Influence of a Virtual Exercise Program throughout Pregnancy during the COVID-19 Pandemic on Perineal Tears and Episiotomy Rates: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
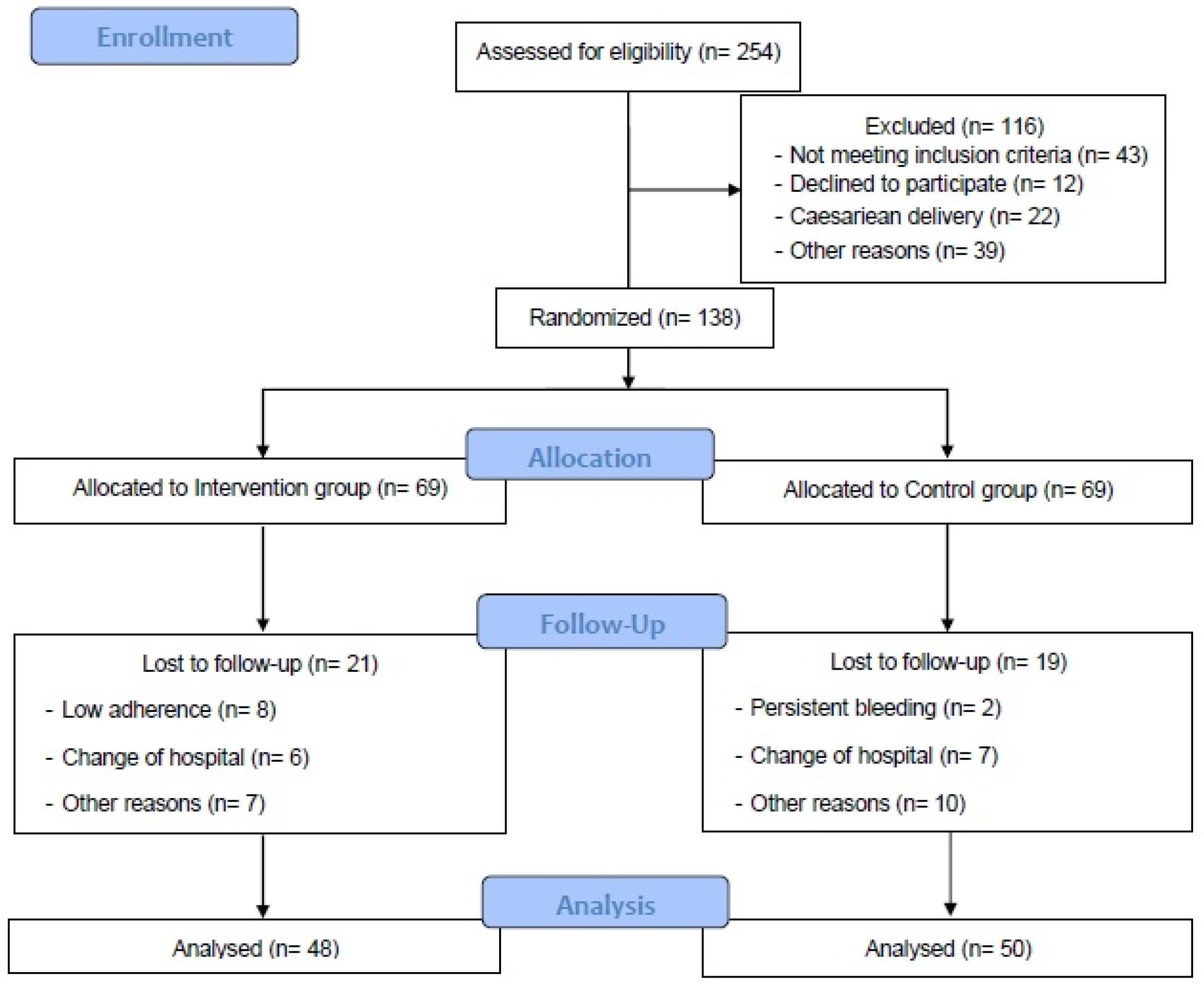
2.2. Participants and Randomization Process
2.3. Intervention
2.4. Control Group
2.5. Outcomes
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Åhlund, S.; Rådestad, I.; Zwedberg, S.; Lindgren, H. Perineal pain the first year after childbirth and uptake of post-partum check-up—A Swedish cohort study. Midwifery 2019, 78, 85–90. [Google Scholar] [CrossRef]
- Edqvist, M.; Hildingsson, I.; Mollberg, M.; Lundgren, I.; Lindgren, H. Midwives’ Management during the Second Stage of Labor in Relation to Second-Degree Tears—An Experimental Study. Birth 2017, 44, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Jose, C.; Díaz-Blanco, Á.; Barakat, R.; Coterón, J.; Refoyo, I. Physical activity during pregnancy is associated with a lower number of perineal tears. Transl. Sport Med. 2021, 4, 38–45. [Google Scholar] [CrossRef]
- Ballesteros-Meseguer, C.; Carrillo-García, C.; Meseguer-de-Pedro, M.; Canteras-Jordana, M.; Martínez-Roche, M.E. Episiotomy and its relationship to various clinical variables that influence its performance. Rev. Lat.-Am. Enferm. 2016, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christianson, L.M.; Bovbjerg, V.E.; McDavitt, E.C.; Hullfish, K.L. Risk factors for perineal injury during delivery. Am. J. Obstet. Gynecol. 2003, 189, 255–260. [Google Scholar] [CrossRef]
- Dahlen, H.G.; Ryan, M.; Homer, C.S.E.; Cooke, M. An Australian prospective cohort study of risk factors for severe perineal trauma during childbirth. Midwifery 2007, 23, 196–203. [Google Scholar] [CrossRef]
- Handa, V.L.; Blomquist, J.L.; McDermott, K.C.; Friedman, S.; Muñoz, A. Pelvic floor disorders after vaginal birth: Effect of episiotomy, perineal laceration, and operative birth. Obstet. Gynecol. 2012, 119, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeman, L.; Fullilove, A.M.; Borders, N.; Manocchio, R.; Albers, L.L.; Rogers, R.G. Postpartum Perineal Pain in a Low Episiotomy Setting: Association with Severity of Genital Trauma, Labor Care, and Birth Variables. Birth 2009, 36, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Gebuza, G.; Kaźmierczak, M.; Gdaniec, A.; Mieczkowska, E.; Gierzsewska, M.; Dombrowska-Pali, A.; Banaszkiewicz, M.; Maleńcyzk, M. Episiotomy and perineal tear risk factors in a group of 4493 women. Health Care Women Int. 2018, 39, 663–683. [Google Scholar] [CrossRef] [PubMed]
- Tegerstedt, G.; Miedel, A.; Mæhle-Schmidt, M.; Nyrén, O.; Hammarström, M. Obstetric risk factors for symptomatic prolapse: A population-based approach. Am. J. Obstet. Gynecol. 2006, 194, 75–81. [Google Scholar] [CrossRef]
