Association between Vitamin D Supplementation and Mental Health in Healthy Adults: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. The Registration and Design
2.2. The Eligibility and Inclusion
- (1)
- Studied adult population;
- (2)
- Applied vitamin D supplementation of the specified dose;
- (3)
- Outcome including any mental health aspect assessed based on any method (including both subjective questionnaire and medical diagnosis).
- (1)
- Studies conducted in animal models;
- (2)
- Studies conducted in a specific populations of individuals with any specific physical health problems (any physical symptom, disease, or disorder defining the studied group);
- (3)
- Studies conducted in subjects with intellectual disabilities;
- (4)
- Studies conducted in subjects with eating disorders;
- (5)
- Studies conducted in subjects with neurological disorders (e.g., Alzheimer’s disease, epilepsy).
- (6)
- Studies assessing influence of combined multiple nutrients supplemented;
- (7)
- Studies assessing influence of maternal vitamin D supplementation on mental health in offspring.
2.3. The Search Strategy
2.4. Procedure of Data Extraction
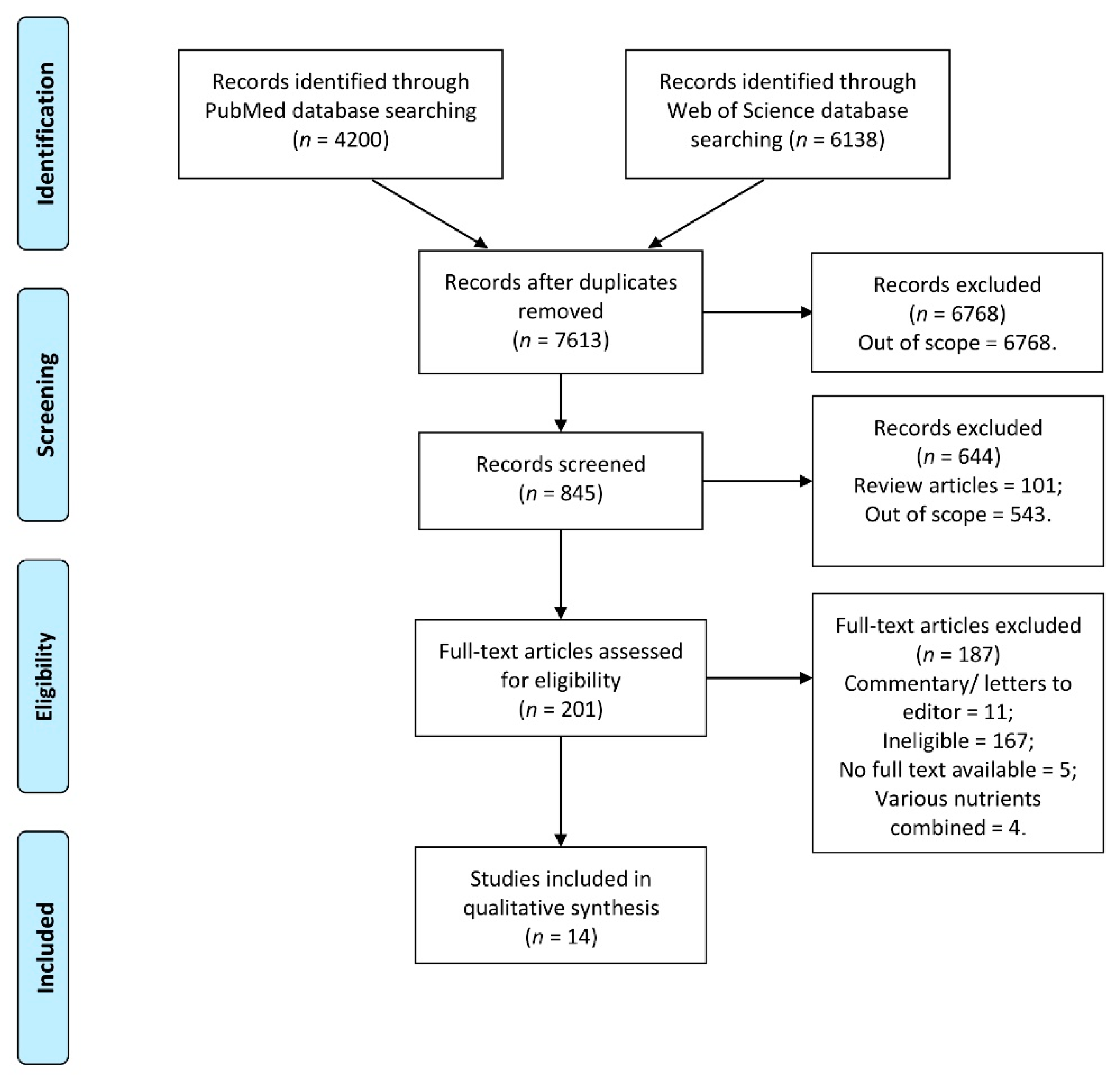
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hill, T.R.; Aspray, T.J. The role of vitamin D in maintaining bone health in older people. Ther. Adv. Musculoskelet. Dis. 2017, 9, 89–95. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of Vitamin D beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef]
- Zmijewski, M.A. Vitamin D and Human Health. Int. J. Mol. Sci. 2019, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K.; et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am. J. Public Health 2014, 104, e43–e50. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, D.T. The Big Vitamin D Mistake. J. Prev. Med. Public Health 2017, 50, 278–281. [Google Scholar] [CrossRef]
- Holick, M.F. Evidence-based D-bate on health benefits of vitamin D revisited. Dermatoendocrinology 2012, 4, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Haines, S.T.; Park, S.K. Vitamin D supplementation: What’s known, what to do, and what’s needed. Pharmacotherapy 2012, 32, 354–382. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Gamble, G.D.; Reid, I.R. Calcium and vitamin D supplements and health outcomes: A reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am. J. Clin. Nutr. 2011, 94, 1144–9114. [Google Scholar] [CrossRef]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am. J. Clin. Nutr. 2007, 85, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Mathyssen, C.; Rafiq, R.; de Jongh, R.T.; Camargo, C.A.; Griffiths, C.J.; Janssens, W.; Martineau, A.R. Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax 2019, 74, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Sizar, O.; Khare, S.; Goyal, A.; Bansal, P.; Givler, A. Vitamin D Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532266/#_NBK532266_pubdet_ (accessed on 12 March 2021).
