Median Nerve Neural Mobilization Adds No Additional Benefit When Combined with Cervical Lateral Glide in the Treatment of Neck Pain: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
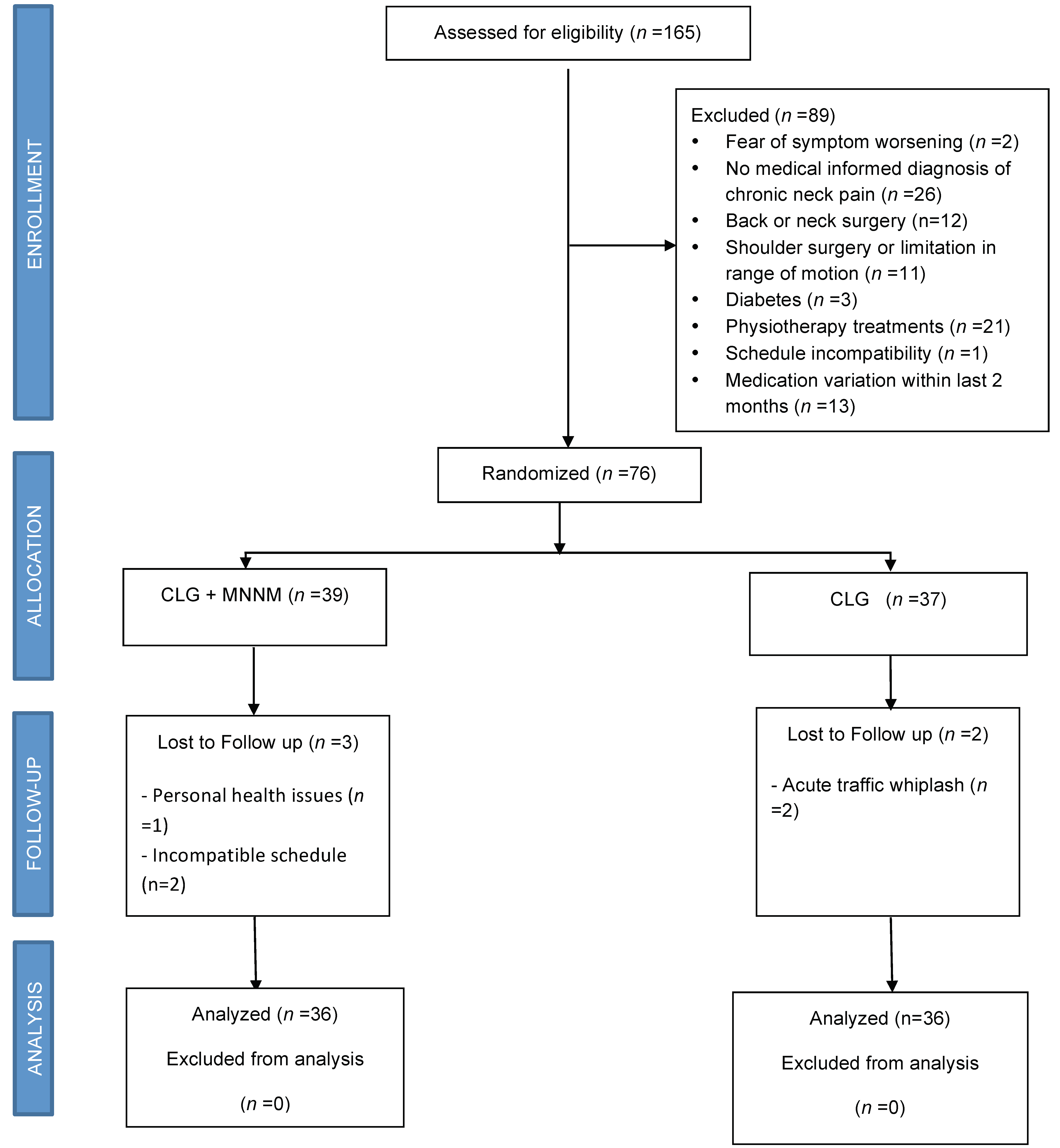
2.2. Randomization and Blinding
2.3. Follow-Up
2.4. Median Nerve Mechanosensitivity
2.5. Widespread Pain Assessment
2.5.1. Pain Intensity
2.5.2. Body Pain Distribution
2.5.3. Pressure Pain threshold (PPT)
2.6. Cervical Function
2.7. TSK-11
2.8. Participants
2.9. Intervention
2.10. Cervical Lateral Glide and Median Nerve Mobilization (CLG + MNNM)
2.11. Cervical Lateral Glide in Isolation (Isolated CLG)
2.12. Sample Size
2.13. Statistical Analysis
3. Results
3.1. Median Nerve Mechanosensitivity
3.2. Widespread Pain Assessment
3.3. Cervical Function and Kinesiophobia
4. Discussion
4.1. Median Nerve Mechanosensitivity
4.2. Widespread Pain Assessment
4.3. Cervical Function and Kinesiofobia
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palacios-Ceña, D.; Alonso-Blanco, C.; Hernández-Barrera, V.; Carrasco-Garrido, P.; Jiménez-García, R.; Fernández-de-Las-Peñas, C. Prevalence of neck and low back pain in community-dwelling adults in Spain: An updated population-based national study (2009/10–2011/12). Eur. Spine J. 2015, 24, 482–492. [Google Scholar] [CrossRef]
- GBD 2013 DALYs; HALE Collaborators; Murray, C.J.L.; Barber, R.M.; Foreman, K.J.; Abbasoglu Ozgoren, A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet Lond. Engl. 2015, 386, 2145–2191. [Google Scholar] [CrossRef]
- Hoy, D.; March, L.; Woolf, A.; Blyth, F.; Brooks, P.; Smith, E.; Vos, T.; Barendregt, J.; Blore, J.; Murray, C.; et al. The global burden of neck pain: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1309–1315. [Google Scholar] [CrossRef]
- Carroll, L.J.; Hogg-Johnson, S.; van der Velde, G.; Haldeman, S.; Holm, L.W.; Carragee, E.J.; Hurwitz, E.L.; Côté, P.; Nordin, M.; Peloso, P.M.; et al. Course and prognostic factors for neck pain in the general population: Results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. J. Manip. Physiol. Ther. 2009, 32, S87–S96. [Google Scholar] [CrossRef] [PubMed]
- Hogg-Johnson, S.; van der Velde, G.; Carroll, L.J.; Holm, L.W.; Cassidy, J.D.; Guzman, J.; Côté, P.; Haldeman, S.; Ammendolia, C.; Carragee, E.; et al. The burden and determinants of neck pain in the general population: Results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. J. Manip. Physiol. Ther. 2009, 32, S46–S60. [Google Scholar] [CrossRef]
- Haldeman, S.; Carroll, L.; Cassidy, J.D. Findings from the bone and joint decade 2000 to 2010 task force on neck pain and its associated disorders. J. Occup. Environ. Med. 2010, 52, 424–427. [Google Scholar] [CrossRef]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. PAIN 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Gangavelli, R.; Nair, N.S.; Bhat, A.K.; Solomon, J.M. Cervicobrachial pain—How Often is it Neurogenic? J. Clin. Diagn. Res. 2016, 10, YC14–YC16. [Google Scholar] [CrossRef]