- De Souza, A.; Dwyer, P.L.; Charity, M.; Thomas, E.; Ferreira, C.H.J.; Schierlitz, L. The effects of mode delivery on postpartum sexual function: A prospective study. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1410–1418. [Google Scholar] [CrossRef]
- Leeman, L.; Rogers, R.; Borders, N.; Teaf, D.; Qualls, C. The Effect of Perineal Lacerations on Pelvic Floor Function and Anatomy at 6 Months Postpartum in a Prospective Cohort of Nulliparous Women. Birth 2016, 43, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, R.G.; Leeman, L.M.; Migliaccio, L.; Albers, L.L. Does the severity of spontaneous genital tract trauma affect postpartum pelvic floor function? Int. Urogynecol. J. 2008, 19, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Tähtinen, R.M.; Cartwright, R.; Tsui, J.F.; Aaltonen, R.L.; Aoki, Y.; Cárdenas, J.L.; El Dib, R.; Joronen, K.M.; Al Juaid, S.; Kalantan, S.; et al. Long-term Impact of Mode of Delivery on Stress Urinary Incontinence and Urgency Urinary Incontinence: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 148–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, M.C.; Gauthier, R.J.; Robbins, J.M.; Kaczorowski, J.; Jorgensen, S.H.; Franco, E.D.; Johnson, B.; Waghorn, K.; Gelfand, M.M.; Guralnick, M.S.; et al. Relationship of episiotomy to perineal trauma and morbidity, sexual dysfunction, and pelvic floor relaxation. Am. J. Obstet. Gynecol. 1994, 171, 591–598. [Google Scholar] [CrossRef]
- Neels, H.; De Wachter, S.; Wyndaele, J.J.; Wyndaele, M.; Vermandel, A. Does pelvic floor muscle contraction early after delivery cause perineal pain in postpartum women? Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 208, 1–5. [Google Scholar] [CrossRef]
- Eason, E.; Labrecque, M.; Marcoux, S.; Mondor, M. Anal incontinence after childbirth. Can. Med. Assoc. J. 2002, 166, 326–330. [Google Scholar]
- Trutnovsky, G.; Kamisan, A.I.; Martin, A.; Dietz, H.P. Delivery mode and pelvic organ prolapse: A retrospective observational study. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 1551–1556. [Google Scholar] [CrossRef] [Green Version]
- MacArthur, C.; Winter, H.R.; Bick, D.E.; Knowles, H.; Lilford, R.; Henderson, C.; Lancashire, R.J.; Braunholtz, D.A.; Gee, H. Effects of redesigned community postnatal care on womens’ health 4 months after birth: A cluster randomised controlled trial. Lancet 2002, 359, 378–385. [Google Scholar] [CrossRef]
- Peralta, F.; Bavaro, J.B. Severe perineal lacerations after vaginal delivery: Are they an anesthesiologist’s problem? Curr. Opin. Anesthesiol. 2018, 31. [Google Scholar] [CrossRef]
