Abstract
Immunotherapy with checkpoint inhibitors has prompted a major change not only in cancer treatment but also in medical imaging. In parallel with the implementation of new drugs modulating the immune system, new response criteria have been developed, aiming to overcome clinical drawbacks related to the new, unusual, patterns of response characterizing both solid tumors and lymphoma during the course of immunotherapy. The acknowledgement of pseudo-progression, hyper-progression, immune-dissociated response and so forth, has become mandatory for all imagers dealing with this clinical scenario. A long list of acronyms, i.e., irRC, iRECIST, irRECIST, imRECIST, PECRIT, PERCIMT, imPERCIST, iPERCIST, depicts the enormous effort made by radiology and nuclear medicine physicians in the last decade to optimize imaging parameters for better prediction of clinical benefit in immunotherapy regimens. Quite frequently, a combination of clinical-laboratory data with imaging findings has been tested, proving the ability to stratify patients into various risk groups. The next steps necessarily require a large scale validation of the most robust criteria, as well as the clinical implementation of immune-targeting tracers for immuno-PET or the exploitation of radiomics and artificial intelligence as complementary tools during the course of immunotherapy administration. For the present review article, a summary of PET/CT role for immunotherapy monitoring will be provided. By scrolling into various cancer types and applied response criteria, the reader will obtain necessary information for better understanding the potentials and limitations of the modality in the clinical setting.
1. Introduction
Starting with the first outstanding results of the anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) antibody, Ipilimumab, in melanoma [1] and following use of antibodies against programmed cell death protein 1 (PD-1) and its ligand PD-L1 (nivolumab, pembrolizumab, atezolizumab) in non-small cell lung cancer (NSCLC) [2,3,4,5], immunotherapy with checkpoint inhibitors has gradually changed the management of malignant tumors by improving the long term benefit and survival. Clinicians have become acquainted along the way with new ways of considering clinical benefit, meaning to recognize objective progression not necessarily as an upfront sign of treatment failure. From an imaging point of view, new semantic artifices have been implemented to help handle the variegated patterns of response that accompany treatment with immune checkpoint inhibitors (ICI). It is therefore not surprising that the number of response criteria has consequently increased, both for morphological and metabolic imaging (Table 1). For the present review article, a summary of the role of PET/CT for immunotherapy monitoring will be provided. By scrolling into various cancer types and applied response criteria, the reader will obtain necessary information for better understanding the potentials and limitations of the modality in the clinical setting.

Table 1.
Summary of anatomic and metabolic criteria for immunotherapy response assessment.
2. New Concepts in Tumor Response during Immunotherapy
Born to overcome the limitations of conventional criteria, and driven by the need to avoid unnecessary treatment withdrawal, immunotherapy-derived response criteria have embraced concepts such as pseudo-progression, hyper-progression or dissociated progression to move beyond the immunotherapy era. Although previously described as an unconventional response pattern in gliomas treated with chemoradiotherapy [17], pseudo-progression is now more broadly associated with ICI and corresponds to the appearance of new lesions or the occurrence of tumor enlargement during therapy, followed by disease regression or stabilization at subsequent imaging [18]. The phenomenon is more frequent during anti-CTLA-4 therapy and tends to affect fewer cancer patients treated with anti-PD-1/L1 agents. Nevertheless, the rate of pseudo-progression in general does not exceed 10% [19,20].
Hyper-progression, on the other hand, refers to a very peculiar pattern of response to ICI, and was firstly described in 2016 by Champiat et al. [21]. Its occurrence ranges from 4% to 29%, proving a large variability of cases according to the casuistics [20,22]. Substantially, hyperprogressive disease (HPD) corresponds to a massive increase of tumor burden, over twice the amount compared to (prior to) treatment start. Notwithstanding, controversies exist on the exact way HPD is defined in clinical practice. While Champiat et al. [21] defined HPD as a twofold or greater increase of tumor growth rate (TGR) during immunotherapy [20], other authors used different descriptions. For instance, Kato et al. defined HPD as a time to treatment failure (TTF) < 2 months, a 50% increase in tumor burden compared to pre-immunotherapy imaging obtained within 2 months of the treatment initiation, and > 2-fold increase in progression pace [20,23]. In other cases, like for Saâda-Bouzid et al., HPD could be computed based on tumor growth kinetic ratio (TGKR), where TGK is defined as the difference of the sum of the largest diameters of target lesions per unit of time, which in the case of HPD has to be ≥ 2 when compared to baseline [20,24,25]. More simply, Matos et al. [26] used as parameter for HPD a 40% increase of the sum of the target lesions from baseline to the first evaluation and/or an increase of 20% plus the appearance of new lesions in two different organs [27]. Although comparison only to baseline imaging, without utilization of data before treatment start, has made some authors define as “fast progression” rather than “hyperprogression” the cases reported by later authors [24,25,26], strictly speaking the occurrence of this “non-response”, is in any of the cases, a dramatic failure. In fact, patients with this type of progression during ICI (call it “hyper-” or “fast”) have a worse outcome with a significantly shorter survival rate [20,21,22,23,24,25,26,28].
To add further confusion to the already intricate situation, recently a new pattern of tumor behavior during ICI has been described in advanced lung cancer [29,30]; this consists of a “dissociated response”, i.e., a contemporary shrinkage of some tumor lesions along with the increase of others in various organs [18], which occurs in around 10% of patients [31]. Given the potential benefit still obtainable for patients showing an immune dissociated response (iDR), some authors [30] have suggested iDR as a surrogate marker of favorable outcome and treatment efficiency [31].
Along with the abovementioned new patterns of response, immunotherapy with ICI can determine several immunologically mediated alterations of healthy tissues and organs, also known as immune-related adverse events (irAEs) [18]. The incidence of these events is higher for anti-CTLA-4 antibodies (80%) and during combination therapy, while it reaches in general 27% for anti-PD-1 and 17% for anti-PD-L1 regimens [18,32]. The occurrence of irAEs, based on the severity of the event, might require immediate ICI discontinuation [33,34]. This will not necessarily prevent fatality, which is surprisingly related to colitis in 70% of the cases treated with anti-CTLA-4, followed by pneumonitis (35%), hepatitis (22%) and neurotoxicities (15%) for anti-PD-1/anti-PD-L1 antibodies [33,34]. From an imaging point of view, irAE interpretation can sometimes be as challenging as other unconventional patterns of response described during ICI. Given the potentially fatal events related to their occurrence, it is fundamental to be aware of their appearance and describe them promptly in the report and to the clinician treating the patient (Figure 1) [35]. Notwithstanding, there is also a positive aspect with irAEs, which is their potential predictive role for treatment benefit. Indeed, being an expression of immune system response, although abnormal and undesirable in most cases, irAEs represent a precognitive sign of longer progression-free (PFS) and overall survival (OS) [36]. From first reports to later meta-analyses, irAE development seems to be positively associated with overall response rate (ORR), PFS, and OS in patients treated with immunotherapy, regardless of lesion site, type of ICI and irAE [36,37], although, grade 3 or higher toxicities have resulted prognostically in worse OS [37].
