Increased Serum Levels of S100A4 and S100A15 in Individuals Suffering from Hidradenitis Suppurativa
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
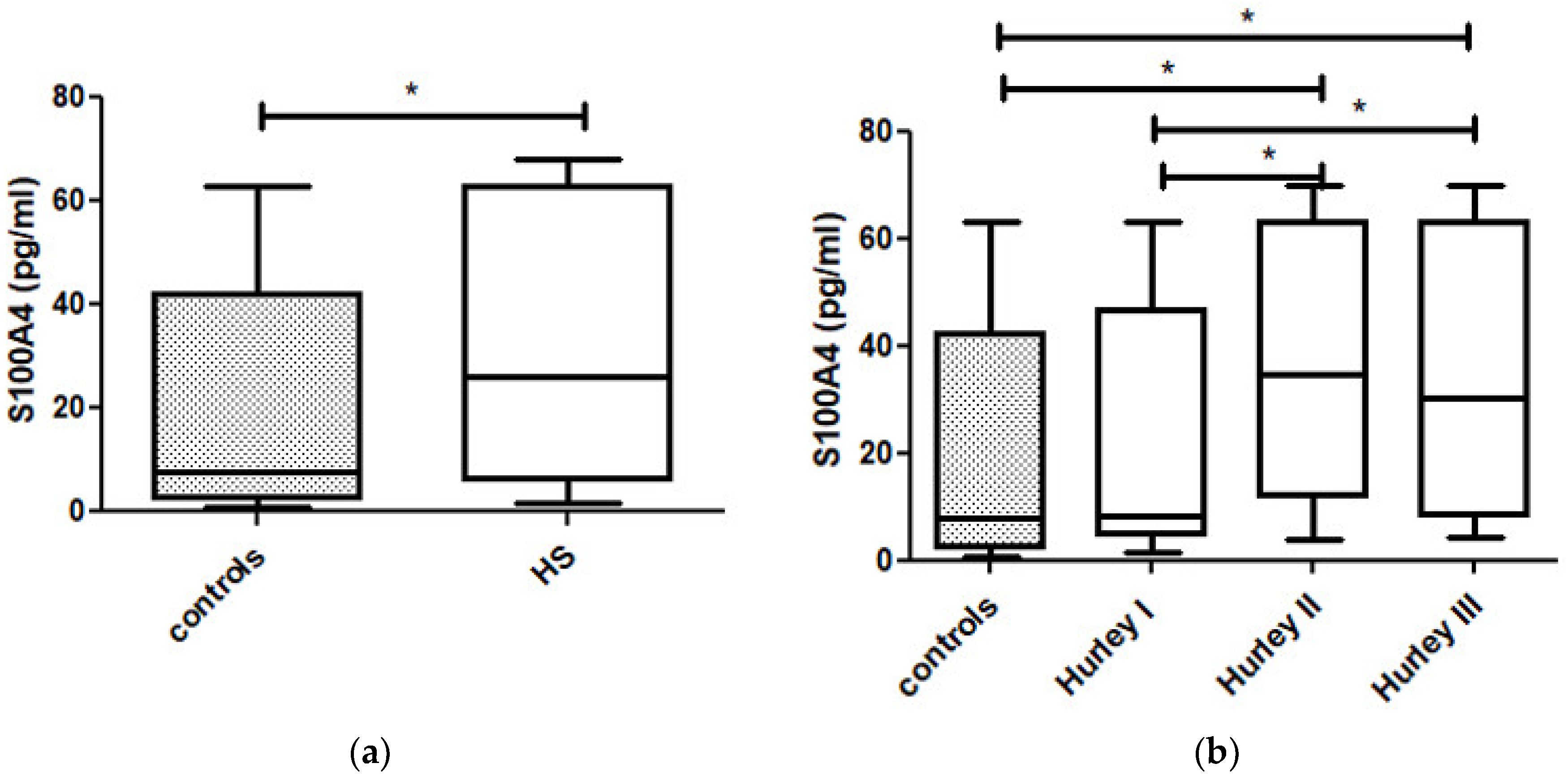
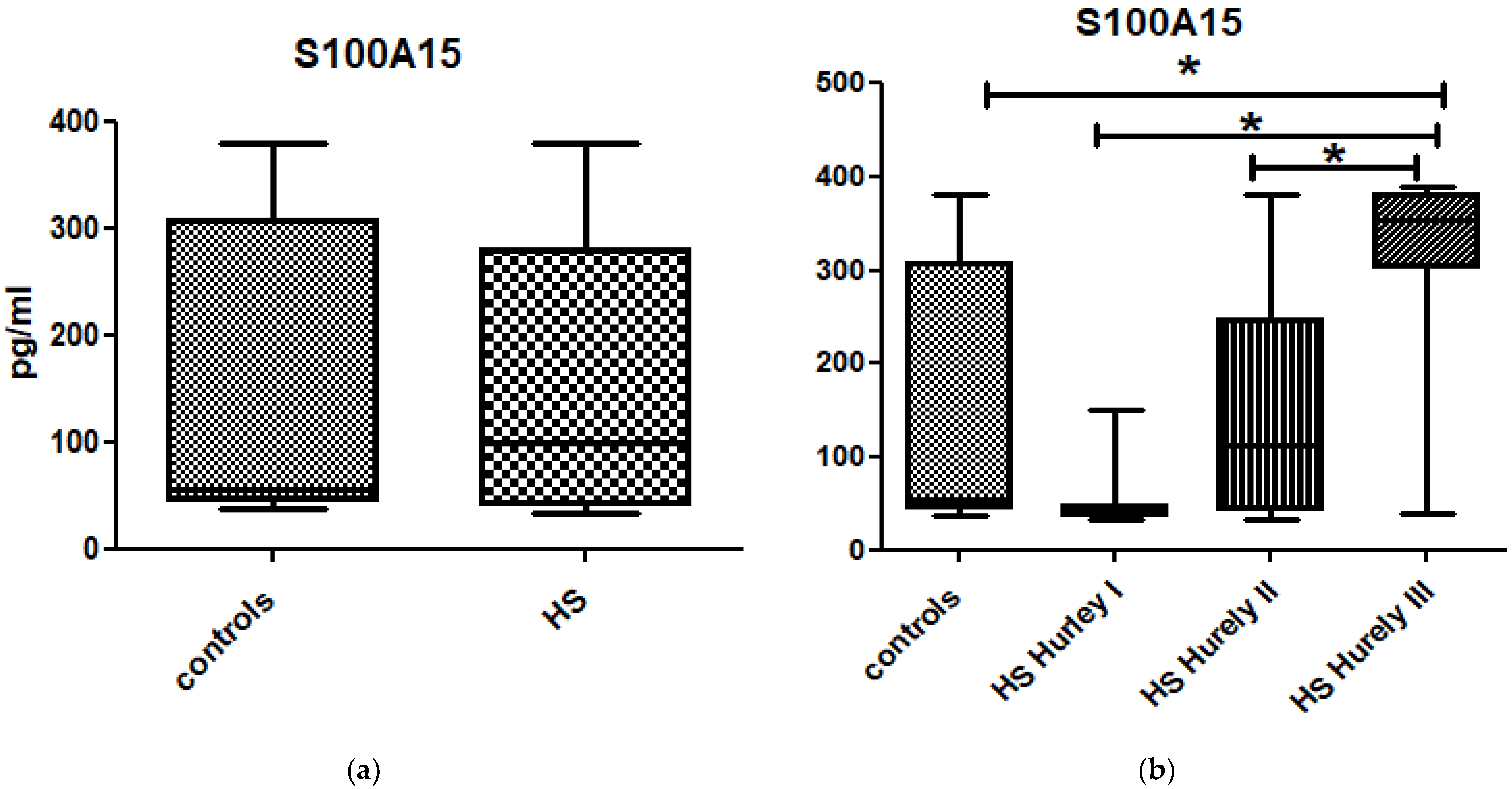
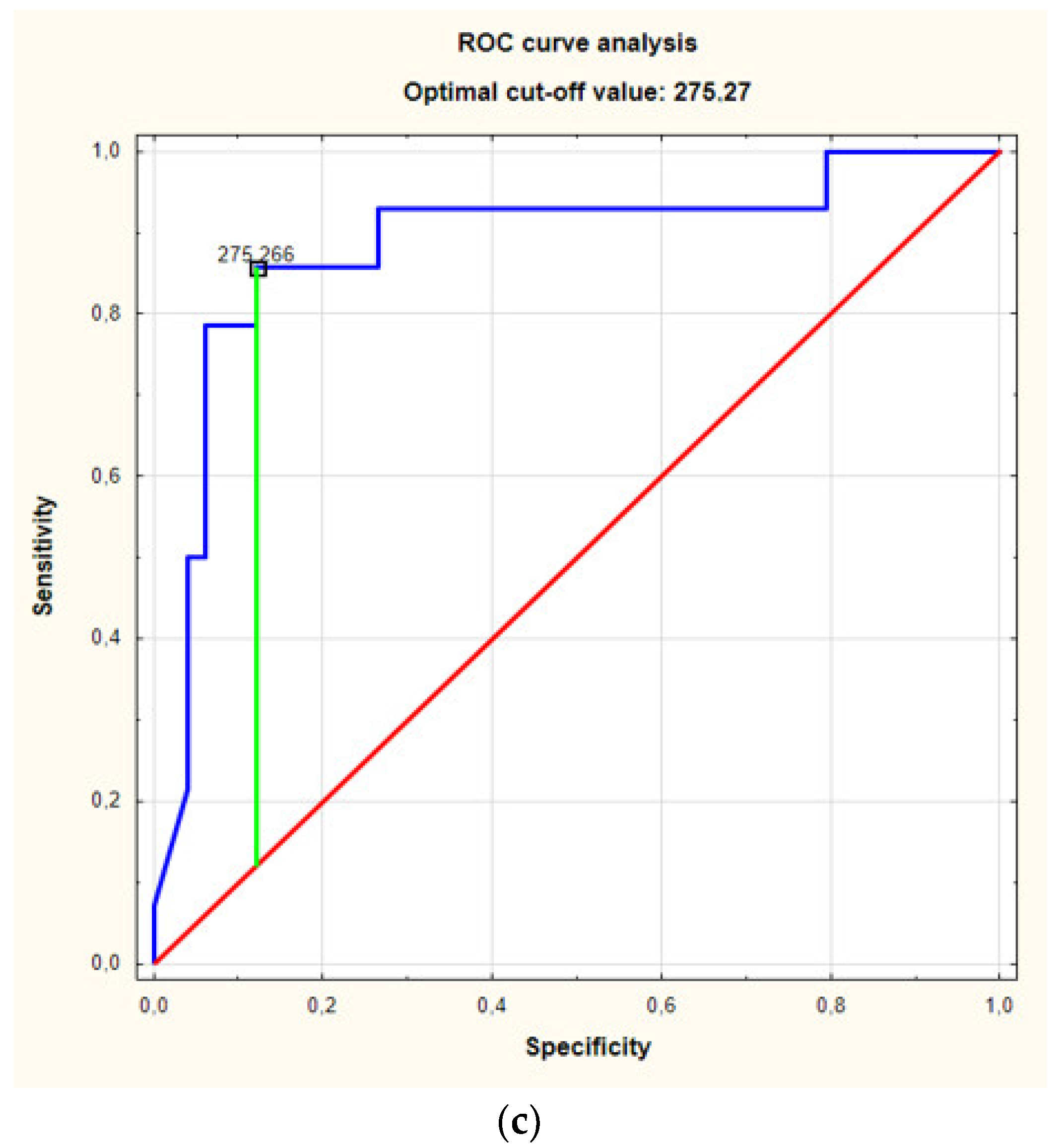
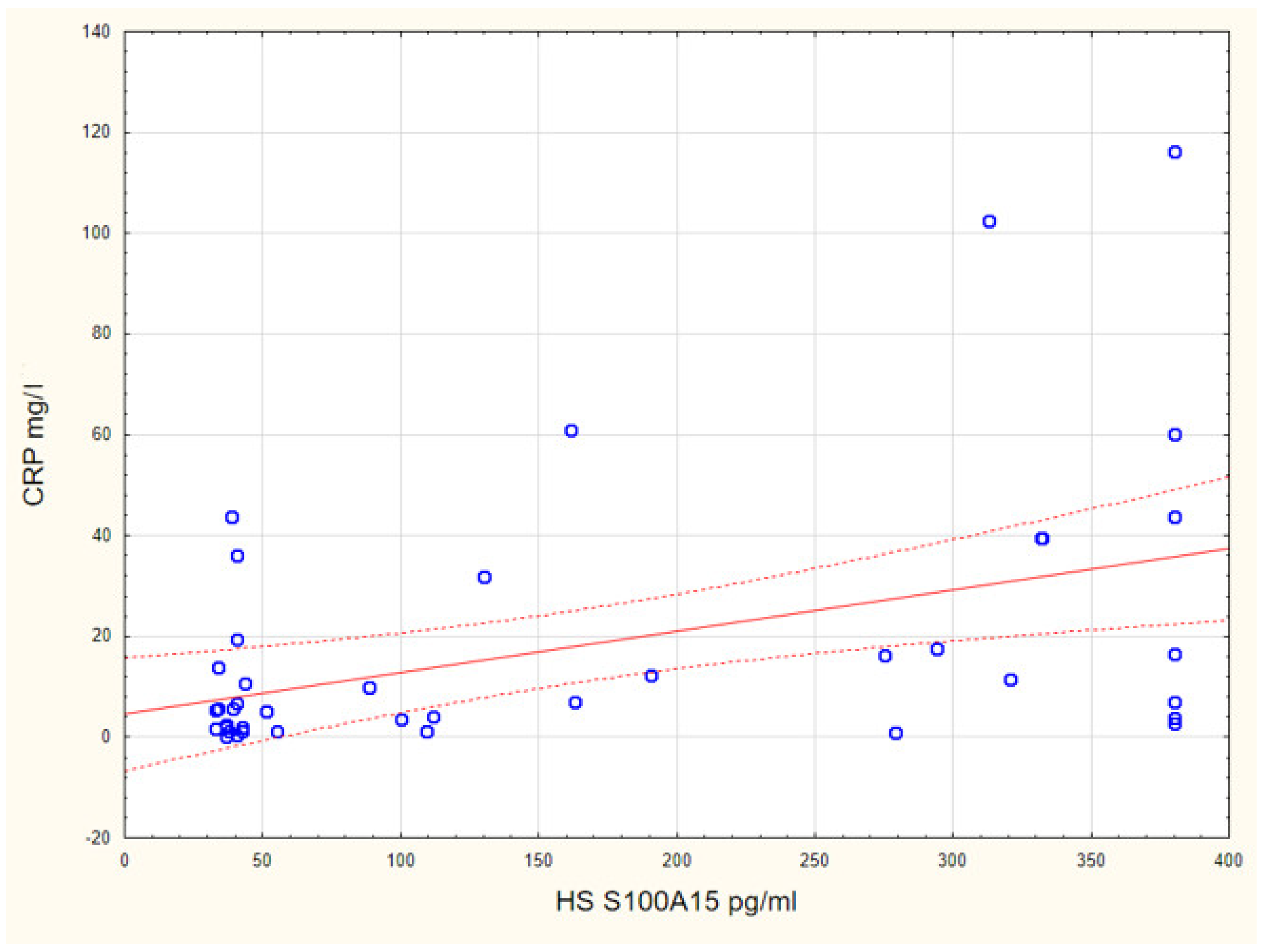
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, T.V.; Damiani, G.; Orenstein, L.A.V.; Hamzavi, I.; Jemec, G.B. Hidradenitis suppurativa: An update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 50–61. [Google Scholar] [CrossRef]
- Poli, F.; Jamec, G.B.; Revuz, J. Clinical presentation. In Hidradenitis Suppurativa; Jamec, G.B., Revuz, J., Leyden, J.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 11–24. [Google Scholar]
- Prens, E.; Deckers, I. Pathophysiology of hidradenitis suppurativa: An update. J. Am. Acad. Dermatol. 2015, 73, S8–S11. [Google Scholar] [CrossRef]
- Kelly, G.; Sweeney, C.M.; Tobin, A.-M.; Kirby, B. Hidradenitis suppurativa: The role of immune dysregulation. Int. J. Dermatol. 2014, 53, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.L.; Karl, I.; Giner, T.; Poppe, H.; Schmidt, M.; Presser, D.; Goebeler, M.; Bauer, B. Keratinocytes and neutrophils are important sources of pro-inflammatory molecules in hidradenitis suppurativa. Br. J. Dermatol. 2016, 174, 514–521. [Google Scholar] [CrossRef]