- Leon-Larios, F.; Corrales-Gutierrez, I.; Casado-Mejía, R.; Suarez-Serrano, C. Influence of a pelvic floor training programme to prevent perineal trauma: A quasi-randomised controlled trial. Midwifery 2017, 50, 72–77. [Google Scholar] [CrossRef]
- Ayaz, R.; Hocaoğlu, M.; Günay, T.; Yardımcı, O.D.; Turgut, A.; Karateke, A. Anxiety and depression symptoms in the same pregnant women before and during the COVID-19 pandemic. J. Perinat. Med. 2020, 48, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Juan, J.; Gil, M.M.; Rong, Z.; Zhang, Y.; Yang, H.; Poon, L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: Systematic review. Ultrasound Obstet. Gynecol. 2020, 56, 15–27. [Google Scholar] [CrossRef]
- Davenport, M.H.; Ruchat, S.M.; Sobierajski, F.; Poitras, V.J.; Gray, C.E.; Yoo, C.; Skow, R.J.; Jaramillo García, A.; Barrowman, N.; Meah, V.L.; et al. Impact of prenatal exercise on maternal harms, labour and delivery outcomes: A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Aguado, A.; Rodríguez, D.; Flor, F.; Sicras, A.; Ruiz, A.; Prados-Torres, A. Distribución del gasto sanitario en atención primaria según edad y sexo: Un análisis retrospectivo. Atención Primaria 2012, 44, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.M.; Davies, G.A.; Poitras, V.; Gray, C.; Jaramillo, A.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sports Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Barakat, R.; Díaz-Blanco, A.; Franco, E.; Rollán-Malmierca, A.; Brik, M.; Varga, M.; Silva, C.; Sánchez-Polán, M.; Gil, J.; Perales, M.; et al. Guías clínicas para el ejercicio físico durante el embarazo. Progr. Obstet. Ginecol. 2019, 62, 464–471. [Google Scholar] [CrossRef]
- Birsner, M.L.; Gyamfi-Bannerman, C. Physical activity and exercise during pregnancy and the postpartum period ACOG committee opinion summary, number 804. Obstet. Gynecol. 2020, 135, E178–E188. [Google Scholar]
- Schantz, C. Quelles interventions au cours de la grossesse diminuent le risque de lésions périnéales ? RPC Prévention et protection périnéale en obstétrique CNGOF. Gynécol. Obstet. Fertil. Sénol. 2018, 46, 922–927. [Google Scholar] [CrossRef]
- Schreiner, L.; Crivelatti, I.; de Oliveira, J.M.; Nygaard, C.C.; Dos Santos, T.G. Systematic review of pelvic floor interventions during pregnancy. Int. J. Gynecol. Obstet. 2018, 143, 10–18. [Google Scholar] [CrossRef]
- Barakat, R. An exercise program throughout pregnancy: Barakat model. Birth Defects Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Barakat, R.; Pelaez, M.; Cordero, Y.; Perales, M.; López, C.; Coterón, J.; Mottola, M.F. Exercise during pregnancy protects against hypertension and macrosomia: Randomized clinical trial. Am. J. Obstet. Gynecol. 2016, 214, 649.e1–649.e8. [Google Scholar] [CrossRef]
