1. Introduction
In current obstetrical practice, antenatal consultations are commonly considered as an opportunity to screen for cervical cancer. High-grade dysplasia is most frequently diagnosed during the childbearing years, with an incidence of 8.1/1000 women aged 25–29 years [
1]. Cervical intraepithelial neoplasia (CIN) is diagnosed in 1–7% of pregnant women [
2,
3], among whom the prevalence of high-grade lesions (defined as CIN2+) is 0.5% [
4]. The progression of high-grade lesions during pregnancy to microinvasive lesions ranges from 0% to 13% [
5,
6]. Human papilloma virus (HPV) infections appear to be more frequent among pregnant women, which may be related to the immunotolerance observed in pregnancy [
7,
8].
According to international criteria [
9], pregnant patients with cytological abnormalities should be investigated by colposcopy in order to exclude a cancer by targeted biopsy. Although assessment of the cervix in pregnancy may be complicated due to pelvic congestion, colposcopic criteria remain the same as for non-pregnant women. In addition, the transformation zone is well visualized due to the eversion of the endocervix as pregnancy progresses. Cytology, colposcopy, and targeted biopsies are as reliable during pregnancy as for non-pregnant-women [
10].
As regards the evolution of cervical dysplasia during pregnancy, the literature has shown a trend toward increased postpartum regression [
11]. This regression is possibly related either to the return of the immune system or to a stimulation of it. A previous article suggested that during vaginal delivery, the cervical microlesions induced inflammation and, consequently, regression of the lesions. However, these results remain controversial.
Through a unicentric cohort of pregnant women diagnosed with high-grade cervical dysplasia during pregnancy, the aim of this study was to describe the evolution of high-grade cervical dysplasia during pregnancy and the postpartum period and to determine the factors that can be associated with dysplasia regression.
2. Materials and Methods
We retrospectively reviewed the medical records of pregnant women with high-grade lesions who were referred to the colposcopy unit of our tertiary hospital between January 2000 and September 2017. High-grade lesions were defined either cytologically by high squamous intraepithelial lesion/atypical squamous cells that cannot exclude HSIL (HSIL/ASC-H) or histologically with high-grade cervical intraepithelial neoplasia (CIN 2 and CIN 3), during pregnancy. All CIN 2 and CIN 3 high-grade histologic dysplasia were classified as CIN2+. Other data abstracted from the medical record included parity, tobacco use, diabetes, presence of HPV, first/second/third trimester feature, delivery route and postpartum feature.
Patients were excluded in cases of incomplete cytological or histological ante/postpartum data about the evolution of dysplastic lesions or in cases of the presence of a factor that could affect the patient’s immunity (immunosuppressive treatment or acquired/innate immunodeficiency).
Cervical biopsies were not systematically performed but were required in cases of significant discordance between cervical cytology and colposcopic impression or those with colposcopic evidence of invasion. To ensure the expertise of the colposcopy assessment, all cases of high-grade lesions were supervised by a colposcopy expert (MJG), either by the image taken by the colpophotograph or directly during the colposcopy. The follow-up was based on a comparison with the images taken during the previous consultation.
When a diagnosis of high-grade dysplasia was confirmed in a pregnant woman, a quarterly follow-up was scheduled on the basis of typical obstetrical trimesters. At each visit, a cervical PAP smear and a colposcopy were performed, and an additional directed cervical biopsy was carried out in cases of suspected higher-grade lesions. The mode of delivery and other procedures were recorded as well.
In our cohort, the postpartum colposcopic follow-up was performed within six to eight weeks after childbirth. Postpartum regression was defined cytologically or histologically by a reduction of at least one-degree in severity from the antepartum diagnosis, such as HSIL regressed to LSIL/normal or CIN2+ regressed to CIN 1/normal. The histopathological findings in patients who underwent loop electrosurgical excision procedure (LEEP procedure) due to extensive lesions or cytology/colposcopic discordance were included to the analysis.
Qualitative data were compared by using the chi-square test and quantitative data by using the student t-test. Results are presented as 95% confidence intervals (95%CIs) or numbers (percentages). p values lower than 0.05 were retained as significant. Patients with CIN regression were compared to those with CIN persistence during the postpartum period. Relevant covariates associated with cervical dysplasia regressions that were significant (p < 0.05) in the univariate analysis were considered in a backward selection procedure to fit a multivariable model. A logistic regression model was applied to determine independent predictive factors for high-grade cervical dysplasia regression after delivery. All statistical analyses were carried out using XLStat Biomed software (AddInsoft V19.4, Paris, France). This study was approved by the ethical research committee of the canton de Vaud (25 July 2019, ID N 2017-01375).