- Scala, E.; Di Caprio, R.; Cacciapuoti, S.; Caiazzo, G.; Fusco, A.; Tortorella, E.; Fabbrocini, G.; Balato, A. A new T helper 17 cytokine in hidradenitis suppurativa: Antimicrobial and proinflammatory role of interleukin-26. Br. J. Dermatol. 2019, 181, 1038–1045. [Google Scholar] [CrossRef]
- Scala, E.; Cacciapuoti, S.; Garzorz-Stark, N.; Megna, M.; Marasca, C.; Seiringer, P.; Volz, T.; Eyerich, K.; Fabbrocini, G. Hidradenitis Suppurativa: Where We Are and Where We Are Going. Cells 2021, 10, 2094. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Katsambas, A.; Antoniou, C. Hidradenitis suppurrativa (acne inversa) as a systemic disease. Clin. Dermatol. 2014, 32, 397–408. [Google Scholar] [CrossRef]
- Garg, A.; Malviya, N.; Strunk, A.; Wright, S.; Alavi, A.; Alhusayen, R.; Alikhan, A.; Daveluy, S.D.; Delorme, I.; Goldfarb, N.; et al. Comorbidity screening in hidradenitis suppurativa: Evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J. Am. Acad. Dermatol. 2021, 23, S0190. [Google Scholar] [CrossRef] [PubMed]
- Skroza, N.; Mambrin, A.; Proietti, I.; Balduzzi, V.; Bernardini, N.; Marchesiello, A.; Michelini, S.; Tolino, E.; Volpe, S.; Maddalena, P.; et al. Evaluation of Cardiovascular Risk in Hidradenitis Suppurativa Patients Using Heart Rate Variability (HRV) Analysis. Cardiovasc. Ther. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Wieland, C.; Vogl, T.; Ordelman, A.; Vloedgraven, H.; Verwoolde, L.; Rensen, J.; Roth, J.; Boer, J.; Hessels, J. Myeloid marker S100A8/A9 and lymphocyte marker, soluble interleukin 2 receptor: Biomarkers of hidradenitis suppurativa disease activity? Br. J. Dermatol. 2013, 168, 1252–1258. [Google Scholar] [CrossRef]