- Röhrig, B.; du Prel, J.B.; Wachtlin, D.; Kwiecien, R.; Blettner, M. Sample size calculation in clinical trials: Part 13 of a series on evaluation of scientific publications. Dtsch. Ärztebl. Int. 2010, 107, 552–556. [Google Scholar] [CrossRef]
- Thubert, T.; Cardaillac, C.; Fritel, X.; Winer, N.; Dochez, V. Définitions, épidémiologie et facteurs de risque des lésions périnéales du 3e et 4e degrés. RPC Prévention et protection périnéale en obstétrique CNGOF. Gynécol. Obstet. Fertil. Sénol. 2018, 46. [Google Scholar] [CrossRef]
- Dietz, H.P.; Schierlitz, L. Pelvic floor trauma in childbirth—Myth or reality? Aust. N. Z. J. Obstet. Gynaecol. 2005, 45, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.B.; Anjum, N.; Hoodbhoy, Z.; Khoso, R. Episiotomy and its complications: A cross sectional study in secondary care hospital. J. Pak. Med. Assoc. 2020, 70, 2036–2038. [Google Scholar] [CrossRef]
- Voldner, N.; Frøslie, K.F.; Haakstad, L.A.; Bø, K.; Henriksen, T. Birth complications, overweight, and physical inactivity. Acta Obstet. Gynecol. Scand. 2009, 88, 550–555. [Google Scholar] [CrossRef]
- Uccella, S.; Manzoni, P.; Marconi, N.; Toscani, C.; Biasoli, S.; Cianci, S.; Franchi, M.; Sorice, P.; Bertoli, F.; Zorzato, P.C. Impact of Sport Activity and Physical Exercise on Obstetrical and Perineal Outcomes at Delivery: A Prospective Study. Am. J. Perinatol. 2019, 36, S83–S90. [Google Scholar] [CrossRef] [Green Version]
- López-Bueno, R.; Calatayud, J.; Casaña, J.; Casajús, J.A.; Smith, L.; Tully, M.A.; Andersen, L.L.; López-Sánchez, G.F. COVID-19 Confinement and Health Risk Behaviors in Spain. Front. Psychol. 2020, 11, 1426. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, L.; De Vivo, M.; Hayes, L.; Hesketh, K.R.; Mills, H.; Newham, J.J.; Olander, E.K.; Smith, D.M. Encouraging Physical Activity during and after Pregnancy in the COVID-19 Era, and beyond. Int. J. Environ. Res. Public Health 2020, 17, 7304. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, K.M.; Hung, P.; Alberg, A.J.; Hair, N.L.; Liu, J. Variations in health behaviors among pregnant women during the COVID-19 pandemic. Midwifery 2021, 95, 102929. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Liang, C.C.; Wu, D.; Chang, S.D.; Chueh, H.Y.; Chao, A.S. Prevalence and contributing factors of severe perineal damage following episiotomy-assisted vaginal delivery. Taiwan J. Obstet. Gynecol. 2014, 53, 481–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, M.H.; Meah, V.L.; Ruchat, S.M.; Davies, G.A.; Skow, R.J.; Barrowman, N.; Adamo, K.B.; Poitras, V.J.; Gray, C.E.; Jaramillo García, A.; et al. Impact of prenatal exercise on neonatal and childhood outcomes: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1386–1396. [Google Scholar] [CrossRef]