- Kozma, C.M.; Provenzano, D.A.; Slaton, T.L.; Patel, A.A.; Benson, C.J. Complexity of Pain Management Among Patients with Nociceptive or Neuropathic Neck, Back, or Osteoarthritis Diagnoses. J. Manag. Care Pharm. 2014, 20, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.R.; Aker, P.D.; Goldsmith, C.H.; Peloso, P. Physical medicine modalities for mechanical neck disorders. Cochrane Database Syst. Rev. 2000, 18, CD000961. [Google Scholar] [CrossRef] [PubMed]
- Aker, P.D.; Gross, A.R.; Goldsmith, C.H.; Peloso, P. Conservative management of mechanical neck pain: Systematic overview and meta-analysis. BMJ 1996, 313, 1291–1296. [Google Scholar] [CrossRef]
- Thoomes, E.J.; Scholten-Peeters, W.; Koes, B.; Falla, D.; Verhagen, A.P. The effectiveness of conservative treatment for patients with cervical radiculopathy: A systematic review. Clin. J. Pain 2013, 29, 1073–1086. [Google Scholar] [CrossRef]
- Thoomes, E.J. Effectiveness of manual therapy for cervical radiculopathy, a review. Chiropr. Man. Therap. 2016, 24, 45. [Google Scholar] [CrossRef]
- Boyles, R.; Toy, P.; Mellon, J.; Hayes, M.; Hammer, B. Effectiveness of manual physical therapy in the treatment of cervical radiculopathy: A systematic review. J. Man. Manip. Ther. 2011, 19, 135–142. [Google Scholar] [CrossRef]
- Ellis, R.F.; Hing, W.A. Neural mobilization: A systematic review of randomized controlled trials with an analysis of therapeutic efficacy. J. Man. Manip. Ther. 2008, 16, 8–22. [Google Scholar] [CrossRef]
- Basson, A.; Olivier, B.; Ellis, R.; Coppieters, M.; Stewart, A.; Mudzi, W. The Effectiveness of Neural Mobilization for Neuromusculoskeletal Conditions: A Systematic Review and Meta-analysis. J. Orthop. Sport Phys. Ther. 2017, 47, 593–615. [Google Scholar] [CrossRef]
- Costello, M.; Puentedura, E.L.J.; Cleland, J.; Ciccone, C.D. The immediate effects of soft tissue mobilization versus therapeutic ultrasound for patients with neck and arm pain with evidence of neural mechanosensitivity: A randomized clinical trial. J. Man. Manip. Ther. 2016, 24, 128–140. [Google Scholar] [CrossRef]
- Kim, D.-G.; Chung, S.H.; Jung, H.B. The effects of neural mobilization on cervical radiculopathy patients’ pain, disability, ROM, and deep flexor endurance. J. Back Musculoskelet. Rehabil. 2017, 30, 951–959. [Google Scholar] [CrossRef]
- Rodríguez-Sanz, D.; López-López, D.; Unda-Solano, F.; Romero-Morales, C.; Sanz-Corbalán, I.; Beltran-Alacreu, H.; Calvo-Lobo, C. Effects of Median Nerve Neural Mobilization in Treating Cervicobrachial Pain: A Randomized Waiting List–Controlled Clinical Trial. Pain Pract. 2018, 18, 431–442. [Google Scholar] [CrossRef]
- Rodríguez-Sanz, D.; Calvo-Lobo, C.; Unda-Solano, F.; Sanz-Corbalán, I.; Romero-Morales, C.; López-López, D. Cervical Lateral Glide Neural Mobilization Is Effective in Treating Cervicobrachial Pain: A Randomized Waiting List Controlled Clinical Trial. Pain Med. 2017, 18, 2492–2503. [Google Scholar] [CrossRef]
- Sanz, D.R.; Solano, F.U.; López, D.L.; Corbalan, I.S.; Morales, C.R.; Lobo, C.C. Effectiveness of median nerve neural mobilization versus oral ibuprofen treatment in subjects who suffer from cervicobrachial pain: A randomized clinical trial. Arch. Med. Sci. 2017, 14, 871–879. [Google Scholar] [CrossRef]