3. Results
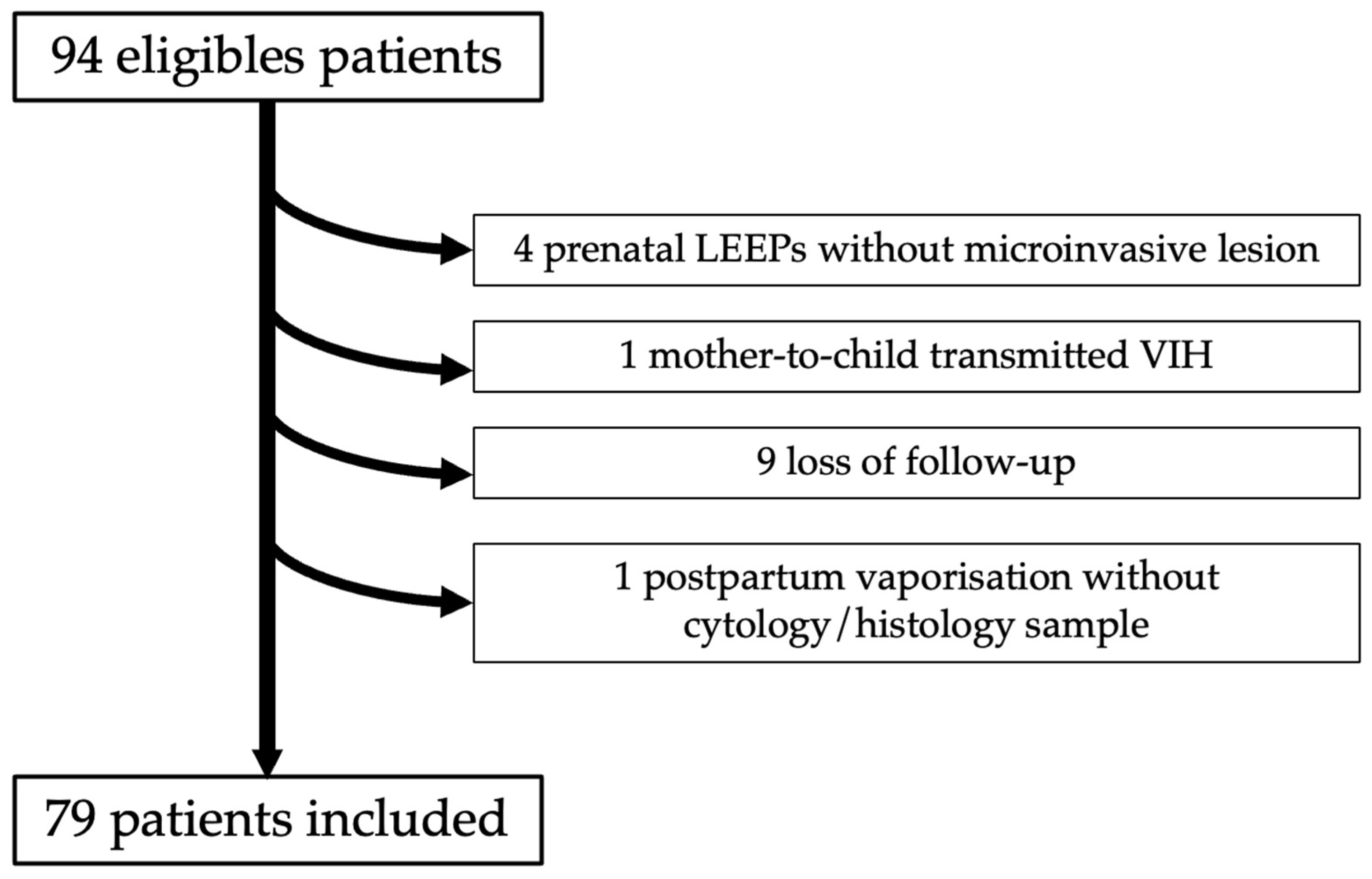
Ninety-four women diagnosed with high-grade dysplasia were identified. Among them, four patients with a cytological HSIL were excluded due to an extensive lesion during the antenatal period with colposcopic neoplasia suspicion, who subsequently underwent a LEEP procedure. Finally, only CIN 2+ lesions without invasive lesions were found in these four cases. No cases received corticosteroid treatment in our cohort; only one patient was known to have mother-to-child HIV infection with CD4+ in the normal range throughout her pregnancy. After exclusion of 15 patients, 79 patients fulfilled the inclusion criteria and were analyzed (
Figure 1). The characteristics of the study population are presented in
Table 1 and
Table 2.