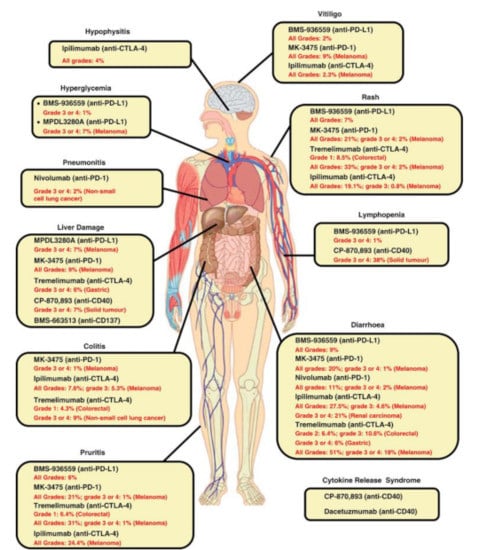
Figure 1.
Spectrum of irAEs associated with immunomodulatory antibodies (available via license: Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported, as published by Liu J, et al. Clin. Transl. Immunol. 2014, 3, e22) [35].
3. Response Assessment in Solid Tumors Treated with Checkpoint Inhibitors
Keeping in mind the abovementioned peculiarities of imagine interpretation during ICI, imagers require adequate instruments to assess immunotherapy benefit, which from a metabolic point of view consists mainly in the use [18F]FDG PET/CT for response assessment (Figure 2 and Figure 3). As previously anticipated, quite an extensive number of response criteria have been proposed for this purpose in recent years (Table 1). During initial studies, consolidated criteria, such as EORTC (European Organization for Research and Treatment of Cancer) [11] and PERCIST (PET Response Criteria in Solid Tumors) [12], have represented the simplest way to assess tumor response, followed later by subsequent adaptations to ICI. This is the case in the instance of PECRIT criteria (PET/CT Criteria for early prediction of Response to Immune checkpoint inhibitor Therapy), introduced by Cho et al. [16], which combine both morphologic (contemplating a change in the sum of diameters of target lesions according to RECIST 1.1) and metabolic response (i.e., a reduction in the SULpeak > 15.5% for the hottest lesion on PET) to assess clinical benefit of ICI. Other authors have introduced PERCIMT (PET Response Evaluation Criteria for IMmunoTherapy), firstly described in melanoma patients [13]. Herein, the appearance of up to four new lesions, depending on their size (Table 1), can be tolerated to obtain clinical benefit (CB) and support treatment continuation [13,38]. More recently, other alternative approaches to PERCIST have been used, including iPERCIST [15] and immunotherapy-modified PERCIST5 (imPERCIST) [14]. For the latter, the definition of a progressive metabolic disease (PMD) becomes less stringent, requiring in fact an increase in the sum of SULpeaks of 30%, with new lesions being eventually included in the sum of SULpeak [14,18]. The principle behind all these new adaptations is substantially the same: to avoid unnecessary and premature treatment withdrawal during immunotherapy. but can we depict one of them as the best response criteria for response assessment during ICI? Actually, not. Some reports have attempted to compare various methods, particularly in melanoma and NSCLC patients [14,38,39,40,41,42,43], proving the superiority of some of the utilized criteria over others (Table 2). Ultimately, all available response criteria, metabolic or morphological, retain the capability to predict response and outcome. What makes one criteria better than the other is most likely to be the interpretation ability of the imager and the correct contextualization of the results into clinical practice. This should not limit, in any case, the continuous research in the field, since robust data must be produced to optimize response criteria for response assessment during ICI, not forgetting the absolute necessity to ascertain the perfect timing for treatment discontinuation for patients to receive long-term clinical benefit.
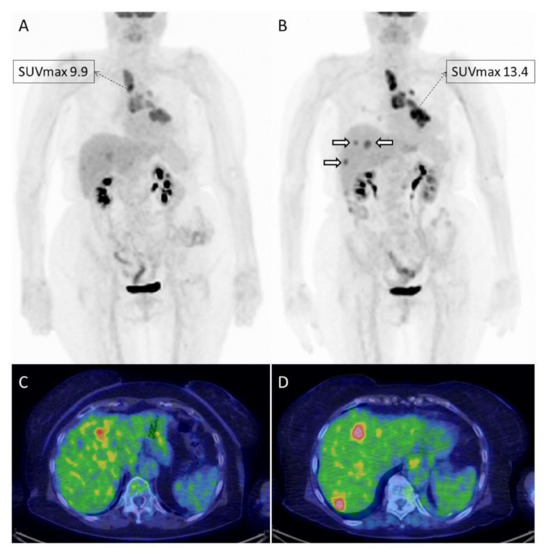
Figure 2.
Example of a 78-year old female with advanced NSCLC treated with nivolumab and imaged with [18F]FDG PET/CT at baseline (A,C) and after 4 cycles of therapy (B,D). The patient resulted in overall stable on morphological imaging performed prior to PET/CT, which on the contrary documented a progressive metabolic disease. In fact, the tumor had an increase in metabolism (SUVmax and MTV), and showed the appearance of new lesions in the liver ((B); white hollowed arrows), only partially detectable on baseline imaging.
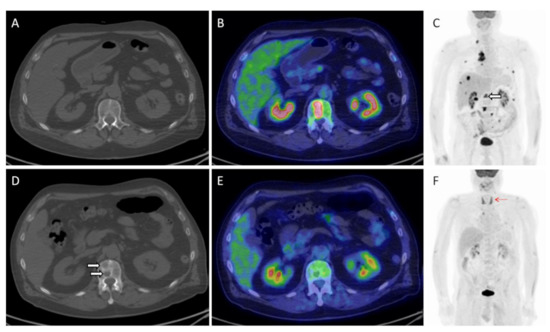
Figure 3.
Herein, the imaging findings of a 66-year old male with metastatic NSCLC investigated before (A–C) and after 3 cycles of pembrolizumab (D–F). An overall response to treatment is easily visible on MIP (maximal intensity projection) images (C,F), including a complete metabolic remission of all bony lesions ((C); white hollowed arrow). On the contrary, morphological imaging proved the appearance of a new bone lesion in the first lumbar vertebra ((A,D); white arrows), which in fact corresponded to a healed metastasis on PET/CT (B,E). Note also the appearance of diffuse thyroid uptake ((F); red arrow), consistent with thyroiditis, one of the irAEs that typically predicts treatment response and good patient’s outcome.

Table 2.
Summary of major studies investigating PET/CT for immunotherapy response assessment and outcome prediction.
4. Combined Parameters for Outcome Prediction
To date, special attention has been given to other parameters obtainable from [18F]FDG PET/CT during ICI. Not just standardized uptake value (SUV), but also metabolic tumor volume (MTV) and total lesion glycolysis (TLG), have been investigated at baseline and during treatment as absolute values or as variations to predict response and outcome [28,47,51,53,54,55,56,58,59,66,67,68,69,70,71,72,73,74,76,77,78,79,82,83,86,87,88]. While SUV appears to be inversely correlated to response to ICI [66,70,80] with higher SUV values being in some reports indicative of treatment benefit, on the other hand higher MTV and TLG values result in negative predictive factors for patient outcome during ICI (Table 2). Recently, a linear positive correlation between SUVmax and tumor mutational burden (TMB), which represents one of the prognostic markers of response to immunotherapy, has been reported (p < 0.001) [95]. These data are in line with previous findings reporting a paradoxically higher SUV in patients responding to ICI, particularly referring to NSCLC [66,70,96]. This evidence also reflects other observations showing a positive correlation between SUV and checkpoints (i.e., PD-L1 or PD-1) and the immune infiltrate [96,97,98,99,100] in lung and other cancer types.