- Chauhan, K.; Shahrokhi, M.; Huecker, M.R. Vitamin D. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441912/ (accessed on 12 March 2021).
- Vellekkatt, F.; Menon, V. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J. Postgrad. Med. 2019, 65, 74–80. [Google Scholar] [CrossRef]
- Shaffer, J.A.; Edmondson, D.; Taggart Wasson, L.; Falzon, L.; Homma, K.; Ezeokoli, N.; Li, P.; Davidson, K.W. Vitamin D supplementation for depressive symptoms: A systematic review and meta-analysis of randomized controlled trials. Psychosom. Med. 2014, 76, 190–196. [Google Scholar] [CrossRef]
- Spedding, S. Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 2014, 6, 1501–1518. [Google Scholar] [CrossRef]
- Gowda, U.; Mutowo, M.P.; Smith, B.J.; Wluka, A.E.; Renzaho, A.M. Vitamin D supplementation to reduce depression in adults: Meta-analysis of randomized controlled trials. Nutrition 2015, 31, 421–429. [Google Scholar] [CrossRef]
- Li, G.; Mbuagbaw, L.; Samaan, Z.; Falavigna, M.; Zhang, S.; Adachi, J.D.; Cheng, J.; Papaioannou, A.; Thabane, L. Efficacy of vitamin D supplementation in depression in adults: A systematic review. J. Clin. Endocrinol. Metab. 2014, 99, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Huang, Y.C.; Huang, W.L. The effect of vitamin D supplement on negative emotions: A systematic review and meta-analysis. Depress. Anxiety 2020, 37, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Senior, P.A.; Mager, D.R. Vitamin D supplementation and health-related quality of life: A systematic review of the literature. J. Acad. Nutr. Diet. 2015, 115, 406–418. [Google Scholar] [CrossRef]
- Głąbska, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Guzek, D. The Influence of Vitamin D Intake and Status on Mental Health in Children: A Systematic Review. Nutrients 2021, 13, 952. [Google Scholar] [CrossRef] [PubMed]
- Guzek, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Głąbska, D. Influence of Vitamin D Supplementation on Mental Health in Diabetic Patients: A Systematic Review. Nutrients 2021, 13, 3678. [Google Scholar] [CrossRef]
- Głąbska, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Guzek, D. Vitamin D Supplementation and Mental Health in Inflammatory Bowel Diseases and Irritable Bowel Syndrome Patients: A Systematic Review. Nutrients 2021, 13, 3662. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) Assessing Risk of Bias in Non-Randomized Studies. Chapter 13.5.2.3. Available online: http://handbook-5-1.cochrane.org/ (accessed on 28 July 2021).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 28 July 2021).
