Genomic Med. 2020, 8, e1535. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.J.; Liu, K.; Zhuang, P.; Tian, R.; Liu, S.; Clairmont, C.; Lin, X.; Sherman, H.; Zhu, J.; Wang, Y.; et al. The Effects of Limosilactobacillus reuteri LR-99 Supplementation on Body Mass Index, Social Communication, Fine Motor Function, and Gut Microbiome Composition in Individuals with Prader-Willi Syndrome: A Randomized Double-Blinded Placebo-Controlled Trial. Probiotics Antimicrob Proteins 2021, 13, 1508–1520. [Google Scholar] [CrossRef]
- Kong, X.J.; Wan, G.; Tian, R.; Liu, S.; Liu, K.; Clairmont, C.; Lin, X.; Zhang, X.; Sherman, H.; Zhu, J.; et al. The Effects of Probiotic Supplementation on Anthropometric Growth and Gut Microbiota Composition in Patients with Prader-Willi Syndrome: A Randomized Double-Blinded Placebo-Controlled Trial. Front. Nutr. 2021, 8, 587974. [Google Scholar] [CrossRef]
- Alyousif, Z.; Miller, J.L.; Sandoval, M.Y.; MacPherson, C.W.; Nagulesapillai, V.; Dahl, W.J. The effects of Bifidobacterium animalis ssp. lactis B94 on gastrointestinal wellness in adults with Prader-Willi syndrome: Study protocol for a randomized controlled trial. Trials 2018, 19, 256. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, C.; Wu, H.; Wang, R.; Shen, J.; Wang, L.; Zhao, Y.; Pang, X.; Zhang, X.; Zhao, L.; et al. Genomic Microdiversity of Bifidobacterium pseudocatenulatum Underlying Differential Strain-Level Responses to Dietary Carbohydrate Intervention. mBio 2017, 8, e02348-16. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, R.; Li, H.; Zhang, C.; Zhao, L.; Zhang, M. miRNA-Gene Regulatory Network in Gnotobiotic Mice Stimulated by Dysbiotic Gut Microbiota Transplanted from a Genetically Obese Child. Front. Microbiol. 2019, 10, 1517. [Google Scholar] [CrossRef]
- Li, H.; Zhao, L.; Zhang, M. Gut Microbial SNPs Induced by High-Fiber Diet Dominate Nutrition Metabolism and Environmental Adaption of Faecalibacterium prausnitzii in Obese Children. Front. Microbiol. 2021, 12, 683714. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2015, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010, 138, 1772–1782. [Google Scholar] [CrossRef]
- Yassour, M.; Lim, M.Y.; Yun, H.S.; Tickle, T.L.; Sung, J.; Song, Y.M.; Lee, K.; Franzosa, E.A.; Morgan, X.C.; Gevers, D.; et al. Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes. Genome Med. 2016, 8, 17. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, F.; Brunkwall, L.; Ericson, U.; Nilsson, P.M.; Almgren, P.; Fernandez, C.; Melander, O.; Orho-Melander, M. Connection Between BMI-Related Plasma Metabolite Profile and Gut Microbiota. J. Clin. Endocrinol. Metab. 2018, 103, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia genus associated with visceral fat accumulation in adults 20-76 years of age. NPJ Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Li, S.C.; Hsu, W.F.; Chang, J.S.; Shih, C.K. Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis Shows a Stronger Anti-Inflammatory Effect than Individual Strains in HT-29 Cells. Nutrients 2019, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef]
| Author, Year | Study Type | Aim | Target Population | Results | GRADE * |
|---|---|---|---|---|---|
| Dahl, 2021 [28] | Observational, cross-sectional | PWS adults vs. controls | Mean age 34.9 ± 10.2 years (n = 25) | Lower abundance of Blautia. Higher RF39 (Tenericutes phyla), Ruminococcaceae, Alistipes, Erysipelotrichacaea, Parabacteriodes, Odoribacter. | 2+ |