- Calvo-Lobo, C.; Unda-Solano, F.; López-López, D.; Sanz-Corbalán, I.; Romero-Morales, C.; Palomo-López, P.; Seco-Calvo, J.; Rodríguez-Sanz, D. Is pharmacologic treatment better than neural mobilization for cervicobrachial pain? A randomized clinical trial. Int. J. Med. Sci. 2018, 15, 456–465. [Google Scholar] [CrossRef]
- Isabel de-la-Llave-Rincón, A.; Puentedura, E.J.; Fernández-de-Las-Peñas, C. Clinical presentation and manual therapy for upper quadrant musculoskeletal conditions. J. Man. Manip. Ther. 2011, 19, 201–211. [Google Scholar] [CrossRef]
- Cobos-Carbó, A.; Augustovski, F. Declaración CONSORT 2010, actualización de la lista de comprobación para informar ensayos clínicos aleatorizados de grupos paralelos. Med. Clin. Barc. 2011, 137, 213–215. [Google Scholar] [CrossRef]
- World-Medical-Association. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Schmid, A.B.; Brunner, F.; Luomajoki, H.; Held, U.; Bachmann, L.M.; Künzer, S.; Coppieters, M.W. Reliability of clinical tests to evaluate nerve function and mechanosensitivity of the upper limb peripheral nervous system. BMC Musculoskelet. Disord. 2009, 10, 11. [Google Scholar] [CrossRef]
- Wainner, R.S.; Fritz, J.M.; Irrgang, J.J.; Boninger, M.L.; Delitto, A.; Allison, S. Reliability and diagnostic accuracy of the clinical examination and patient self-report measures for cervical radiculopathy. Spine Phila Pa 1976 2003, 28, 52–62. [Google Scholar] [CrossRef]
- Kleinrensink, G.J.; Stoeckart, R.; Vleeming, A.; Snijders, C.J.; Mulder, P.G.H. Mechanical tension in the median nerve. The effects of joint positions. Clin. Biomech. Bristol. Avon. 1995, 10, 240–244. [Google Scholar] [CrossRef][Green Version]
- Nee, R.J.; Jull, G.A.; Vicenzino, B.; Coppieters, M.W. The Validity of Upper-Limb Neurodynamic Tests for Detecting Peripheral Neuropathic Pain. J. Orthop. Sport Phys. Ther. 2012, 42, 413–424. [Google Scholar] [CrossRef]
- Coppieters, M.; Stappaerts, K.; Janssens, K.; Jull, G. Reliability of detecting “onset of pain” and “submaximal pain” during neural provocation testing of the upper quadrant. Physiother. Res. Int. 2002, 7, 146–156. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S. Studies Comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for Assessment of Pain Intensity in Adults: A Systematic Literature Review. J. Pain Symptom. Manag. 2011, 41, 1073. [Google Scholar] [CrossRef]
- Kahl, C.; Cleland, J.A. Visual analogue scale, numeric pain rating scale and the McGill pain Questionnaire: An overview of psychometric properties. Phys. Ther. Rev. 2005, 10, 123–128. [Google Scholar] [CrossRef]
- Rosier, E.M.; Iadarola, M.J.; Coghill, R.C. Reproducibility of pain measurement and pain perception. Pain 2002, 98, 205–216. [Google Scholar] [CrossRef]