- You, S.; Kong, T.H.; Han, W. The Effects of short-term and long-term hearing changes on music exposure: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2020, 17, 2091. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R.; Kimball, S.; Hu, A.; Walfish, P.G. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr. J. 2004, 19, 8. [Google Scholar] [CrossRef]
- Shipowick, C.D.; Moore, C.B.; Corbett, C.; Bindler, R. Vitamin D and depressive symptoms in women during the winter: A pilot study. Appl. Nurs. Res. 2009, 22, 221–225. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Powers, S.I.; Spangler, L.; Brunner, R.L.; Michael, Y.L.; Larson, J.C.; Millen, A.E.; Bueche, M.N.; Salmoirago-Blotcher, E.; Liu, S.; et al. Vitamin D intake from foods and supplements and depressive symptoms in a diverse population of older women. Am. J. Clin. Nutr. 2011, 94, 1104–1112. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Jacka, F.N.; Dodd, S.; Nicholson, G.; Berk, M. Annual high-dose vitamin D3 and mental well-being: Randomised controlled trial. Br. J. Psychiatry 2011, 198, 357–364. [Google Scholar] [CrossRef]
- Kjærgaard, M.; Waterloo, K.; Wang, C.E.; Almås, B.; Figenschau, Y.; Hutchinson, M.S.; Svartberg, J.; Jorde, R. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: Nested case-control study and randomised clinical trial. Br. J. Psychiatry 2012, 201, 360–368. [Google Scholar] [CrossRef]
- Cheema, M.R.; Chaudhry, A.Y. Quality-of-life indicators and falls due to vitamin D deficiency. Int. J. Gen. Med. 2016, 22, 21–25. [Google Scholar] [CrossRef][Green Version]
- Patil, R.; Karinkanta, S.; Tokola, K.; Kannus, P.; Sievänen, H.; Uusi-Rasi, K. Effects of Vitamin D and Exercise on the Wellbeing of Older Community-Dwelling Women: A Randomized Controlled Trial. Gerontology 2016, 62, 401–408. [Google Scholar] [CrossRef]
- Vaziri, F.; Nasiri, S.; Tavana, Z.; Dabbaghmanesh, M.H.; Sharif, F.; Jafari, P. A randomized controlled trial of vitamin D supplementation on perinatal depression: In Iranian pregnant mothers. BMC Pregnancy Childbirth 2016, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Choukri, M.A.; Conner, T.S.; Haszard, J.J.; Harper, M.J.; Houghton, L.A. Effect of vitamin D supplementation on depressive symptoms and psychological wellbeing in healthy adult women: A double-blind randomised controlled clinical trial. J. Nutr. Sci. 2018, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Kubiak, J. No improvement in depressive symptoms by vitamin D supplementation: Results from a randomised controlled trial. J. Nutr. Sci. 2018, 22, e30. [Google Scholar] [CrossRef]
- Krokosz, D.; Lipowski, M.; Aschenbrenner, P.; Ratkowski, W. Personality Traits and Vitamin D3 Supplementation Affect Mood State 12 h Before 100 km Ultramarathon Run. Front. Psychol. 2018, 29, 980. [Google Scholar] [CrossRef]
- De Koning, E.J.; Lips, P.; Penninx, B.W.J.H.; Elders, P.J.M.; Heijboer, A.C.; den Heijer, M.; Bet, P.M.; van Marwijk, H.W.J.; van Schoor, N.M. Vitamin D supplementation for the prevention of depression and poor physical function in older persons: The D-Vitaal study, a randomized clinical trial. Am. J. Clin. Nutr. 2019, 110, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Gugger, A.; Marzel, A.; Orav, E.J.; Willett, W.C.; Dawson-Hughes, B.; Theiler, R.; Freystätter, G.; Egli, A.; Bischoff-Ferrari, H.A. Effect of Monthly High-Dose Vitamin D on Mental Health in Older Adults: Secondary Analysis of a RCT. J. Am. Geriatr. Soc. 2019, 67, 1211–1217. [Google Scholar] [CrossRef]