- Donato, R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 2003, 60, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Batycka-Baran, A.; Maj, J.; Wolf, R.; Szepietowski, J. The New Insight into the Role of Antimicrobial Proteins-Alarmins in the Immunopathogenesis of Psoriasis. J. Immunol. Res. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Cao, Y.; Hong, F.; Conlon, D.; Sidur, L.; Smith, K.; Fang, Y.; Cuff, C.; Kaymakcalan, Z.; Ruzek, M. Potential predictive biomarkers of adalimumab response in patients with hidradenitis suppurativa. Br. J. Dermatol. 2021, 185, 804–814. [Google Scholar] [CrossRef]
- Wolf, R.; Ruzicka, T.; Yuspa, S.H. Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: Highly homologous but distinct in regulation and function. Amino Acids 2010, 41, 789–796. [Google Scholar] [CrossRef]
- Fei, F.; Qu, J.; Li, C.; Wang, X.; Li, Y.; Zhang, S. Role of metastasis-induced protein S100A4 in human non-tumor pathophysiologies. Cell Biosci. 2017, 7, 64. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Liu, S.; Qin, Z. Extracellular S100A4 as a key player in fibrotic diseases. J. Cell. Mol. Med. 2020, 24, 5973–5983. [Google Scholar] [CrossRef] [Green Version]
- Tomcik, M.; Palumbo-Zerr, K.; Zerr, P.; Avouac, J.; Dees, C.; Sumova, B.; Distler, A.; Beyer, C.; Cerezo, L.A.; Becvar, R.; et al. S100A4 amplifies TGF-beta-induced fibroblast activation in systemic sclerosis. Ann. Rheum. Dis. 2015, 74, 1748–1755. [Google Scholar] [CrossRef] [Green Version]