Of special interest also is the risk stratification of patients based on volumetric parameters already obtained at baseline, with patients having a higher MTV and TLG being at higher risk of poor outcome or HPD compared to others [53,55,71,73,74,76,80]. In this context, to further improve the predictive role, a combination of metabolic tumor burden (MTV and TLG) with other clinical parameters has been performed. In particular, circulating inflammatory markers, such as neutrophyl-to-lymphocyte ratio (NLR) and its derived value (dNLR) have proved to better stratify patients undergoing immunotherapy with ICI into risk groups (i.e., higher values predicting poor outcome), both at baseline and after treatment start [71,72,76,80]. Similarly, the combination of volumetric parameters on PET with circulating tumor cells (CTC) count and soluble PD-L1 [72,75,83], or lactate dehydrogenase (LDH) [88] has been reported to be as useful for risk stratification. Thanks to the capability of [18F]FDG PET/CT to depict underlying immunological status, expressed as bone marrow or lymphatic organ activation (i.e., bone marrow-to-liver ratio, spleen-to-liver ratio) or by the development of irAEs, it is also possible to combine metabolic and immunological parameters to improve response prediction and outcome [48,49,50,51,52,53,56,57,81].
The downside of the previously mentioned findings, despite being fascinating and promising, is that most of the original data derive from retrospective analyses or from limited, single centered, prospective cohorts (Table 2). Consequently, their clinical relevance remains circumscribed to theory, until large prospective multicentric imaging trials are properly conducted.
5. Next Generation Imaging for Immunotherapy in Cancer
Radiomics and artificial intelligence (AI) have become a constant mantra in applied sciences, and this includes, necessarily, medical imaging. Automated machine or deep learning algorithms also represent the next frontier of imaging for immunotherapy in cancer, since they might be able to extract precious information, invisible to the naked eye or to conventional measurements. We have known for some years that image heterogeneity is a marker of underlying histological and genetic complexity; but which features could be better associated with specific tumor aspects still requires thorough investigation. What emerges from initial reports published so far on radiomics and AI in the context of immunotherapy setting is that no unique parameter or feature can be defined as superior (Table 2). While features like “skewness” and “kurtosis”, well known from other types of treatment, might represent a marker of treatment failure during ICI in lung cancer [90], for other authors either Small Run Emphasis (SRE), multiparametric radiomics signature (mpRS), cytolytic activity score (CytAct), deeply learned score (DLS), or long zone emphasis (LZE) [89,91,92,93,94] can be as effective. What is missing in this clinical scenario is a solid ground truth, which can only be obtainable from preliminary reports validating imaging parameters with targets specifically relevant for immunotherapy, as in the case of PD-L1 expression. Unfortunately, evidence in this regard is extremely limited, particularly when concerning metabolic imaging [94,101].
On the other hand, PET imaging during immunotherapy implies another frontier of development, with radiolabeled immune-based tracers, also known as Immuno-PET. This includes the targeting with radiolabeled antibodies, antibody fragments, or small proteins of checkpoints (i.e., CTLA-4, PD-1, PD-L1) [102,103,104,105,106], tumor infiltrating lymphocytes (ex. CD3, CD4, CD8) [107,108,109,110], cytokines (ex. IL-2) [111], enzymes (ex. Granzyme B, dCK deoxycytidine kinase, dGK deoxyguanosine kinase) [112,113,114,115], and potentially any other element involved in immune system response [116]. The possibility of detecting non-invasively checkpoint expression prior to the administration of ICI, as well as the identification on the entire tumor mass of the amount and pattern of distribution of immune cells, can have priceless clinical implications [106,110]. The same compound used for treatment, ex. ipilimumab, nivolumab, pembrolizumab, atezolizumab, and so forth, [105,106,117,118,119], would be labeled and imaged with PET to detect the actual targeting of tumor sites (Figure 4). Similarly, it would be able to detect the status of lymphocyte activation, exhaustion or cytotoxic capacity by simply injecting radiolabeled molecules targeting enzymes like Granzyme B, a downstream effector of tumoral cytotoxic T cells [113,115,120], or by checking the deoxyribonucleotide kinase activity [112,114]. The majority of data belong mostly to the preclinical setting, with ongoing research aiming to translate the results from bench to clinical practice [106,119,121]. The hope is that in the near future the data will be mature enough to implement immuno-PET into the diagnostic pathway for cancer patient candidates to undergo immunotherapy with checkpoint inhibitors.
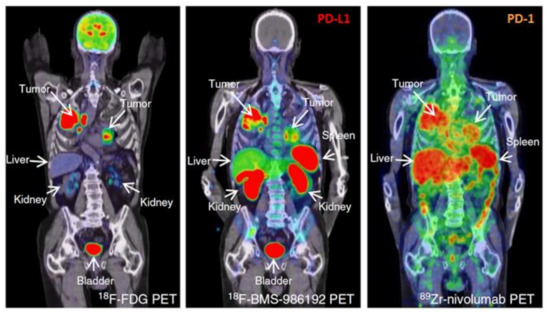
Figure 4.
Comparison of [18F]FDG PET/CT with anti-PD-L1 (18F-BMS-986192) and anti-PD-1 (89Zr-labeled Nivolumab) immuno-PET images in the same patient with NSCLC. Along the high glucose metabolism of the tumor in both lungs and mediastinal lymph nodes, a heterogeneous tracer uptake for 18F-BMS-986192 PET/CT and 89Zr-labeled Nivolumab PET/CT within and between tumors is demonstrated. Modified from Niemeijer AN et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun 2018;9:4664. [106]; Licensed under a Creative Commons license: http://creativecommons.org/licenses/by/4.0/) Last access date: 2 November 2021.
6. Endnote Remarks
The introduction of immunotherapy in cancer treatment has represented a turning point in medical oncology, but also a new challenge for diagnostic imaging. The multitude of adapted response criteria and the numerous research studies published within a relatively short period of time demonstrate the capability of our community to face challenges and find solutions. From a nuclear medicine point of view, practical directives/guidelines are in the pipeline, along with previously published position papers or comments [122,123] on how to deal with the assessment of tumor response in the era of checkpoint inhibitors. The battlefield should, anyhow, move to clinical validation and recognition by the medical oncology community, which remains skeptical and firmly anchored to morphological criteria. Superior data are required in this regard, since non-inferiority would not be sufficient, given the larger availability of radiological devices (i.e., CT) and the reduced costs of the procedures compared to PET imaging. The astonishing technological leap of the last decade might be the game changer (immune-PET, Radiomics, AI), along with the improved awareness among nuclear medicine physicians of the clinical trial requirements in case of imaging studies, which should represent the backbone of any novel clinical indication or new tracer development.
Funding
This paper received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on motivated request to the corresponding author.
Acknowledgments
Fondazione AIRC is acknowledged for previous support in research by means of the grant nr. 18923 provided to Egesta Lopci.
Conflicts of Interest
E.L. reports receiving grants from AIRC (Associazione Italiana per la Ricerca sul Cancro) and from the Italian Ministry of Health, and faculty remuneration from ESMIT (European School of Multimodality Imaging and Therapy) and MI&T congressi. No other potential conflicts of interest relevant to this article exist.