- Lipowski, M.; Walczak-Kozłowska, T.; Lipowska, M.; Kortas, J.; Antosiewicz, J.; Falcioni, G.; Ziemann, E. Improvement of Attention, Executive Functions, and Processing Speed in Elderly Women as a Result of Involvement in the Nordic Walking Training Program and Vitamin D Supplementation. Nutrients 2019, 11, 1311. [Google Scholar] [CrossRef]
- Stokes, C.S.; Lammert, F. Vitamin D supplementation: Less controversy, more guidance needed. F1000Res 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Utri, Z.; Głąbska, D. Vitamin D Intake in a Population-Based Sample of Young Polish Women, Its Major Sources and the Possibility of Meeting the Recommendations. Foods 2020, 9, 1482. [Google Scholar] [CrossRef]
- Australian Government. National Health and Medical Research Council Vitamin D—Nutrient Reference Values. Available online: https://www.nrv.gov.au/nutrients/vitamin-d (accessed on 25 August 2021).
- Reference Values—DACH-Referenzwerte—Schweizerische Gesellschaft für Ernährung. Available online: http://www.sge-ssn.ch/grundlagen/lebensmittel-und-naehrstoffe/naehrstoffempfehlungen/dachreferenzwerte/ (accessed on 25 August 2021).
- Utri, Z.; Głąbska, D. Salmon Intake Intervention in the Vulnerable Group of Young Polish Women to Maintain Vitamin D Status during the Autumn Season. Sustainability 2020, 12, 2829. [Google Scholar] [CrossRef]
- National Academies Press (US). Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Collection: Reports Funded by National Institutes of Health; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Aspray, T.J.; Bowring, C.; Fraser, W.; Gittoes, N.; Javaid, M.K.; Macdonald, H.; Patl, S.; Selby, P.; Tanna, N.; Francis, R.M. National Osteoporosis Society Vitamin D Guideline Summary. Age Ageing 2014, 43, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Gallo, S.; Comeau, K.; Vanstone, C.; Agellon, S.; Sharma, A.; Jones, G.; L’Abbé, M.; Khamessan, A.; Rodd, C.; Weiler, H. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: A randomized trial. JAMA 2013, 309, 1785–1792. [Google Scholar] [CrossRef]
- Chel, V.; Wijnhoven, H.A.; Smit, J.H.; Ooms, M.; Lips, P. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos. Int. 2008, 19, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Spiro, A.; Buttriss, J.L. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef]
- Penckofer, S.; Kouba, J.; Byrn, M.; Estwing Ferrans, C. Vitamin D and depression: Where is all the sunshine? Issues Ment. Health Nurs. 2010, 31, 385–393. [Google Scholar] [CrossRef]
- Głąbska, D.; Guzek, D.; Groele, B.; Gutkowska, K. Fruit and Vegetable Intake and Mental Health in Adults: A Systematic Review. Nutrients 2020, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Guzek, D.; Głąbska, D.; Groele, B.; Gutkowska, K. Fruit and Vegetable Dietary Patterns and Mental Health in Women: A Systematic Review. Nutr. Rev. 2021, 26, nuab007. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Meseguer, J.; Tortosa-Martínez, J.; Cortell-Tormo, J.M. The Benefits of Physical Exercise on Mental Disorders and Quality of Life in Substance Use Disorders Patients. Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 3680. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Healthy adults | Children and adolescents, individuals with any specific physical health problems, intellectual disabilities, eating disorders, or neurological disorders |
| Intervention/exposure | Participants assessed during vitamin D supplementation | Combined multiple nutrients supplemented |
| Comparison | Influence on a mental health outcomes assessed while compared with baseline/placebo/various doses and regimens | Lack of comparison |