- Klingelhöfer, J.; Senolt, L.; Baslund, B.; Nielsen, G.H. Up-regulation of metastasis-promoting S100A4 (Mts-1) in rheumatoid ar-thritis: Putative involvement in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2007, 56, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Zibert, J.R.; Skov, L.; Thyssen, J.P.; Jacobsen, G.K.; Grigorian, M. Significance of the S100A4 protein in psoriasis. J. Investig. Dermatol. 2010, 130, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Andrés Cerezo, L.; Kuncová, K.; Mann, H.; Tomčík, M.; Zámečník, J.; Lukanidin, E.; Neidhart, M.; Gay, S.; Grigorian, M.; Vencovský, J.; et al. The metastasis promoting protein S100A4 is increased in idiopathic inflammatory myopathies. Rheumatology 2011, 50, 1766–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batycka-Baran, A.; Koziol-Galczynska, M.; Bieniek, A.; Wolf, R.; Łaczmański, Ł.; Szepietowski, J. Expression of koebnerisin (S100A15) and calgranulin A (S100A8) in lesional and perilesional skin in patients suffering from hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e402–e404. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, Z.; Zwicker, S.; Bureik, D.; Peric, M.; Koglin, S.; Batycka-Baran, A.; Prinz, J.C.; Ruzicka, T.; Schauber, J.; Wolf, R. Vitamin D Analog Calcipotriol Suppresses the Th17 Cytokine–Induced Proinflammatory S100 “Alarmins” Psoriasin (S100A7) and Koebnerisin (S100A15) in Psoriasis. J. Investig. Dermatol. 2012, 132, 1416–1424. [Google Scholar] [CrossRef] [Green Version]