Abbreviations
| PERCIST | PET Response Criteria in Solid Tumors |
| PECRIT | PET/CT Criteria for Early Prediction of Response to Immune Checkpoint Inhibitor Therapy (combined RECIST 1.1 and PERCIST) |
| PERCIMT | PET Response Evaluation Criteria for Immunotherapy |
| CMR | complete metabolic response |
| PMR | partial metabolic response |
| SMD | stable metabolic disease |
| PMD | progressive metabolic disease |
| SULpeak | lean body mass corrected SUV peak |
| UPMD | unconfirmed progressive metabolic disease |
| CPMD | confirmed progressive metabolic disease. |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| irRC | immune-related Response Criteria |
| CR | complete response |
| PR | partial response |
| SD | stable disease |
| PD | progressive disease |
| iUPD | initially unconfirmed progressive disease |
| iCPD | confirmed progressive disease |
| CB | clinical benefit |
| EORTC | European Organization for Research and Treatment of Cancer (EORTC5, includes the sum of SUVmax) |
| MTV | metabolic tumor volume |
| wbMTV | whole body MTV |
| TMTV | total metabolic tumor volume |
| WB-MATV | whole body metabolically active tumor volume |
| TLG | total lesions glycolysis |
| iDR | immune dissociated-response |
| ETD | early treatment discontinuation |
| BLR | bone marrow-to-liver SUVmax ratio |
| SLR | spleen-to-liver SUVmax ratio |
| dNLR | derived neutrophils-to-lymphocytes ratio |
| LDH | lactate dehydrogenase |
| FD | fractal dimension |
| ICI | immune checkpoint inhibitors |
| irAEs | immune-related adverse events |
| IMPI | immune-metabolic-prognostic index |
| ATB | antibiotic |
| ADC | apparent diffusion coefficient |
| SRE | Small Run Emphasis |
| mpRS | multiparametric radiomics signature |
| DLS | deeply learned score |
| DCB | durable clinical benefit |
| PFS | progression-free survival |
| OS | overall survival |
| DCR | disease control rate |
| ORR | overall response rate |
| Muc-M | mucosal melanoma |
| Cut-M | cutaneous melanoma |
| sPD-L1 | soluble PD-L1. |
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, W.R.; Sosman, A.J.; Haanen, B.J.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, C.J.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [Green Version]
- Nishino, M.; Gargano, M.; Suda, M.; Ramaiya, N.H.; Hodi, F.S. Optimizing immune-related tumor response assessment: Does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J. Immunother. Cancer 2014, 2, 17. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; Ballinger, M.; Lyons, B.; Soria, J.C.; Nishino, M.; Tabernero, J.; Powles, T.; Smith, D.; Hoos, A.; McKenna, C.; et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining guidelines to assess the clinical benefit of cancer immunotherapy. J. Clin. Oncol. 2018, 36, 850–858. [Google Scholar] [CrossRef]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, H.; Sachpekidis, C.; Winkler, J.; Kopp-Schneider, A.; Haberkorn, U.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 376–383. [Google Scholar] [CrossRef]
- Ito, K.; Teng, R.; Schöder, H.; Humm, J.L.; Ni, A.; Michaud, L.; Nakajima, R.; Yamashita, R.; Wolchok, J.D.; Weber, W.A. 18F-FDG PET/CT for monitoring of ipilimumab therapy in patients with metastatic melanoma. J. Nucl. Med. 2019, 60, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Goldfarb, L.; Duchemann, B.; Chouahnia, K.; Zelek, L.; Soussan, M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: Introduction of iPERCIST. EJNMMI Res. 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lipson, E.J.; Im, H.J.; Rowe, S.P.; Gonzalez, E.M.; Blackford, A.; Chirindel, A.; Pardoll, D.M.; Topalian, S.L.; Wahl, R.L. Prediction of response to immune checkpoint inhibitor therapy using early time-point (18)F-FDG PET/CT imaging in patients with advanced melanoma. J. Nucl. Med. 2017, 58, 1421–1428. [Google Scholar] [CrossRef] [Green Version]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Castello, A.; Lopci, E. Update on tumor metabolism and patterns of response to immunotherapy. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Borcoman, E.; Nandikolla, A.; Long, G.; Goel, S.; Le Tourneau, C. Patterns of Response and Progression to immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 169–178. [Google Scholar] [CrossRef]
- Onesti, C.E.; Freres, P.; Jerusalem, G. Atypical patterns of response to immune checkpoint inhibitors: Interpreting pseudoprogression and hyperprogression in decision making for patients’ treatment. J. Thorac. Dis. 2019, 11, 35–38. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; Le Moulec, S.; et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single Agent Chemotherapy. JAMA Oncol. 2018, 4, 1543–1552. [Google Scholar] [CrossRef]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin. Cancer Res. 2017, 23, 4242–4250. [Google Scholar] [CrossRef] [Green Version]
- Saâda-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D.; et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1605–1611. [Google Scholar] [CrossRef]
- Lo Russo, G.; Moro, M.; Sommariva, M.; Cancila, V.; Boeri, M.; Centonze, G.; Ferro, S.; Ganzinelli, M.; Gasparini, P.; Huber, V.; et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin. Cancer Res. 2019, 25, 989–999. [Google Scholar] [CrossRef] [Green Version]
- Matos, I.; Martin-Liberal, J.; García-Ruiz, A.; Hierro, C.; Ochoa de Olza, M.; Viaplana, C.; Azaro, A.; Vieito, M.; Braña, I.; Mur, G.; et al. Capturing hyperprogressive disease with immune-checkpoint inhibitors using RECIST 1.1 criteria. Clin. Cancer Res. 2020, 26, 1846–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caramella, C.; Ferrara, R.; Besse, B. Clarification of Definitions of Hyperprogressive Disease During Immunotherapy-Reply. JAMA Oncol. 2021, 7, 137. [Google Scholar] [CrossRef]
- Castello, A.; Rossi, S.; Toschi, L.; Mazziotti, E.; Lopci, E. Hyperprogressive Disease in Patients With Non-Small Cell Lung Cancer Treated With Checkpoint Inhibitors: The Role of (18)FFDG PET/CT. J. Nucl. Med. 2020, 61, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Tazdait, M.; Mezquita, L.; Lahmar, J.; Ferrara, R.; Bidault, F.; Ammari, S.; Balleyguier, C.; Planchard, D.; Gazzah, A.; Soria, J.C.; et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur. J. Cancer 2018, 88, 38–47. [Google Scholar] [CrossRef]
- Humbert, O.; Cadour, N.; Paquet, M.; Schiappa, R.; Poudenx, M.; Chardin, D.; Borchiellini, D.; Benisvy, D.; Ouvrier, M.J.; Zwarthoed, C.; et al. 18FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: Frequency and clinical significance of atypical evolutive patterns. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1158–1167. [Google Scholar] [CrossRef]