- Viannay, P.; de la Codre, F.; Brochard, C.; Thubert, T.; Meurette, G.; Legendre, G.; Venara, A. Management and consequences of obstetrical anal sphincter injuries: Review. J. Visc. Surg. 2021, 158, 231–241. [Google Scholar] [CrossRef]
- Woodley, S.J.; Lawrenson, P.; Boyle, R.; Cody, J.D.; Mørkyed, S.; Kernohan, A.; Hay-Smith, E.J.C. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst. Rev. 2020, 5, CD007471. [Google Scholar] [CrossRef]
- Huber, M.; Malers, E.; Tunón, K. Pelvic floor dysfunction one year after first childbirth in relation to perineal tear severity. Sci. Rep. 2021, 11, 12560. [Google Scholar] [CrossRef] [PubMed]
- Feria-Ramírez, C.; Gonzalez-Sanz, J.D.; Molina-Luque, R.; Molina-Recio, G. The Effects of the Pilates Method on Pelvic Floor Injuries during Pregnancy and Childbirth: A Quasi-Experimental Study. Int. J. Environ. Res. Public Health 2021, 18, 6995. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Blanque, R.; Sanchez-Garcia, J.C.; Sánchez-López, A.M.; Expósito-Ruiz, M.; Aguilar-Cordero, M.J. Randomized Clinical Trial of an Aquatic Physical Exercise Program During Pregnancy. J. Obstet. Gynecol. Neonatal Nurs. 2019, 48, 321–331. [Google Scholar] [CrossRef]
| Maternal Characteristics | |||
|---|---|---|---|
| Variable | Intervention Group (n = 48) | Control Group (n = 50) | p-Value |
| Age (years) | 33.15 ± 4.82 | 33.54 ± 4.87 | 0.690 |
| Maternal height (m) | 1.63 ± 0.05 | 1.63 ± 0.06 | 0.630 |
| Maternal weight (kg) | 61.90 ± 10.57 | 67.33 ± 18.81 | 0.085 |
| BMI (n/%) | 22.92 ± 5.10 | 24.58 ± 3.87 | 0.072 |
| <18.5 | 5/10.4 | 2/4.0 | 0.330 |
| 18.5–24.9 | 30/62.5 | 29/58.0 | |
| 25–29.9 | 11/22.9 | 13/26.0 | |
| >30 | 2/4.6 | 6/12.0 | |
| Parity † (n/%) | |||
| None | 31/64.6 | 36/76.6 | 0.278 |
| One | 11/22.9 | 9/19.1 | |
| Two or more | 6/12.5 | 2/4.3 | |
| Smoking during pregnancy | |||
| No | 46/95.8 | 42/89.4 | 0.227 |
| Yes | 2/4.2 | 5/10.6 | |
| Occupation (n/%) | |||
| Active job | 26/54.2 | 20/43.5 | 0.564 |
| Sedentary job | 16/33.3 | 18/39.1 | |
| Homemaker | 6/12.5 | 8/17.4 | |
| Previous miscarriage (n/%) | |||
| None | 31/64.6 | 36/73.5 | 0.076 |
| One | 14/29.2 | 6/12.2 | |
| Two or more | 3/6.3 | 7/14.3 | |
| Episiotomy | PTT | MD | BW | BL | HC | MA | MW | Parity | Smoking | |
|---|---|---|---|---|---|---|---|---|---|---|
| Episiotomy | 1 | |||||||||
| PTT | −0.364 * | 1 | ||||||||
| MD | 0.246 | −0.072 | 1 | |||||||
| BW | 0.168 | −0.017 | 0.342 * | 1 | ||||||
| BL | 0.308 | −0.120 | 0.378 * | 0.736 * | 1 | |||||