| Peng, 2020 [27] | Observational, cross-sectional | PWS children vs. controls | Mean age 6.2 years (range 3–17) (n = 25) | Higher Prevotella and lower Oscillospira. Higher Candida and lower Saccharomyces. Hyperphagia scores associated with fungal α-diversity. | 2++ |
| Garcia-Ribera, 2020 [26] | Observational, cross-sectional | PWS obese vs.PWS normal weight | Mean age 12.0 ± 4.0 years (range 5–18) (n = 31) | Lower phylogenetic diversity, lower Alistipes and Murimonas abundance, and higher Klebsiella abundance in obesity. | 2+ |
| Olsson, 2019 [25] | Observational, cross-sectional | PWS obese vs. obese controls | Mean age 29.4 ± 7.8 years (n = 17) | Higher phylogenetic diversity in PWS. Higher Akkermansia, Desulfovibrio, and Archaea. Lower Dorea. | 2++ |
| Zhang, 2015 [29] | Intervention | PWS children with obesity vs. obese controls | Mean age 9.3 years (range 5–16) (n = 17) | No major differences in microbiota diversity and composition. | 1− |
| Author, Year | Study Type | Aim | Intervention | Target Population | Time Period | Results | GRADE * |
|---|---|---|---|---|---|---|---|
| Kong, 2021 [32] | RCT, parallel group | Weight, height, ASQ3, GARS-3 | Probiotic L. reuteri (LR-99) | Mean age 5.4 ± 4.3 years (n = 71) | 12 weeks | Decrease in BMI, improvement in social communication and interaction, fine motor function, and total ASQ-3 score | 1+ |
| Kong, 2021 [33] | RCT, parallel group | Weight, height, ASQ3, ABC, RRB, SRS-2, CGI-I | Probiotic B. Lactis (BL-11) | Mean age 4.2 ± 3.1 years (n = 68) | 12 weeks | No change in weight, increase in height, improvement in CGI-I | 1+ |
| Alyousif, 2020 [31] | RCT, crossover | Stool characteristics and frequency, gastrointestinal symptoms | Probiotic B. Lactis (B94) | Mean age 34.9 ± 10.2 years (n = 25) | 4 weeks | No changes in laxation. | 1− |
| Amat-Bou, 2020 [30] | RCT, crossover | Adiposity, lipid and glucose metabolism, hyperphagia, CBCL | Probiotic B. Lactis (BPL1) | Mean age 10.4 ± 5.0 years (n = 35) | 12 weeks | Decreased abdominal adiposity, improvement in fasting insulin concentration. Modest improvements in CBCL. | 1− |
| Zhang, 2015 [29] | Single group pre-/post-intervention | Microbiota composition, BMI, glucose and lipid homeostasis | Dietary (prebiotics, rich in non-digestive CH) | Mean age 9.3 years (range 5–16) (n = 17) | 12 weeks | Decrease in weight and inflammation, improved glucose and lipid homeostasis | 1− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramon-Krauel, M.; Amat-Bou, M.; Serrano, M.; Martinez-Monseny, A.F.; Lerin, C. Targeting the Gut Microbiome in Prader-Willi Syndrome. J. Clin. Med. 2021, 10, 5328. https://doi.org/10.3390/jcm10225328
Ramon-Krauel M, Amat-Bou M, Serrano M, Martinez-Monseny AF, Lerin C. Targeting the Gut Microbiome in Prader-Willi Syndrome. Journal of Clinical Medicine. 2021; 10(22):5328. https://doi.org/10.3390/jcm10225328
Chicago/Turabian StyleRamon-Krauel, Marta, Montse Amat-Bou, Mercedes Serrano, Antonio F. Martinez-Monseny, and Carles Lerin. 2021. "Targeting the Gut Microbiome in Prader-Willi Syndrome" Journal of Clinical Medicine 10, no. 22: 5328. https://doi.org/10.3390/jcm10225328
APA StyleRamon-Krauel, M., Amat-Bou, M., Serrano, M., Martinez-Monseny, A. F., & Lerin, C. (2021). Targeting the Gut Microbiome in Prader-Willi Syndrome. Journal of Clinical Medicine, 10(22), 5328. https://doi.org/10.3390/jcm10225328