Patients had at least two antenatal examinations in 90% of cases (71/79) and all had a postpartum evaluation. High-grade lesions were diagnosed by cytology in 87% of cases (69/79), of which 45% (31/69) were histologically confirmed, whereas the remaining 13% (10/79) were found on biopsies that revealed CIN2+ after initial LSIL cytology. During colposcopic follow-up, no cases of invasive lesions were found. Delivery routes were vaginal in 68% of cases (54/79) and by C-section in 32% of cases (25/79). Among the patients who had a vaginal delivery, 27 delivered spontaneously, 14 were induced for an obstetric indication and 13 were unspecified.
At postpartum colposcopic follow up, all patients underwent colposcopy with cytology/histology except for three. These three cases underwent a LEEP procedure immediately postpartum due to CIN2+ extensive antenatal lesions, which was confirmed by the pathology. Of the 27 patients whose postpartum cytology revealed an HSIL, 18 (66.7%) histological samples confirmed a high-grade lesion (either by directed biopsy or conization result) and 6 (22%) samples did not. In the 34 cytological specimens showing an LSIL/ASCUS/normal lesion, only 20 (59%) histological specimens were collected, of which 10 (50%) confirmed a high-grade lesion. For patients who only underwent colposcopic evaluation (15/79), 13 histological samples were taken, 8 (62%) of which confirmed a HSIL lesion. Sixteen patients were only diagnosed cytologically and not histologically, of which only 3 (11%) were in the HSIL group. A total of 47 biopsies and 42 conizations were performed with a rate of 53% (25/47) and 79% (33/42), respectively, of persistent high-grade lesions. Seventy-one percent (56/79) of our cohort had a histological diagnosis at postpartum colposcopic follow up. The overall regression rate in our cohort was 43% (34/79).
Univariate analysis revealed that parity (
p = 0.04), diabetes (
p = 0.04) and third trimester cytology (
p = 0.009) were associated with dysplasia regression. Age, smoking and delivery route did not impact on postpartum CIN regression rate (
Table 3 and
Table 4). By multivariate analysis, nulliparity (OR = 4.35; 95%CI= (1.03–18.42);
p= 0.046) and presence of HSIL at third-trimester cervical cytology (OR = 0.17; 95%CI = (0.04–0.72);
p = 0.016) were identified as independent predictive factors for dysplasia regression (
Table 5).
4. Discussion
The aim of this study was to assess the clinical interest in knowing which factors would allow targeting patients at potential risk of maintaining a high-grade lesion or progressing to a cancerous lesion in the postpartum period and avoiding loss to follow up for adequate treatment.
In our cohort, we reported a regression rate of high-grade lesion at 43% and no case of invasive lesions at postpartum colposcopy follow up. This rate is similar to those reported in the literature of 17–69% [
4,
5,
12,
13]. However, these studies show large disparities in terms of diagnostic criteria and therapeutic management. Considering all grades of dysplasia, caution should be paid to the interpretation of these data, since overall regression may design initial high-grade or low-grade lesions [
6,
11,
14,
15,
16,
17,
18].
The correlation between cytology and final diagnosis within one degree of severity in pregnant woman has been found to be 78% [
10], with cytology having an 88% positive predictive value for high-grade lesions [
10]. Regarding colposcopic impressions, the results are consistent with final diagnoses in 73% of cases within one degree of severity [
19]. In our cohort, we found a prenatal biopsy rate of 45% due to suspicion of cancerous lesion. This is due to overestimation of lesions related to colposcopic changes in pregnancy, which often leads to systematic sampling despite the examiner’s experience. This is described by Fader et al. [
20], in their large study based on correlations between colposcopic impression and final diagnosis by colposcopy experts; out of 62 samples taken for colposcopic suspicion of CIN2+/neoplasia, only 55% confirmed a high grade lesion. The overestimation of the severity of lesions when performing colposcopies among pregnant women is the main reason for the non-concordance [
19]. In our cohort, 34 of the 45 patients with diagnoses of persistent HSIL/CIN2+ at postpartum colposcopy follow up had a LEEP procedure confirming high-grade non-invasive dysplastic lesions.