- Humbert, O.; Chardin, D. Dissociated Response in Metastatic Cancer: An Atypical Pattern Brought into the Spotlight with Immunotherapy. Front. Oncol. 2020, 10, 566297. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Blake, S.J.; Smyth, M.J.; Teng, M.W.I. Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin. Transl. Immunol. 2014, 3, e22. [Google Scholar] [CrossRef] [PubMed]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non–Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef]
- Hussaini, S.; Chehade, R.; Boldt, R.G.; Raphael, J.; Blanchette, P.; Maleki Vareki, S.; Fernandes, R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—A systematic review and meta-analysis. Cancer Treat. Rev. 2020, 92, 102134. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Anwar, H.; Winkler, J.; Kopp-Schneider, A.; Larribere, L.; Haberkorn, U.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. The role of interim 18F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1289–1296. [Google Scholar] [CrossRef]
- Amrane, K.; Le Goupil, D.; Quere, G.; Delcroix, O.; Gouva, S.; Schick, U.; Salaun, P.Y.; Abgral, R.; Alavi, Z.; Keromnes, N.; et al. Prediction of response to immune checkpoint inhibitor therapy using 18F-FDG PET/CT in patients with melanoma. Medicine 2019, 98, e16417. [Google Scholar] [CrossRef]
- Beer, L.; Hochmair, M.; Haug, A.R.; Schwabel, B.; Kifjak, D.; Wadsak, W.; Fuereder, T.; Fabikan, H.; Fazekas, A.; Schwab, S.; et al. Comparison of RECIST, iRECIST, and PERCIST for the evaluation of response to PD-1/PD-L1 blockade therapy in patients with non-small cell lung cancer. Clin. Nucl. Med. 2019, 44, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Bauckneht, M.; Genova, C.; Rijavec, E.; Biello, F.; Mennella, S.; Dal Bello, M.G.; Cittadini, G.; Bruzzi, P.; Piva, R.; et al. Comparison Between 18F-FDG PET-Based and CT-Based Criteria in Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J. Nucl. Med. 2020, 61, 990–998. [Google Scholar] [CrossRef]
- Ayati, N.; Lee, S.T.; Zakavi, S.R.; Cheng, M.; Lau, W.F.E.; Parakh, S.; Pathmaraj, K.; Scott, A.M. Response Evaluation and Survival Prediction After PD-1 Immunotherapy in Patients with Non-Small Cell Lung Cancer: Comparison of Assessment Methods. J. Nucl. Med. 2021, 62, 926–933. [Google Scholar] [CrossRef]
- Castello, A.; Rossi, S.; Toschi, L.; Lopci, E. Comparison of Metabolic and Morphological Response Criteria for Early Prediction of Response and Survival in NSCLC Patients Treated With Anti-PD-1/PD-L1. Front. Oncol. 2020, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.Y.; Menzies, A.M.; Saunders, C.A.; Liniker, E.; Ramanujam, S.; Guminski, A.; Kefford, R.F.; Long, G.V.; Carlino, M.S. Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment. Cell Melanoma Res. 2016, 29, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Seith, F.; Forschner, A.; Schmidt, H.; Pfannenberg, C.; Gückel, B.; Nikolaou, K.; la Fougère, C.; Garbe, C.; Schwenzer, N. 18F-FDG-PET detects complete response to PD1-therapy in melanoma patients two weeks after therapy start. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Emmett, L.; Lo, S.; Liu, V.; Kapoor, R.; Carlino, M.S.; Guminski, A.D.; Long, G.V.; Menzies, A.M. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann. Oncol. 2018, 29, 2115–2120. [Google Scholar] [CrossRef]
- Ito, K.; Schöder, H.; Teng, R.; Humm, J.L.; Ni, A.; Wolchok, J.D.; Weber, W.A. Prognostic value of baseline metabolic tumor volume measured on 18F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Werner, T.J.; Gholami, S.; Margalit, O.; Baruch, E.; Markel, G.; Eshet, Y.; Houshmand, S.; Shacham-Shmueli, E.; Mitchell, T.C.; et al. Physiologic colonic fluorine-18-fluorodeoxyglucose uptake may predict response to immunotherapy in patients with metastatic melanoma. Melanoma Res. 2019, 29, 318–321. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Larribère, L.; Kopp-Schneider, A.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Can benign lymphoid tissue changes in 18F-FDG PET/CT predict response to immunotherapy in metastatic melanoma? Cancer Immunol. Immunother. 2019, 68, 297–303. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Kopp-Schneider, A.; Hakim-Meibodi, L.; Dimitrakopoulou-Strauss, A.; Hassel, J.C. 18F-FDG PET/CT longitudinal studies in patients with advanced metastatic melanoma for response evaluation of combination treatment with vemurafenib and ipilimumab. Melanoma Res. 2019, 29, 178–186. [Google Scholar] [CrossRef]
- Seban, R.D.; Nemer, J.S.; Marabelle, A.; Yeh, R.; Deutsch, E.; Ammari, S.; Moya-Plana, A.; Mokrane, F.Z.; Gartrell, R.D.; Finkel, G.; et al. Prognostic and theranostic 18F-FDG PET biomarkers for anti-PD1 immunotherapy in metastatic melanoma: Association with outcome and transcriptomics. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2298–2310. [Google Scholar] [CrossRef]
- Nobashi, T.; Baratto, L.; Reddy, S.A.; Srinivas, S.; Toriihara, A.; Hatami, N.; Yohannan, T.K.; Mittra, E. Predicting Response to Immunotherapy by Evaluating Tumors, Lymphoid Cell-Rich Organs, and Immune-Related Adverse Events Using FDG-PET/CT. Clin. Nucl. Med. 2019, 44, e272–e279. [Google Scholar] [CrossRef] [PubMed]
- Seban, R.D.; Moya-Plana, A.; Antonios, L.; Yeh, R.; Marabelle, A.; Deutsch, E.; Schwartz, L.H.; Gómez, R.G.H.; Saenger, Y.; Robert, C.; et al. Prognostic 18F-FDG PET biomarkers in metastatic mucosal and cutaneous melanoma treated with immune checkpoint inhibitors targeting PD-1 and CTLA-4. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Iravani, A.; Osman, M.M.; Weppler, A.M.; Wallace, R.; Galligan, A.; Lasocki, A.; Hunter, M.O.; Akhurst, T.; Hofman, M.S.; Lau, P.K.H.; et al. FDG PET/CT for tumoral and systemic immune response monitoring of advanced melanoma during first-line combination ipilimumab and nivolumab treatment. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, R.; Zaba, L.C.; Rosenberg, J.; Reddy, S.A.; Nobashi, T.W.; Davidzon, G.; Aparici, C.M.; Nguyen, J.; Moradi, F.; Iagaru, A.; et al. Prognostic value of volumetric PET parameters at early response evaluation in melanoma patients treated with immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2787–2795. [Google Scholar] [CrossRef]
- Wong, A.; Callahan, J.; Keyaerts, M.; Neyns, B.; Mangana, J.; Aberle, S.; Herschtal, A.; Fullerton, S.; Milne, D.; Iravani, A.; et al. 18 F-FDG PET/CT based spleen to liver ratio associates with clinical outcome to ipilimumab in patients with metastatic melanoma. Cancer Imaging 2020, 20, 36. [Google Scholar] [CrossRef]