| Outcome | Any aspect of mental health associated with any area of the broad spectrum of general mental health | Patients assessed for cognitive function |
| Study design | Peer-reviewed articles published in English, including: randomized controlled trials, randomized crossover trials, cohort studies, case-control studies, and cross-sectional studies | Articles not published in English, reviews, meta-analyses, expert opinions, letters to editor, comments, studies in animal models, methodological articles, case reports, and conference reports |
| Ref. | Authors, Year | Study Design | Country/Location | Studied Group | Time |
|---|---|---|---|---|---|
| [29] | Vieth et al., 2004 | Blinded, randomized trial | Canada/Toronto | Outpatients of endocrinology clinic with 25(OH)D < 61 nmol/L (Study 1) | Winter 2001–2002 (Study 1) |
| Outpatients of endocrinology clinic with 25(OH)D < 51 nmol/L (Study 2) | Winter 2002–2003 (Study 2) | ||||
| [30] | Shipowick et al., 2009 | Quasi-experimental pilot study | United States of America/Washington | Female patients treated at a medical clinic for vitamin D deficiency or insufficiency | Not specified |
| [31] | Bertone-Johnson et al., 2011 | Cross-sectional prospective analysis within Women’s Health Initiative Observational Study (WHI OS) | United States of America | Women aged 50–79 participating in WHI OS | 1993–1998 |
| [32] | Sanders et al., 2011 | Double-blind, randomized, placebo-controlled trial within Vital D study | Australia/Barwon and Mornington Peninsula regions | Community-dwelling women aged at least 70 years participating in Vital D study | 2005–2008 |
| [33] | Kjaergaard et al., 2012 | Nested case-control study and randomized clinical trial | Norway/Tromsø | Adults 30–75 years old with serum 25(OH)D levels below the 20 percentile (55 nmol/L) or above the 75 percentile (70 nmol/L) from the sixth Tromsø study | October 2009–November 2010 |
| [34] | Cheema and Chaudhry, 2016 | Prospective study | United Kingdom | Patients admitted with a fall with or without sustaining a fragility fracture post fall | Not specified |
| [35] | Patil et al., 2016 | Randomized double-blind, placebo-controlled intervention trial with vitamin D and exercise (DEX trial) | Finland | Older Finnish women participating in DEX trial | 2010–2013 |
| [36] | Vaziri et al., 2016 | Randomized controlled trial | Iran/Shiraz | Pregnant women under prenatal care in Hafez hospital | November 2014–October 2015 |
| [37] | Choukri et al., 2018 | Double-blind, placebo-controlled, randomized clinical trial | New Zealand/Dunedin | Healthy women aged 18–40 years | February–October 2013 |
| [38] | Jorde and Kubiak, 2018 | Randomized controlled trial within Tromsø study population | Norway/Tromsø | Adults aged 40–80 years participating in Tromsø Study | 2015–2016 |
| [39] | Krokosz et al., 2018 | Double-blind, placebo-controlled, randomized study | Poland | Experienced marathon and ultramarathon male runners (aged 31–50 years) taking part in a 100 km track run | Not specified |
| [40] | de Koning et al., 2019 | Randomized placebo-controlled clinical trial within D-Vitaal Study | Netherlands/Amsterdam | Community-dwelling older individuals with functional limitations participating in D-Vitaal Study | 2013–2016 |
| [41] | Gugger et al., 2019 | 1-year double-blind randomized clinical trial | Switzerland/Zurich | Community-dwelling older adults with a prior low-trauma fall in the previous year | January 2010–May 2011 |