- Barbero, M.; Moresi, F.; Leoni, D.; Gatti, R.; Egloff, M.; Falla, D. Test-retest reliability of pain extent and pain location using a novel method for pain drawing analysis. Eur. J. Pain. 2015, 19, 1129–1138. [Google Scholar] [CrossRef]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.B.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef]
- Rebbeck, T.; Moloney, N.; Azoory, R.; Hubscher, M.; Waller, R.; Gibbons, R.; Beales, D. Clinical Ratings of Pain Sensitivity Correlate With Quantitative Measures in People With Chronic Neck Pain and Healthy Controls: Cross-Sectional Study. Phys. Ther. 2015, 95, 1536–1546. [Google Scholar] [CrossRef]
- Backonja, M.-M.; Walk, D.; Edwards, R.R.; Sehgal, N.; Moeller-Bertram, T.; Wasan, A.; Irving, G.; Argoff, C.; Wallace, M. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin. J. Pain 2009, 25, 641–647. [Google Scholar] [CrossRef]
- Konopka, K.H.; Harbers, M.; Houghton, A.; Kortekaas, R.; van Vliet, A.; Timmerman, W.; den Boer, J.A.; Struys, M.M.R.F.; van Wijhe, M. Bilateral sensory abnormalities in patients with unilateral neuropathic pain; A quantitative sensory testing (QST) study. PLoS ONE 2012, 7, e37524. [Google Scholar]
- Loeser, J.; Arendt-Nielsen, L.; Baron, R.; Basbaum, A.; Bond, M.; Breivik, H.; Clauw, D.; De Laat, A.; Dworkin, R.; Giamberardino, M.; et al. Pain Terms, A Current List with Definitions and Notes on Usage. In Classification of Chronic Pain; IASP Press: Seattle, WA, USA, 2011; pp. 209–214. [Google Scholar]
- Sterling, M.; Treleaven, J.; Jull, G.A. Responses to a clinical test of mechanical provocation of nerve tissue in whiplash associated disorder. Man. Ther. 2002, 7, 89–94. [Google Scholar] [CrossRef]
- Lascurain-Aguirrebena, I.; Newham, D.; Critchley, D.J. Mechanism of action of spinal mobilizations a systematic review. Spine Phila Pa 1976 2016, 41, 159–172. [Google Scholar] [CrossRef]
- Salom-Moreno, J.; Ortega-Santiago, R.; Cleland, J.A.; Palacios-Ceña, M.; Truyols-Domínguez, S.; Fernández-de-las-Peñas, C. Immediate changes in neck pain intensity and widespread pressure pain sensitivity in patients with bilateral chronic mechanical neck pain: A randomized controlled trial of thoracic thrust manipulation vs non-thrust mobilization. J. Manip. Physiol. Ther. 2014, 37, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Bisset, L.M.; Evans, K.; Tuttle, N. Reliability of 2 protocols for assessing pressure pain threshold in healthy young adults. J. Manip. Physiol. Ther. 2015, 38, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Prushansky, T.; Handelzalts, S.; Pevzner, E. Reproducibility of pressure pain threshold and visual analog scale findings in chronic whiplash patients. Clin. J. Pain 2007, 23, 339–345. [Google Scholar] [CrossRef]
- Walton, D.M.; Macdermid, J.C.; Nielson, W.; Teasell, R.W.; Chiasson, M.; Brown, L. Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J. Orthop. Sports Phys. Ther. 2011, 41, 644–650. [Google Scholar] [CrossRef]
- Fischer, A.A. Algometry in Diagnosis of Musculoskeletal Pain and Evaluation of Treatment Outcome: An Update. J. Musculoskelet. Pain 1998, 6, 5–32. [Google Scholar] [CrossRef]