| HC | 0.370 * | −0.259 | 0.197 | 0.743 * | 0.631 * | 1 | ||||
| MA | 0.030 | 0.041 | −0.026 | 0.006 | −0.042 | 0.112 | 1 | |||
| MW | 0.063 | −0.182 | −0.029 | 0.259 | 0.238 | 0.261 | −0.225 | 1 | ||
| Parity | 0.052 | −0.381 * | −0.204 | −160 | −0.038 | −143 | 0.349 * | 0.166 | 1 | |
| Smoking | −0.100 | −0.063 | −0.063 | −0.115 | −0.053 | −0.279 | −0.052 | −0.155 | 0.006 | 1 |
| Episiotomy | PTT | MD | BW | BL | HC | MA | MW | Parity | Smoking | |
|---|---|---|---|---|---|---|---|---|---|---|
| Episiotomy | 1 | |||||||||
| PTT | −0.384 * | 1 | ||||||||
| MD | 0.577 * | −0.453 * | 1 | |||||||
| BW | 0.145 | 0.333 * | −0.117 | 1 | ||||||
| BL | 0.031 | 0.127 | −0.111 | 0.769 * | 1 | |||||
| HC | 0.304 | 0.049 | 0.180 | 0.641 * | 0.471 * | 1 | ||||
| MA | 0.031 | −0.099 | 0.203 | 0.068 | 0.021 | 0.020 | 1 | |||
| MW | −0.105 | −0.074 | 0.035 | −0.149 | −0.116 | −0.223 | 0.112 | 1 | ||
| Parity | −0.036 | −0.010 | 0.007 | 0.201 | 0.262 | 0.275 | 0.209 | −0.047 | 1 | |
| Smoking | 0.043 | 0.092 | 0.049 | −0.136 | −0.320 | −0.327 | 0.077 | −0.118 | −0.179 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Jose, C.; Sánchez-Polán, M.; Díaz-Blanco, Á.; Pérez-Medina, T.; Carrero Martínez, V.; Alzola, I.; Barakat, R.; Refoyo, I.; Mottola, M.F. Influence of a Virtual Exercise Program throughout Pregnancy during the COVID-19 Pandemic on Perineal Tears and Episiotomy Rates: A Randomized Clinical Trial. J. Clin. Med. 2021, 10, 5250. https://doi.org/10.3390/jcm10225250
Silva-Jose C, Sánchez-Polán M, Díaz-Blanco Á, Pérez-Medina T, Carrero Martínez V, Alzola I, Barakat R, Refoyo I, Mottola MF. Influence of a Virtual Exercise Program throughout Pregnancy during the COVID-19 Pandemic on Perineal Tears and Episiotomy Rates: A Randomized Clinical Trial. Journal of Clinical Medicine. 2021; 10(22):5250. https://doi.org/10.3390/jcm10225250
Chicago/Turabian StyleSilva-Jose, Cristina, Miguel Sánchez-Polán, Ángeles Díaz-Blanco, Tirso Pérez-Medina, Vanessa Carrero Martínez, Irune Alzola, Rubén Barakat, Ignacio Refoyo, and Michelle F. Mottola. 2021. "Influence of a Virtual Exercise Program throughout Pregnancy during the COVID-19 Pandemic on Perineal Tears and Episiotomy Rates: A Randomized Clinical Trial" Journal of Clinical Medicine 10, no. 22: 5250. https://doi.org/10.3390/jcm10225250
APA StyleSilva-Jose, C., Sánchez-Polán, M., Díaz-Blanco, Á., Pérez-Medina, T., Carrero Martínez, V., Alzola, I., Barakat, R., Refoyo, I., & Mottola, M. F. (2021). Influence of a Virtual Exercise Program throughout Pregnancy during the COVID-19 Pandemic on Perineal Tears and Episiotomy Rates: A Randomized Clinical Trial. Journal of Clinical Medicine, 10(22), 5250. https://doi.org/10.3390/jcm10225250