In our univariate analysis, there was a statistically significant result for the variable gestational diabetes; however, this should be interpreted with caution due to the small patient cohort (eight patients). In contrast, our results support the idea that the parity status may have an impact on HSIL/CIN regression rate during pregnancy. This finding is concordant with the findings reported by Hong et al. [
21]. The authors highlighted that the persistence of high-grade lesions was more frequent in multiparous patients (OR: 10.52; 95%CI: 1.36–81.01;
p = 0.004) [
21]. Compared to multiparous patients, nulliparous patients would also have longer exposure estrogen impregnation related to cervical ripening [
22], as well as related proinflammatory cytokine signaling, leading to increased local vascularization and a recrudescence of immune cells such as myeloid-derived immune cells and lymphocyte cells. These cells may participate in inflammation and recovery processes [
23]. The density of CD4+ T-lymphocytes was shown to increase by 4-fold in pregnant patients who are not in labor and by 10-fold in pregnant patients in labor [
24].
There is some evidence that persistent, high-risk HPV infections in the postpartum period may be an underlying factor in patients with either persistent or progressing lesions [
21]. Pregnancy leads to physiological changes that induce temporary immunomodulatory effects in downregulating the expression of inflammatory chemokines [
25] increasing susceptibility to HPV infection. Hong et al. [
21] showed that the persistence of high-grade lesions were more frequent in patients with persistent, high-grade HPV infections (OR = 5.25; 95%CI: 2.26–12.18;
p < 0.001).
However, no differences were found between both groups in terms of presence of high-risk HPV. As for non-pregnant women, it would be interesting to investigate other markers, such as P16 and Ki67, and to assess their prognostic value for more accurately identifying patients at risk of developing invasive lesions. Nonetheless, there is no consensus on the use of P16 and Ki67 during pregnancy, and the influences of hormones and immunity on these proteins remain unknown. The literature is scarce with only one study that has been published; furthermore, it was based on a small series of cases [
26].
Some authors raised the hypothesis that cervical desquamation related to vaginal delivery resulted in an inflammatory response that leads to increased regressions of cervical intraepithelial lesions in the postpartum period [
11]. However, the role of the delivery route is still subject to debate and remains controversial. As suggested by our results, the route of delivery had no influence on HSIL/CIN 2+ regression rate. This result is concordant with that reported by Yost et al. [
5], who reported no difference in regression rates according to mode of delivery in a prospective study of 153 histologically diagnosed high-grade dysplasia.
This study has several limitations. First, the retrospective design and the small sample size lead to insufficient power to extrapolate these results. Second, our study is mainly based on colposcopic and cytological criteria, histological samplings being carried out only when a lesion is suspected of being cancerous. Although cytology has a positive predictive value of 88% for high-grade lesions in pregnant women, histology remains the gold standard for the diagnosis of high-grade lesions, thus excluding high-grade cytological lesions lacking histological confirmation [
4,
5,
6,
12,
14,
15,
21,
27]. This implies that if the sample does not contain the high-grade lesion, this minimizes the risk and results in these patients not being monitored during pregnancy and postpartum [
28]. However, the results of the current study underlined that cytological regression at the third trimester was predictive of HSIL/CIN regression after delivery. In addition, Ueda et al. reported the same observations as those underlined in our study, with a spontaneous regression in a quarter of their cohort in the second and third trimester before delivery. Persistent HPV infection is necessary for the development of precancers and cancers. Although our study does not report HPV typing, there is a ripening of the cervix due to hormonal change and, consequently, a return of the inflammatory system in the third trimester. We support the idea that this process allows clearance of the HPV infection and, consequently, a regression of the lesion. Cytology in the third trimester will allow this change to be observed and, therefore, predict the evolution of the lesions.