- Wolf, R.; Howard, O.M.Z.; Dong, H.-F.; Voscopoulos, C.; Boeshans, K.; Winston, J.; Divi, R.; Gunsior, M.; Goldsmith, P.; Ahvazi, B.; et al. Chemotactic Activity of S100A7 (Psoriasin) Is Mediated by the Receptor for Advanced Glycation End Products and Potentiates Inflammation with Highly Homologous but Functionally Distinct S100A15. J. Immunol. 2008, 181, 1499–1506. [Google Scholar] [CrossRef] [Green Version]
- Jenei, A.; Dajnoki, Z.; Medgyesi, B.; Gáspár, K.; Béke, G.; Kinyó, Á.; Méhes, G.; Hendrik, Z.; Dinya, T.; Törőcsik, D.; et al. Apocrine Gland–Rich Skin Has a Non-Inflammatory IL-17–Related Immune Milieu, that Turns to Inflammatory IL-17–Mediated Disease in Hidradenitis Suppurativa. J. Investig. Dermatol. 2019, 139, 964–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frew, J.W.; Hawkes, J.E.; Krueger, J.G. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Research 2018, 7, 1930. [Google Scholar] [CrossRef]
- Batycka-Baran, A.; Hattinger, E.; Zwicker, S.; Summer, B.; Howard, O.Z.; Thomas, P.; Szepietowski, J.; Ruzicka, T.; Prinz, J.C.; Wolf, R. Leukocyte-derived koebnerisin (S100A15) and psoriasin (S100A7) are systemic mediators of inflammation in psoriasis. J. Dermatol. Sci. 2015, 79, 214–221. [Google Scholar] [CrossRef]