- Vanderweeën, L.; Oostendorp, R.A.B.; Vaes, P.; Duquet, W. Pressure algometry in manual therapy. Man. Ther. 1996, 1, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.P.; Bandy, W.D. Intrarater reliability of CROM measurement of cervical spine active range of motion in persons with and without neck pain. J. Orthop. Sports Phys. Ther. 2008, 38, 640–645. [Google Scholar] [CrossRef]
- Vernon, H. The Neck Disability Index: State-of-the-art, 1991–2008. J. Manip. Physiol. Ther. 2008, 31, 491–502. [Google Scholar] [CrossRef]
- Macdermid, J.C.; Walton, D.M.; Côté, P.; Santaguida, P.L.; Gross, A.; Carlesso, L.; ICON. Use of outcome measures in managing neck pain: An international multidisciplinary survey. Open Orthop. J. 2013, 7, 506. [Google Scholar] [CrossRef]
- Murphy, D.R.; Lopez, M. Neck and back pain specific outcome assessment questionnaires in the Spanish language: A systematic literature review. Spine J. 2013, 13, 1667–1674. [Google Scholar] [CrossRef]
- Andrade Ortega, J.A.; Delgado Martínez, A.D.; Almécija Ruiz, R. Validation of the Spanish version of the Neck Disability Index. Spine Phila Pa 1976. 2010, 35, E114–E118. [Google Scholar] [CrossRef] [PubMed]
- Hudes, K. The Tampa Scale of Kinesiophobia and neck pain, disability and range of motion: A narrative review of the literature. J. Can. Chiropr. Assoc. 2011, 55, 222–232. [Google Scholar]
- Gómez-Pérez, L.; López-Martínez, A.E.; Ruiz-Párraga, G.T. Psychometric Properties of the Spanish Version of the Tampa Scale for Kinesiophobia (TSK). J. Pain. 2011, 12, 425–435. [Google Scholar] [CrossRef]
- Roelofs, J.; Goubert, L.; Peters, M.L.; Vlaeyen, J.W.S.; Crombez, G. The Tampa Scale for Kinesiophobia: Further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. Eur. J. Pain 2004, 8, 495–502. [Google Scholar] [CrossRef]
- Nee, R.J.; Vicenzino, B.; Jull, G.A.; Cleland, J.A.; Coppieters, M.W. A novel protocol to develop a prediction model that identifies patients with nerve-related neck and arm pain who benefit from the early introduction of neural tissue management. Contemp. Clin. Trials 2011, 32, 760–770. [Google Scholar] [CrossRef]
- Guzman, J.; Hurwitz, E.L.; Carroll, L.J.; Haldeman, S.; Cote, P.; Carragee, E.J.; Peloso, P.M.; van der Velde, G.; Holm, L.W.; Hogg-Johnson, S.; et al. A New Conceptual Model of Neck Pain Linking Onset, Course, and Care: The Bone and Joint Decade 2000 –2010 Task Force on Neck Pain and Its Associated Disorders. Spine Phila Pa 1976 2008, 33, S14–S23. [Google Scholar] [CrossRef]
- Nee, R.J.; Vicenzino, B.; Jull, G.A.; Cleland, J.A.; Coppieters, M.W. Neural tissue management provides immediate clinically relevant benefits without harmful effects for patients with nerve-related neck and arm pain: A randomised trial. J. Physiother. 2012, 58, 23–31. [Google Scholar] [CrossRef]
- Khalil, H.; Quinn, L.; van Deursen, R.; Martin, R.; Rosser, A.; Busse, M. Adherence to Use of a Home-Based Exercise DVD in People With Huntington Disease: Participants’ Perspectives. Phys. Ther. 2012, 92, 69–82. [Google Scholar] [CrossRef]