5. Conclusions
In conclusion, our study follows the ASCCP (American Society of Colposcopy and Cervical Pathology) guidelines. Although assessment of high-grade dysplastic lesions may be postponed in the postpartum period, our observations revealed that the clinical value of surveillance for high-grade dysplasia during pregnancy is to identify lesions with a potential risk of progression to cervical carcinoma in the postpartum period. Our results highlighted that parity status may have an impact on the regression of dysplasia during pregnancy. Cervical cytology should be performed in the third trimester to identify patients at risk of persistent CIN after delivery. Therefore, larger cohorts are required to confirm these results. A prospective study, including biomarkers such as P16 and Ki67, as well as immune cell characteristics/density and HPV typing would be relevant to gain more knowledge and increase the accuracy of diagnosis and management of high-grade cervical lesions during pregnancy and postpartum.
Author Contributions
Y.G.: data acquisition, conception, design, analysis and interpretation, drafting, critical revision. M.J.-G.: conception, design, analysis and interpretation, drafting, critical revision. V.B.: analysis and interpretation, critical revision. K.L.: analysis and interpretation, critical revision. P.M.: conception, interpretation, critical revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the canton de Vaud, Switzerland, (25 July 2019, ID N 2017-01375).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
The authors want to acknowledge the support of Mia Cespedes from Réseau Hospitalier Neuchâtelois Pourtalès for collecting clinical data and Stefan Gerber, former head of the colposcopy unit.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Insinga, R.P.; Glass, A.G.; Rush, B.B. Diagnoses and outcomes in cervical cancer screening: A population-based study. Am. J. Obstet. Gynecol. 2004, 191, 105–113. [Google Scholar] [CrossRef]
- Douvier, S.; Filipuzzi, L.; Sagot, P. Management of cervical intra-epithelial neoplasm during pregnancy. Gynecol. Obstet. Fertil. 2003, 31, 851–855. [Google Scholar] [CrossRef]
- Selleret, L.; Mathevet, P. Precancerous cervical lesions during pregnancy: Diagnostic and treatment. J. Gynecol. Obstet. Biol. Reprod. 2008, 37 (Suppl. 1), S131–S138. [Google Scholar] [CrossRef]
- Coppolillo, E.F.; DE Ruda Vega, H.M.; Brizuela, J.; Eliseth, M.C.; Barata, A.; Perazzi, B.E. High-grade cervical neoplasia during pregnancy: Diagnosis, management and postpartum findings. Acta Obstet. Gynecol. Scand. 2013, 92, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Yost, N.P.; Santoso, J.T.; McIntire, D.D.; Iliya, F.A. Postpartum regression rates of antepartum cervical intraepithelial neoplasia II and III lesions. Obstet. Gynecol. 1999, 93, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, S.; Gehrsitz, C.; Mehlhorn, G.; Beckmann, M.W. Management and course of histologically verified cervical carcinoma in situ during pregnancy. Acta Obstet. Gynecol. Scand. 2006, 85, 1134–1137. [Google Scholar] [CrossRef]
- Fife, K.H.; Katz, B.P.; Roush, J.; Handy, V.D.; Brown, D.R.; Hansell, R. Cancer-associated human papillomavirus types are selectively increased in the cervix of women in the first trimester of pregnancy. Am. J. Obstet. Gynecol. 1996, 174, 1487–1493. [Google Scholar] [CrossRef]
- Domža, G.; Gudlevičienė, Z.; Didžiapetrienė, J.; Valuckas, K.P.; Kazbarienė, B.; Drąsutienė, G. Human papillomavirus infection in pregnant women. Arch. Gynecol. Obstet. 2011, 284, 1105–1112. [Google Scholar] [CrossRef]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.-B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldauf, J.J.; Dreyfus, M.; Ritter, J.; Philippe, E. Colposcopy and directed biopsy reliability during pregnancy: A cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 62, 31–36. [Google Scholar] [CrossRef]
- Ahdoot, D.; Van Nostrand, K.M.; Nguyen, N.J.; Tewari, D.S.; Kurasaki, T.; DiSaia, P.J.; Rose, G.S. The effect of route of delivery on regression of abnormal cervical cytologic findings in the postpartum period. Am. J. Obstet. Gynecol. 1998, 178, 1116–1120. [Google Scholar] [CrossRef]