- Awad, S.M.; Attallah, D.A.; Salama, R.H.; Mahran, A.M.; Abu El-Hamed, E. Serum levels of psoriasin (S100A7) and koebnerisin (S100A15) as potential markers of atherosclerosis in patients with psoriasis. Clin. Exp. Dermatol. 2018, 43, 262–267. [Google Scholar] [CrossRef]
- González-López, M.A.; Hernández, J.L.; Lacalle, M.; Mata, C.; López-Escobar, M.; López-Mejías, R.; Portilla, V.; Fuentevilla, P.; Corrales, A.; González-Vela, M.C.; et al. Increased prevalence of subclinical atherosclerosis in patients with hidradenitis suppurativa (HS). J. Am. Acad. Dermatol. 2016, 75, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Lagrand, W.K.; Visser, C.A.; Hermens, W.T.; Niessen, H.W.; Verheugt, F.W.; Wolbink, G.J.; Hack, C.E. C-reactive protein as a cardiovascular risk factor: More than an epiphenomenon? Circulation 1999, 100, 96–102. [Google Scholar] [CrossRef] [Green Version]


| Patients | Controls | p Value | ||||
|---|---|---|---|---|---|---|
| Gender F/M | 31/30 | 16/14 | p > 0.05 | |||
| Age (years) | 38.5 ± 10.9 | 39.3 ± 10.3 | p > 0.05 | |||
| BMI (kg/m2) | 28.9 ± 4.9 | 25.6 ± 4.2 | * p < 0.05 | |||
| Disease duration (years) | 8.7 ± 7.4 | N/A | ||||
| CRP (mg/L) | 17.4 ± 25.2 | 2.7 ± 2.0 | * p < 0.05 | |||
| Smokers/non-smokers | 39/22 | 5/25 | * p < 0.05 | |||
| Hurley staging | Females | Males | Total | N/A | ||
| Hurley I | 12 | 7 | 19 | |||
| Hurley II | 15 | 13 | 28 | |||
| Hurley III | 4 | 10 | 14 | |||
| N | S100A4 (pg/mL) Mean ± SD | S100A15 (pg/mL) Mean ± SD | CRP (mg/L) Mean ± SD | |