- Bernal-Utrera, C.; Gonzalez-Gerez, J.J.; Anarte-Lazo, E.; Rodriguez-Blanco, C. Manual therapy versus therapeutic exercise in non-specific chronic neck pain: A randomized controlled trial. Trials 2020, 21. [Google Scholar] [CrossRef]
- Vicenzino, B.; Collins, D.; Wright, A. The initial effects of a cervical spine manipulative physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain 1996, 68, 69–74. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Cleland, J.; Palacios-Ceña, M.; Fuensalida-Novo, S.; Alonso-Blanco, C.; Pareja, J.A.; Alburquerque-Sendín, F. Effectiveness of manual therapy versus surgery in pain processing due to carpal tunnel syndrome: A randomized clinical trial. Eur. J. Pain USA 2017, 21, 1266–1276. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; Vincent, K.R.; George, S.Z. A randomized sham-controlled trial of a neurodynamic technique in the treatment of carpal tunnel syndrome. J. Orthop. Sports Phys. Ther. 2009, 39, 709–723. [Google Scholar] [CrossRef]
- Fernández-Carnero, J.; Sierra-Silvestre, E.; Beltran-Alacreu, H.; Gil-Martínez, A.; La Touche, R. Neural Tension Technique Improves Immediate Conditioned Pain Modulation in Patients with Chronic Neck Pain: A Randomized Clinical Trial. Pain Med. 2019, 20, 1227–1235. [Google Scholar] [CrossRef]
- Coppieters, M.W.; Stappaerts, K.H.; Wouters, L.L.; Janssens, K. The immediate effects of a cervical lateral glide treatment technique in patients with neurogenic cervicobrachial pain. J. Orthop. Sports Phys. Ther. 2003, 33, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Beneciuk, J.M.; Bishop, M.D.; George, S.Z. Effects of upper extremity neural mobilization on thermal pain sensitivity: A sham-controlled study in asymptomatic participants. J. Orthop. Sports Phys. Ther. 2009, 39, 428–438. [Google Scholar] [CrossRef]
- Emshoff, R.; Bertram, S.; Emshoff, I. Clinically important difference thresholds of the visual analog scale: A conceptual model for identifying meaningful intraindividual changes for pain intensity. Pain 2011, 152, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Alacreu, H.; Jiménez-Sanz, L.; Fernández Carnero, J.; La Touche, R. Comparison of Hypoalgesic Effects of Neural Stretching vs Neural Gliding: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2015, 38, 644–652. [Google Scholar] [CrossRef]
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J. Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- MacDermid, J.C.; Walton, D.M.; Avery, S.; Blanchard, A.; Etruw, E.; McAlpine, C.; Goldsmith, C.H. Measurement properties of the neck disability index: A systematic review. J. Orthop. Sports Phys. Ther. 2009, 39, 400–417. [Google Scholar] [CrossRef] [PubMed]
| CLGS + MNNM Group n = (72) | CLGS Group n = (72) | p-Value | |
|---|---|---|---|
| Age, years | 44.69 ± 13.48 | 50.72 ± 9.42 | 0.03 * |
| Sex M/F (Female%) | 15/21 (48.8%) | 14/22 (51.2%) | 0.81 |
| Duration of pain (months) | 73.11 ± 53.52 | 61.61 ± 42.09 | 0.31 |
| PPT-C6-H (kg/cm2) | 2.48 ± 1.14 | 2.58 ± 1.09 | 0.71 |
| PPT-C6-CL (kg/cm2) | 2.53 ± 0.83 | 2.74 ± 0.98 | 0.34 |
| PPT-MN-H (kg/cm2) | 2.82 ± 0.95 | 2.91 ± 1.16 | 0.74 |