- Seith, F.; Forschne, R.A.; Weide, B.; Gückel, B.; Schwartz, M.; Schwenck, J.; Othman, A.E.; Fenchel, M.; Garbe, C.; Nikolaou, K.; et al. Is there a link between very early changes of primary and secondary lymphoid organs in 18F-FDG-PET/MRI and treatment response to checkpoint inhibitor therapy? J. Immunother. Cancer 2020, 8, e000656. [Google Scholar] [CrossRef]
- Annovazzi, A.; Vari, S.; Giannarelli, D.; Pasqualoni, R.; Sciuto, R.; Carpano, S.; Cognetti, F.; Ferraresi, V. Comparison of 18F-FDG PET/CT Criteria for the Prediction of Therapy Response and Clinical Outcome in Patients With Metastatic Melanoma Treated With Ipilimumab and PD-1 Inhibitors. Clin. Nucl. Med. 2020, 45, 187–194. [Google Scholar] [CrossRef]
- Nakamoto, R.; Zaba, L.C.; Rosenberg, J.; Arani Reddy, S.; Nobashi, T.W.; Ferri, V.; Davidzon, G.; Mari Aparici, C.; Nguyen, J.; Moradi, F.; et al. Imaging Characteristics and Diagnostic Performance of 2-deoxy-2-[18F] fluoro-D-Glucose PET/CT for Melanoma Patients Who Demonstrate Hyperprogressive Disease When Treated with Immunotherapy. Mol. Imaging Biol. 2021, 23, 139–147. [Google Scholar] [CrossRef]
- Prigent, K.; Lasnon, C.; Ezine, E.; Janson, M.; Coudrais, N.; Joly, E.; Césaire, L.; Stefan, A.; Depontville, M.; Aide, N. Assessing immune organs on 18F-FDG PET/CT imaging for therapy monitoring of immune checkpoint inhibitors: Inter-observer variability, prognostic value and evolution during the treatment course of melanoma patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2573–2585. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Kopp-Schneider, A.; Hassel, J.; Dimitrakopoulou-Strauss, A. Assessment of early metabolic progression in melanoma patients under immunotherapy: An 18F-FDG PET/CT study. EJNMMI Res. 2021, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Hassel, J.C.; Kopp-Schneider, A.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Quantitative Dynamic 18F-FDG PET/CT in Survival Prediction of Metastatic Melanoma under PD-1 Inhibitors. Cancers 2021, 13, 1019. [Google Scholar] [CrossRef]
- Schank, T.E.; Forschner, A.; Sachse, M.M.; Dimitrakopoulou-Strauss, A.; Sachpekidis, C.; Stenzinger, A.; Volckma, R.A.-L.; Enk, A.; Hassel, J.C. Complete Metabolic Response in FDG-PET-CT Scan before Discontinuation of Immune Checkpoint Inhibitors Correlates with Long Progression-Free Survival. Cancers 2021, 13, 2616. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, R.; Zaba, L.C.; Liang, T.; Reddy, S.A.; Davidzon, G.; Aparici, C.M.; Nguyen, J.; Moradi, F.; Iagaru, A.; Franc, B.L. Prognostic Value of Bone Marrow Metabolism on Pretreatment 18F-FDG PET/CT in Patients with Metastatic Melanoma Treated with Anti-PD-1 Therapy. J. Nucl. Med. 2021, 62, 1380–1383. [Google Scholar] [CrossRef]
- Kitajima, K.; Watabe, T.; Nakajo, M.; Ishibashi, M.; Daisaki, H.; Soeda, F.; Tanemura, A.; Kanekura, T.; Yamazaki, N.; Ito, K. Tumor response evaluation in patients with malignant melanoma undergoing immune checkpoint inhibitor therapy and prognosis prediction using 18F-FDG PET/CT: Multicenter study for comparison of EORTC, PERCIST, and imPERCIST. Jpn. J. Radiol 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Grizzi, F.; Castello, A.; Lopci, E. Is it time to change our vision of tumor metabolism prior to immunotherapy? Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Higuchi, T.; Naruse, I.; Arisaka, Y.; Tokue, A.; Altan, B.; Suda, S.; Mogi, A.; Shimizu, K.; Sunaga, N.; et al. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Jreige, M.; Letovanec, I.; Chaba, K.; Renaud, S.; Rusakiewicz, S.; Cristina, V.; Peters, S.; Krueger, T.; de Leval, L.; Kandalaft, L.E.; et al. 18F-FDG PET metabolic-to-morphological volume ratio predicts PDL1 tumour expression and response to PD-1 blockade in non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1859–1868. [Google Scholar] [CrossRef]
- Evangelista, L.; Cuppari, L.; Menis, J.; Bonanno, L.; Reccia, P.; Frega, S.; Pasello, G. 18F-FDG PET/CT in non-small-cell lung cancer patients: A potential predictive biomarker of response to immunotherapy. Nucl. Med. Commun. 2019, 40, 802–807. [Google Scholar] [CrossRef]
- Takada, K.; Toyokawa, G.; Yoneshima, Y.; Tanaka, K.; Okamoto, I.; Shimokawa, M.; Wakasu, S.; Haro, A.; Osoegawa, A.; Tagawa, T.; et al. (18)F-FDG uptake in PET/CT is a potential predictive biomarker of response to anti-PD-1 antibody therapy in non-small cell lung cancer. Sci. Rep. 2019, 9, 13362. [Google Scholar] [CrossRef] [Green Version]
- Seban, R.D.; Mezquita, L.; Berenbaum, A.; Dercle, L.; Botticella, A.; Le Pechoux, C.; Caramella, C.; Deutsch, E.; Grimaldi, S.; Adam, J.; et al. Baseline metabolic tumor burden on FDG PET/CT scans predicts outcome in advanced NSCLC patients treated with immune checkpoint inhibitors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Carbone, F.G.; Rossi, S.; Monterisi, S.; Federico, D.; Toschi, L.; Lopci, E. Circulating Tumor Cells and Metabolic Parameters in NSCLC Patients Treated with Checkpoint Inhibitors. Cancers 2020, 12, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seban, R.D.; Assié, J.B.; Giroux-Leprieur, E.; Massiani, M.A.; Soussan, M.; Bonardel, G.; Chouaid, C.; Playe, M.; Goldfarb, L.; Duchemann, B.; et al. Association of the Metabolic Score Using Baseline FDG-PET/CT and dNLR with Immunotherapy Outcomes in Advanced NSCLC Patients Treated with First-Line Pembrolizumab. Cancers 2020, 12, 2234. [Google Scholar] [CrossRef] [PubMed]
- Chardin, D.; Paquet, M.; Schiappa, R.; Darcourt, J.; Bailleux, C.; Poudenx, M.; Sciazza, A.; Ilie, M.; Benzaquen, J.; Martin, N.; et al. Baseline metabolic tumor volume as a strong predictive and prognostic biomarker in patients with non-small cell lung cancer treated with PD1 inhibitors: A prospective study. J. Immunother. Cancer 2020, 8, e000645. [Google Scholar] [CrossRef]
- Castello, A.; Rossi, S.; Toschi, L.; Mansi, L.; Lopci, E. Soluble PD-L1 in NSCLC Patients Treated with Checkpoint Inhibitors and Its Correlation with Metabolic Parameters. Cancers 2020, 12, 1373. [Google Scholar] [CrossRef]
- Castello, A.; Toschi, L.; Rossi, S.; Mazziotti, E.; Lopci, E. The immune-metabolic-prognostic index and clinical outcomes in patients with non-small cell lung carcinoma under checkpoint inhibitors. J. Cancer Res. Clin. Oncol. 2020, 146, 1235–1243. [Google Scholar] [CrossRef]