- Kärrberg, C.; Brännström, M.; Strander, B.; Ladfors, L.; Rådberg, T. Colposcopically directed cervical biopsy during pregnancy; minor surgical and obstetrical complications and high rates of persistence and regression. Acta Obstet. Gynecol. Scand. 2013, 92, 692–699. [Google Scholar] [CrossRef]
- Mailath-Pokorny, M.; Schwameis, R.; Grimm, C.; Reinthaller, A.; Polterauer, S. Natural history of cervical intraepithelial neoplasia in pregnancy: Postpartum histo-pathologic outcome and review of the literature. BMC Pregnancy Childbirth 2016, 16, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, Y.; Enomoto, T.; Miyatake, T.; Yoshino, K.; Fujita, M.; Miyake, T.; Fujiwara, K.; Muraji, M.; Kanagawa, T.; Kimura, T. Postpartum outcome of cervical intraepithelial neoplasia in pregnant women determined by route of delivery. Reprod. Sci. 2009, 16, 1034–1039. [Google Scholar] [CrossRef]
- Chung, S.M.; Son, G.H.; Nam, E.J.; Kim, Y.H.; Kim, Y.T.; Park, Y.W.; Kwon, J.Y. Mode of delivery influences the regression of abnormal cervical cytology. Gynecol. Obstet. Investig. 2011, 72, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, K.J.; Dainty, L.A.; Dolinsky, B.; Rose, G.S.; Carlson, J.; McHale, M.; Elkas, J.C. Prognosis and recurrence risk for patients with cervical squamous intraepithelial lesions diagnosed during pregnancy. Cancer 2004, 102, 228–232. [Google Scholar] [CrossRef]
- Kaneshiro, B.E.K.; Acoba, J.D.; Holzman, J.; Wachi, K.; Carney, M.E. Effect of delivery route on natural history of cervical dysplasia. Am. J. Obstet. Gynecol. 2005, 192, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Joura, E.; Kohlberger, P. Natural History of Squamous Intraepithelial Lesions in Pregnancy and Mode of Delivery. Anticancer Res. 2018, 38, 2439–2442. [Google Scholar] [CrossRef]
- Economos, K.; Perez Veridiano, N.; Delke, I.; Collado, M.L.; Tancer, M.L. Abnormal cervical cytology in pregnancy: A 17-year experience. Obstet. Gynecol. 1993, 81, 915–918. [Google Scholar]
- Fader, A.N.; Alward, E.K.; Niederhauser, A.; Chirico, C.; Lesnock, J.L.; Zwiesler, D.J.; Guido, R.S.; Lofgren, D.J.; Gold, M.A.; Moore, K.N. Cervical dysplasia in pregnancy: A multi-institutional evaluation. Am. J. Obstet. Gynecol. 2010, 203, 113.e1–113.e6. [Google Scholar] [CrossRef]
- Hong, D.K.; Kim, S.A.; Lim, K.T.; Lee, K.H.; Kim, T.J.; So, K.A. Clinical outcome of high-grade cervical intraepithelial neoplasia during pregnancy: A 10-year experience. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 236, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Minjarez, D.; Yost, N.P.; Word, R.A. Estrogen and progesterone metabolism in the cervix during pregnancy and parturition. J. Clin. Endocrinol. Metab. 2008, 93, 2366–2374. [Google Scholar] [CrossRef] [Green Version]
- Yellon, S.M. Immunobiology of Cervix Ripening. Front. Immunol. 2019, 10, 3156. [Google Scholar] [CrossRef] [Green Version]
- Bokström, H.; Brännström, M.; Alexandersson, M.; Norström, A. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum. Reprod. 1997, 12, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Nancy, P.; Tagliani, E.; Tay, C.-S.; Asp, P.; Levy, D.E.; Erlebacher, A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 2012, 336, 1317–1321. [Google Scholar] [CrossRef] [Green Version]
- Ciavattini, A.; Sopracordevole, F.; Di Giuseppe, J.; Moriconi, L.; Lucarini, G.; Mancioli, F.; Zizzi, A.; Goteri, G. Cervical intraepithelial neoplasia in pregnancy: Interference of pregnancy status with p16 and Ki-67 protein expression. Oncol. Lett. 2017, 13, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppola, A.; Sorosky, J.; Casper, R.; Anderson, B.; Buller, R.E. The clinical course of cervical carcinoma in situ diagnosed during pregnancy. Gynecol. Oncol. 1997, 67, 162–165. [Google Scholar] [CrossRef]
- Benedet, J.L.; Selke, P.A.; Nickerson, K.G. Colposcopic evaluation of abnormal Papanicolaou smears in pregnancy. Am. J. Obstet. Gynecol. 1987, 157, 932–937. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).