| PPT-MN-CL (kg/cm2) | 2.71 ± 1.01 | 3.03 ± 1.08 | 0.20 |
| PPT-AT-H (kg/cm2) | 7.41 ± 3.40 | 7.19 ± 3.17 | 0.77 |
| PPT-AT-CL (kg/cm2) | 6.99 ± 3.34 | 7.01 ± 3.20 | 0.97 |
| Neck-PD | 8.45 ± 6.79 | 8.98 ± 11.67 | 0.81 |
| UL-PD-HL | 5.91 ± 8.38 | 6.24 ± 12.58 | 0.89 |
| UL-PD-CL | 2.24 ± 4.43 | 2.63 ± 8.07 | 0.79 |
| Elbow extension | 49.50 ± 19.9 | 44.36 ± 21.07 | 0.29 |
| NDI (0 to 50) | 33.69 ± 16.64 | 34.83 ± 16.48 | 0.77 |
| VAS (0 to 100 mm) | 42.86 ± 19.13 | 42.78 ± 21.33 | 0.98 |
| CROM (grades) | |||
| Flexion/extension | 105.77 ± 29.59 | 102.30 ± 24.55 | 0.59 |
| Lateral flexion | 69.53 ± 22.25 | 66.97 ± 17.35 | 0.58 |
| Rotation | 115.58 ± 30.85 | 115.52 ± 20.99 | 0.99 |
| Psychological measures | |||
| TSK-11 (11 to 44) | 27.31 ± 7.73 | 28.92 ± 7.94 | 0.38 |
| Group | Baseline | Post-Intervention 5 min | Follow-Up 2 Weeks | Follow-Up 4 Weeks | Mean Difference (95% CI)
| Mean Difference (95% CI)
| |
|---|---|---|---|---|---|---|---|
| ULNT1 (degrees for elbow extension) | |||||||
| Homolateral side pain | CLG + MNNM | 50.85 ± 20.78 | 47.09 ± 20.02 | 40.80 ± 19.72 | 37.31 ± 18.47 |
|
|
| CLG | 43.58 ± 21.31 | 41.08 ± 20.52 | 42.12 ± 20.22 | 35.25 ± 18.94 |
|
| |
| Contralateral side pain | CLG + MNNM | 45.96 ± 18.80 | 47.13 ± 18.28 | 38.53 ± 15.98 | 34.57 ± 16.63 |
|
|
| CLG | 43.24 ± 19.27 | 39.20 ± 18.73 | 38.27 ± 16.38 | 32.88 ± 17.05 |
|
| |
| Median Nerve PPT (kg/cm2) | |||||||
| Homolateral side pain | CLG + MNNM | 2.76 ± 1.06 | 2.82 ± 1.15 | 2.89 ± 1.23 | 3.20 ± 1.36 |
|
|
| CLG | 2.81 ± 1.09 | 2.98 ± 1.18 | 3.19 ± 1.26 | 3.77 ± 1.39 |
|
| |
| Contralateral side pain | CLG + MNNM | 2.67 ± 1.03 | 2.81 ± 1.04 | 3.04 ± 1.08 | 3.53 ± 1.43 |
|
|
| CLG | 2.90 ± 1.06 | 3.13 ± 1.07 | 3.33 ± 1.11 | 3.76 ± 1.47 |
|
| |
| Group | Baseline | Post-Intervention 5 min | Follow-Up 2 Weeks | Follow-Up 4 Weeks | Mean Difference (95% CI)
| Mean Difference (95% CI)
| |
|---|---|---|---|---|---|---|---|
| VAS (0–100 mm) | CLG + MNNM | 44.19 ± 20.61 | 40.71 ± 21.88 | 42.29 ± 21.64 | 31.96 ± 19.89 |
|
|
| CLG | 42.39 ± 21.13 | 38.08 ± 22.43 | 36.60 ± 22.19 | 29.04 ± 20.39 |
|
| |
| Body pain distribution (pain expansion drawing) | |||||||
| Neck pain | CLG + MNNM | 8.27 ± 9.92 | 5.64 ± 7.06 | 7.89 ± 8.45 | 3.93 ± 3.95 |
|
|
| CLG | 8.92 ± 10.17 | 6.36 ± 7.24 | 6.71 ± 8.66 | 4.43 ± 4.05 |
|
| |
| PPT (kg/cm2) | |||||||
| Homolateral C6 | CLG + MNNM | 2.41 ± 1.12 | 2.46 ± 1.07 | 2.70 ± 1.11 | 3.25 ± 1.22 |
|
|
| CLG | 2.51 ± 1.15 | 2.81 ± 1.10 | 3.06 ± 1.13 | 3.66 ± 1.25 |
|
| |
| Contralateral C6 | CLG + MNNM | 2.47 ± 0.91 | 2.63 ± 1.15 | 2.80 ± 0.97 | 3.40 ± 1.27 |
|
|
| CLG | 2.68 ± 0.94 | 2.86 ± 1.18 | 3.08 ± 1.00 | 3.65 ± 1.30 |
|
| |
| Tibial muscle | CLG + MNNM | 6.91 ± 3.06 | 6.92 ± 3.16 | 7.77 ± 2.96 | 7.95 ± 2.78 |
|
|
| CLG | 7.24 ± 3.14 | 7.88 ± 3.24 | 7.59 ± 3.04 | 7.80 ± 2.85 |
|
| |
| CROM (Degrees) | Group | Baseline | Post-Intervention 5 min | Follow-Up 2 Weeks | Follow-Up 4 Weeks | Mean Difference (95% CI)
| Mean Difference (95% CI)
|
|---|---|---|---|---|---|---|---|