- Tao, X.; Li, N.; Wu, N.; He, J.; Ying, J.; Gao, S.; Wang, S.; Wang, J.; Wang, Z.; Ling, Y.; et al. The efficiency of 18F-FDG PET-CT for predicting the major pathologic response to the neoadjuvant PD-1 blockade in resectable non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1209–1219. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Kaira, K.; Yamaguchi, O.; Mouri, A.; Shiono, A.; Miura, Y.; Murayama, Y.; Kobayashi, K.; Kagamu, H.; Kuji, I. Potential of FDG-PET as Prognostic Significance after anti-PD-1 Antibody against Patients with Previously Treated Non-Small Cell Lung Cancer. J. Clin. Med. 2020, 9, 725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umeda, Y.; Morikawa, M.; Anzai, M.; Ameshima, S.; Kadowaki, M.; Waseda, Y.; Shigemi, H.; Tsujikawa, T.; Kiyono, Y.; Okazawa, H.; et al. Predictive value of integrated 18F-FDG PET/MRI in the early response to nivolumab in patients with previously treated non-small cell lung cancer. J. Immunother. Cancer 2020, 8, e000349. [Google Scholar] [CrossRef]
- Seban, R.D.; Assie, J.B.; Giroux-Leprieur, E.; Massiani, M.A.; Soussan, M.; Bonardel, G.; Chouaid, C.; Playe, M.; Goldfarb, L.; Duchemann, B.; et al. FDG-PET biomarkers associated with long-term benefit from first-line immunotherapy in patients with advanced non-small cell lung cancer. Ann. Nucl. Med. 2020, 34, 968–974. [Google Scholar] [CrossRef]
- Cvetkovic, L.; Régis, C.; Richard, C.; Derosa, L.; Leblond, A.; Malo, J.; Messaoudene, M.; Desilets, A.; Belkaid, W.; Elkrief, A.; et al. Physiologic colonic uptake of 18F-FDG on PET/CT is associated with clinical response and gut microbiome composition in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Kitajima, K.; Toriihara, A.; Ishibashi, M.; Nakahara, T.; Daisaki, H.; Ohe, Y.; Honda, R.; Kijima, T.; Hasegawa, S.; et al. 18F-FDG PET/CT for monitoring anti-PD-1 therapy in patients with non-small cell lung cancer using SUV harmonization of results obtained with various types of PET/CT scanners used at different centers. Ann. Nucl. Med. 2021, 35, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Bauckneht, M.; Genova, C.; Rossi, G.; Rijavec, E.; Dal Bello, M.G.; Ferrarazzo, G.; Tagliamento, M.; Donegani, M.I.; Biello, F.; Chiola, S.; et al. The Role of the Immune Metabolic Prognostic Index in Patients with Non-Small Cell Lung Cancer (NSCLC) in Radiological Progression during Treatment with Nivolumab. Cancers 2021, 13, 3117. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Metzenmacher, M.; Kessler, L.; Umutlu, L.; Aigner, C.; Karl, K.O.; Grünwald, V.; Eberhardt, W.; Fendler, W.P.; Herrmann, K.; et al. Complete metabolic response in patients with advanced non-small cell lung cancer with prolonged response to immune checkpoint inhibitor therapy. J. Immunother. Cancer 2021, 9, e002262. [Google Scholar] [CrossRef]
- Castello, A.; Rossi, S.; Toschi, L.; Lopci, E. Impact of Antibiotic Therapy and Metabolic Parameters in Non-Small Cell Lung Cancer Patients Receiving Checkpoint Inhibitors. J. Clin. Med. 2021, 10, 1251. [Google Scholar] [CrossRef]
- Vekens, K.; Everaert, H.; Neyns, B.; Ilsen, B.; Decoster, L. The Value of 18F-FDG PET/CT in Predicting the Response to PD-1 Blocking Immunotherapy in Advanced NSCLC Patients with High-Level PD-L1 Expression. Clin. Lung Cancer 2021, 22, 432–440. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Kim, T.-S.; Kim, S.-K.; Han, J.-Y. Response evaluation after immunotherapy in NSCLC: Early response assessment using FDG PET/CT. Medicine 2020, 99, e23815. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Wang, L.; Yu, J.; Meng, X. Prognostic Significance of SUVmax Combined With Lactate Dehydrogenase in Advanced Lung Cancer Patients Treated With Immune Checkpoint Inhibitor Plus Chemotherapy: A Retrospective Study. Front. Oncol. 2021, 11, 652312. [Google Scholar] [CrossRef]
- Valentinuzzi, D.; Vrankar, M.; Boc, N.; Ahac, V.; Zupancic, Z.; Unk, M.; Skalic, K.; Zagar, I.; Studen, A.; Simoncic, U.; et al. [18F]FDG PET immunotherapy radiomics signature (iRADIOMICS) predicts response of non-small-cell lung cancer patients treated with pembrolizumab. Radiol. Oncol. 2020, 54, 285–294. [Google Scholar] [CrossRef]
- Polverari, G.; Ceci, F.; Bertaglia, V.; Reale, M.L.; Rampado, O.; Gallio, E.; Passera, R.; Liberini, V.; Scapoli, P.; Arena, V.; et al. 18 F-FDG PET Parameters and Radiomics Features Analysis in Advanced Nsclc Treated with Immunotherapy as Predictors of Therapy Response and Survival. Cancers 2020, 12, 1163. [Google Scholar] [CrossRef]
- Mu, W.; Tunali, I.; Gray, J.E.; Qi, J.; Schabath, M.B.; Gillies, R.J. Radiomics of 18F-FDG PET/CT images predicts clinical benefit of advanced NSCLC patients to checkpoint blockade immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Na, K.J.; Choi, H.; Ock, C.Y.; Ha, S.; Kim, M.; Park, S.; Keam, B.; Kim, T.M.; Paeng, J.C.; et al. Tumor immune profiles noninvasively estimated by FDG PET with deep learning correlate with immunotherapy response in lung adenocarcinoma. Theranostics 2020, 10, 10838–10848. [Google Scholar] [CrossRef] [PubMed]
- Flaus, A.; Habouzit, V.; De Leiris, N.; Vuillez, J.P.; Leccia, M.T.; Perrot, J.L.; Prevot, N.; Cachin, F. FDG PET biomarkers for prediction of survival in metastatic melanoma prior to anti-PD1 immunotherapy. Sci. Rep. 2021, 11, 18795. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Jiang, L.; Shi, Y.; Tunali, I.; Gray, J.E.; Katsoulakis, E.; Tian, J.; Gillies, R.J.; Schabath, M.B. Non-invasive measurement of PD-L1 status and prediction of immunotherapy response using deep learning of PET/CT images. J. Immunother. Cancer 2021, 9, e002118. [Google Scholar] [CrossRef]
- Haghighat Jahromi, A.; Barkauskas, D.A.; Zabel, M.; Goodman, A.M.; Frampton, G.; Nikanjam, M.; Hoh, C.K.; Kurzrock, R. Relationship between tumor mutational burden and maximum standardized uptake value in 2-[18F]FDG PET (positron emission tomography) scan in cancer patients. EJNMMI Res. 2020, 10, 150. [Google Scholar] [CrossRef]
- Lopci, E.; Toschi, L.; Grizzi, F.; Rahal, D.; Olivari, L.; Castino, G.F.; Marchetti, S.; Cortese, N.; Qehajaj, D.; Pistillo, D.; et al. Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates for upfront surgery. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Toyokawa, G.; Okamoto, T.; Baba, S.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Akamine, T.; Takamori, S.; Katsura, M.; et al. Metabolic characteristics of programmed cell death-ligand 1-expressing lung cancer on 18 F-fluorodeoxyglucose positron emission tomography/computed tomography. Cancer Med. 2017, 6, 2552–2561. [Google Scholar] [CrossRef]
- Takada, K.; Toyokawa, G.; Tagawa, T.; Kohashi, K.; Akamine, T.; Takamori, S.; Hirai, F.; Shoji, F.; Okamoto, T.; Oda, Y.; et al. Association Between PD-L1 Expression and Metabolic Activity on 18 F-FDG PET/CT in Patients with Small-sized Lung Cancer. Anticancer Res. 2017, 37, 7073–7082. [Google Scholar]