|---|---|---|---|---|
| Controls | 30 | 22.5 ± 23.3 | 153.9 ± 134.0 | 2.7 ± 2.0 |
| Hurley I | 19 | 22.7 ± 22.6 | 50.8 ± 30.9 | 3.9 ± 4.4 |
| Hurley II | 28 | 37.6 ± 24.5 | 151.5 ± 115.7 | 9.6 ± 10.1 |
| Hurley III | 14 | 35.3 ± 24.9 | 317.1 ± 101.0 | 43.1 ± 35.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batycka-Baran, A.; Matusiak, Ł.; Nowicka-Suszko, D.; Szepietowski, J.C.; Baran, W. Increased Serum Levels of S100A4 and S100A15 in Individuals Suffering from Hidradenitis Suppurativa. J. Clin. Med. 2021, 10, 5320. https://doi.org/10.3390/jcm10225320
Batycka-Baran A, Matusiak Ł, Nowicka-Suszko D, Szepietowski JC, Baran W. Increased Serum Levels of S100A4 and S100A15 in Individuals Suffering from Hidradenitis Suppurativa. Journal of Clinical Medicine. 2021; 10(22):5320. https://doi.org/10.3390/jcm10225320
Chicago/Turabian StyleBatycka-Baran, Aleksandra, Łukasz Matusiak, Danuta Nowicka-Suszko, Jacek C. Szepietowski, and Wojciech Baran. 2021. "Increased Serum Levels of S100A4 and S100A15 in Individuals Suffering from Hidradenitis Suppurativa" Journal of Clinical Medicine 10, no. 22: 5320. https://doi.org/10.3390/jcm10225320
APA StyleBatycka-Baran, A., Matusiak, Ł., Nowicka-Suszko, D., Szepietowski, J. C., & Baran, W. (2021). Increased Serum Levels of S100A4 and S100A15 in Individuals Suffering from Hidradenitis Suppurativa. Journal of Clinical Medicine, 10(22), 5320. https://doi.org/10.3390/jcm10225320