| Flexion/ Extension | CLG + MNNM | 101.51 ± 23.53 | 100.86 ± 23.76 | 100.21 ± 20.35 | 101.71 ± 17.74 |
|
|
| CLG | 105.37 ± 24.12 | 109.14 ± 24.35 | 108.76 ± 20.86 | 107.65 ± 18.18 |
|
| |
| Homolateral Rotation | CLG + MNNM | 55.26 ± 13.08 | 58.23 ± 13.40 | 54.82 ± 11.49 | 55.94 ± 12.07 |
|
|
| CLG | 57.87 ± 13.41 | 60.11 ± 13.74 | 59.53 ± 11.78 | 62.83 ± 12.38 * |
|
| |
| Contralateral Rotation | CLG + MNNM | 56.17 ± 12.05 | 60.18 ± 12.71 | 57.45 ± 11.70 | 55.07 ± 12.38 |
|
|
| CLG | 59.51 ± 12.35 | 61.22 ± 13.03 | 61.30 ± 12.00 | 62.83 ± 12.70 * |
|
| |
| Homlateral side flexion | CLG + MNNM | 33.81 ± 9.42 | 35.65 ± 11.20 | 34.03 ± 9.65 | 35.12 ± 8.77 |
|
|
| CLG | 33.85 ± 9.66 | 36.93 ± 11.48 | 34.35 ± 9.89 | 35.69 ± 8.99 |
|
| |
| Contralateral side flexion | CLG + MNNM | 33.12 ± 10.93 | 34.45 ± 10.86 | 34.60 ± 10.27 | 36.52 ± 9.86 |
|
|
| CLG | 34.61 ± 11.21 | 35.36 ± 11.14 | 35.93 ± 10.53 | 37.00 ± 10.10 |
|
|
| Group | Baseline | Follow-Up 2 Weeks | Follow-Up 4 Weeks | Mean Difference (95% CI)
| |
|---|---|---|---|---|---|
| NDI | CLG + MNNM | 17.66 ± 7.94 | 15.69 ± 7.31 | 13.88 ± 6.98 |
|
| CLG | 16.54 ± 8.14 | 14.16 ± 7.49 | 11.73 ± 7.16 |
| |
| TSK-11 | CLG + MNNM | 27.93 ± 7.78 | 25.21 ± 7.78 | 25.43 ± 7.70 |
|
| CLG | 28.35 ± 7.98 | 26.81 ± 7.98 | 24.60 ± 7.90 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Vera, D.; Fernández-Carnero, J.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; López-de-Uralde-Villanueva, I.; Arribas-Romano, A.; Martínez-Lozano, P.; Pecos-Martín, D. Median Nerve Neural Mobilization Adds No Additional Benefit When Combined with Cervical Lateral Glide in the Treatment of Neck Pain: A Randomized Clinical Trial. J. Clin. Med. 2021, 10, 5178. https://doi.org/10.3390/jcm10215178
Martin-Vera D, Fernández-Carnero J, Rodríguez-Sanz D, Calvo-Lobo C, López-de-Uralde-Villanueva I, Arribas-Romano A, Martínez-Lozano P, Pecos-Martín D. Median Nerve Neural Mobilization Adds No Additional Benefit When Combined with Cervical Lateral Glide in the Treatment of Neck Pain: A Randomized Clinical Trial. Journal of Clinical Medicine. 2021; 10(21):5178. https://doi.org/10.3390/jcm10215178
Chicago/Turabian StyleMartin-Vera, Daniel, Josué Fernández-Carnero, David Rodríguez-Sanz, Cesar Calvo-Lobo, Ibai López-de-Uralde-Villanueva, Alberto Arribas-Romano, Pedro Martínez-Lozano, and Daniel Pecos-Martín. 2021. "Median Nerve Neural Mobilization Adds No Additional Benefit When Combined with Cervical Lateral Glide in the Treatment of Neck Pain: A Randomized Clinical Trial" Journal of Clinical Medicine 10, no. 21: 5178. https://doi.org/10.3390/jcm10215178
APA StyleMartin-Vera, D., Fernández-Carnero, J., Rodríguez-Sanz, D., Calvo-Lobo, C., López-de-Uralde-Villanueva, I., Arribas-Romano, A., Martínez-Lozano, P., & Pecos-Martín, D. (2021). Median Nerve Neural Mobilization Adds No Additional Benefit When Combined with Cervical Lateral Glide in the Treatment of Neck Pain: A Randomized Clinical Trial. Journal of Clinical Medicine, 10(21), 5178. https://doi.org/10.3390/jcm10215178