- Chen, R.; Zhou, X.; Liu, J.; Huang, G. Relationship between the expression of PD-1/PD-L1 and 18F-FDG uptake in bladder cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 848–854. [Google Scholar] [CrossRef]
- Hirakata, T.; Fujii, T.; Kurozumi, S.; Katayama, A.; Honda, C.; Yanai, K.; Tokuda, S.; Nakazawa, Y.; Obayashi, S.; Yajima, R.; et al. FDG uptake reflects breast cancer immunological features: The PD-L1 expression and degree of TILs in primary breast cancer. Breast Cancer Res. Treat. 2020, 181, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Sun, D.; Guo, Y.; Guo, Y.; Xiao, J.; Wang, L.; Yao, X. Assessing PD-L1 Expression Level by Radiomic Features From PET/CT in Nonsmall Cell Lung Cancer Patients: An Initial Result. Acad. Radiol. 2020, 27, 171–179. [Google Scholar] [CrossRef]
- Higashikawa, K.; Yagi, K.; Watanabe, K.; Kamino, S.; Ueda, M.; Hiromura, M.; Enomoto, S. (64)Cu-DOTA-anti-CTLA-4 mAb enabled PET visualiza-tion of CTLA-4 on the T-cell infiltrating tumor tissues. PLoS ONE 2014, 9, e109866. [Google Scholar] [CrossRef]
- Natarajan, A.; Mayer, A.T.; Xu, L.; Reeves, R.E.; Gano, J.; Gambhir, S.S. Novel Radiotracer for ImmunoPET Imaging of PD-1 Checkpoint Expression on Tumor Infiltrating Lymphocytes. Bioconjug. Chem. 2015, 26, 2062–2069. [Google Scholar] [CrossRef]
- Hettich, M.; Braun, F.; Bartholomä, M.D.; Schirmbeck, R.; Niedermann, G. High-resolution PET imaging with therapeutic antibody-based PD-1/PD-L1 checkpoint tracers. Theranostics 2016, 6, 1629–1640. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; England, C.G.; Majewski, R.L.; Valdovinos, H.F.; Jiang, D.; Liu, G.; McNeel, D.G.; Nickles, R.J.; Cai, W. ImmunoPET Imaging of CTLA-4 Expression in Mouse Models of Non-small Cell Lung Cancer. Mol. Pharm. 2017, 14, 1782–1789. [Google Scholar] [CrossRef] [Green Version]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.A.M.S.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef]
- Tavaré, R.; McCracken, M.N.; Zettlitz, K.A.; Knowles, S.M.; Salazar, F.B.; Olafsen, T.; Witte, O.N.; Wu, A.M. Engineered antibody fragments for immuno-PET imaging of endogenous CD8+ T cells in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 1108–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavaré, R.; McCracken, M.N.; Zettlitz, K.A.; Knowles, S.M.; Salazar, F.B.; Olafsen, T.; Witte, O.N.; Wu, A.M. Immuno-PET of Murine T Cell Reconstitution Postadoptive Stem Cell Transplantation Using Anti-CD4 and Anti-CD8 Cys-Diabodies. J. Nucl. Med. 2015, 56, 1258–1264. [Google Scholar] [CrossRef] [Green Version]
- Larimer, B.M.; Wehrenberg-Kee, E.; Caraballo, A.; Mahmood, U. Quantitative CD3 PET Imaging Predicts Tumor Growth Response to Anti-CTLA-4 Therapy. J. Nucl. Med. 2016, 57, 1607–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidian, M.; Ingram, J.R.; Dougan, M.; Dongre, A.; Whang, K.A.; LeGall, C.; Cragnolini, J.J.; Bierie, B.; Gostissa, M.; Gorman, J.; et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J. Exp. Med. 2017, 214, 2243–2255. [Google Scholar] [CrossRef]
- Di Gialleonardo, V.; Signore, A.; Glaudemans, A.W.J.M.; Dierckx, R.A.J.O.; De Vries, E.F.J. N-(4-18F-fluorobenzoyl)interleukin-2 for PET of human-activated T lymphocytes. J. Nucl. Med. 2012, 53, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Radu, C.G.; Shu, C.J.; Nair-Gill, E.; Shelly, S.M.; Barrio, J.R.; Satyamurthy, N.; Phelps, M.E.; Witte, O.N. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2′-deoxycytidine analog. Nat. Med. 2008, 14, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Larimer, B.M.; Wehrenberg-Klee, E.; Dubois, F.; Mehta, A.; Kalomeris, T.; Flaherty, K.; Boland, G.; Mahmood, U. Granzyme B PET Imaging as a Predictive Biomarker of Immunotherapy Response. Cancer Res. 2017, 77, 2318–2327. [Google Scholar] [CrossRef] [Green Version]
- Ronald, J.A.; Kim, B.S.; Gowrishankar, G.; Namavari, M.; Alam, I.S.; D’Souza, A.; Nishikii, H.; Chuang, H.Y.; Ilovich, O.; Lin, C.F.; et al. A PET imaging strategy to visualize activated T cells in acute graft-versus-host disease elicited by allogenic hematopoietic cell transplant. Cancer Res. 2017, 77, 2893–2902. [Google Scholar] [CrossRef] [Green Version]
- Larimer, B.M.; Bloch, E.; Nesti, S.; Austin, E.E.; Wehrenberg-Klee, E.; Boland, G.; Mahmood, U. The Effectiveness of Checkpoint Inhibitor Combinations and Administration Timing Can Be Measured by Granzyme B PET Imaging. Clin. Cancer Res. 2019, 25, 1196–1205. [Google Scholar] [CrossRef] [Green Version]
- Krekorian, M.; Fruhwirth, G.O.; Srinivas, M.; Figdor, C.G.; Heskamp, S.; Witney, T.H.; Aarntzen, E.H.J.G. Imaging of T-cells and their responses during anti-cancer immunotherapy. Theranostics 2019, 9, 7924–7947. [Google Scholar] [CrossRef]
- England, C.G.; Ehlerding, E.B.; Hernandez, R.; Rekoske, B.T.; Graves, S.A.; Sun, H.; Liu, G.; McNeel, D.G.; Barnhart, T.E.; Cai, W. Preclinical Pharmacokinetics and Biodistribution Studies of 89Zr-Labeled Pembrolizumab. J. Nucl. Med. 2017, 58, 162–168. [Google Scholar] [CrossRef] [Green Version]
- England, C.G.; Jiang, D.; Ehlerding, E.B.; Rekoske, B.T.; Ellison, P.A.; Hernandez, R.; Barnhart, T.E.; McNeel, D.G.; Huang, P.; Cai, W. 89 Zr-labeled nivolumab for imaging of T-cell infiltration in a humanized murine model of lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 110–120. [Google Scholar] [CrossRef]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. 89 Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef]
- Goggi, J.L.; Tan, Y.X.; Hartimath, S.V.; Jieu, B.; Hwang, Y.Y.; Jiang, L.; Boominathan, R.; Cheng, P.; Yuen, T.Y.; Chin, X.H.; et al. Granzyme B PET Imaging of Immune Checkpoint Inhibitor Combinations in Colon Cancer Phenotypes. Mol. Imaging Biol. 2020, 22, 1392–1402. [Google Scholar] [CrossRef]
- Nienhuis, P.H.; Antunes, I.F.; Glaudemans, A.W.J.M.; Jalving, M.; Leung, D.; Noordzij, W.; Slart, R.H.J.A.; de Vries, E.F.; Hospers, G.A.P. 18F-BMS986192 PET imaging of PD-L1 in metastatic melanoma patients with brain metastases treated with immune checkpoint inhibitors. A pilot study. J. Nucl. Med. 2021. [Google Scholar] [CrossRef]
- Aide, N.; Hicks, R.J.; Le Tourneau, C.; Lheureux, S.; Fanti, S.; Lopci, E. FDG PET/CT for assessing tumour response to immunotherapy: Report on the EANM symposium on immune modulation and recent review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 238–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aide, N.; De Pontdeville, M.; Lopci, E. Evaluating response to immunotherapy with 18F-FDG PET/CT: Where do we stand? Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1019–1021. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).