| [42] | Lipowski et al., 2019 | Intervention program | Poland/Gdansk | Elderly women participating in a Nordic walking training program | October 2018 * |
| Ref. | Authors, Year | Number of Participants (Number of Female Participants) | Age (Mean with SD/Range) | Inclusion Criteria/Exclusion Criteria |
|---|---|---|---|---|
| [29] | Vieth et al., 2004 | 64 (53) (Study 1) 112 (87) (Study 2) | Mean of 48–56 years depending on the sub-group | Inclusion: outpatients of endocrinology clinic; low 25(OH)D that demonstrated a need for supplementation Exclusion: not specified |
| [30] | Shipowick et al., 2009 | 6 (6) | 42.2 ± 13.2 years | Inclusion: female patients; serum 25(OH)D levels below 100 nmol/L Exclusion: mental impairments; dementia; language barriers; using or planned to use tanning beds or other phototherapy; planned on traveling to sunnier, more tropical areas during the winter; taking or planning to take antidepressants |
| [31] | Bertone-Johnson et al., 2011 | 81,189 (81,189) | 50–79 years at baseline | Inclusion: female participants of WHI OS Exclusion: enrolment in a WHI clinical trial; medical conditions likely to result in death within 3 years; previous history of cancers (except nonmelanoma skin cancer); conditions that were likely to interfere with retention in the study; implausible calorie intake (<600 and >5000 kcal/day); missing data |
| [32] | Sanders et al., 2011 | 2258 (2258) | At least 70 years at baseline | Inclusion: female participants of Vital D study; aged at least 70 years at baseline; identified risk factor for hip fracture (maternal hip fracture, self-reported “faller”, fracture since aged 50), and/or high risk of low vitamin D and osteoporosis Exclusion: inability to provide informed consent or falls/fracture data; permanent resident of high-level care facility; albumin-corrected calcium 42.65 mmol/L; vitamin D supplementation 110 µg; other parameters relating to bone health |
| [33] | Kjaergaard et al., 2012 | 357 (179) | 53.6 ± 10.3 years for case group 55.1 ± 9.4 years for control group | Inclusion: participants of the sixth Tromsø study; 30–75 years, serum 25(OH)D levels below the 20 percentile (55 nmol/L) or above the 75 percentile (70 nmol/L) Exclusion: history of known diabetes, coronary heart disease, or stroke in the past 12 months; cancer; kidney stones; any conditions needing medical attention; possible primary hyperparathyroidism (PTH > 5.0 pmol/L combined with serum calcium > 2.50 mmol/L); males with serum creatinine > 130 mmol/L and females with serum creatinine > 110 mmol/L; systolic blood pressure > 174 mmHg or diastolic blood pressure > 104 mmHg; pregnant or lactating women; fertile women below the age of 50 years without adequate contraception; reported use of vitamin D supplements, antidepressants, or other mood stabilizing medication; regular use of a solarium; planned trip to a sunny location in the trial period; Beck Depression Inventory (BDI) score > 29; Montgomery–Åsberg Depression Rating Scale (MADRS) score >34; serious depression in the Structured Clinical Interview for DSM-IV Axis I Disorders–Clinician Version (SCID-CV) |
| [34] | Cheema and Chaudhry, 2016 | 38 (20) | 80.2 ± 12.0 years | Inclusion: patients admitted with falls Exclusion: not specified |
| [35] | Patil et al., 2016 | 409 (409) | Mean of 73–75 years depending on the sub-group | Inclusion: participants of the DEX trial; 70–80 years; home-dwelling women; fallen at least once during the previous 12 months; no contraindications to exercise Exclusion: regular use of vitamin D supplements; moderate to vigorous exercise > 2 h per week |
| [36] | Vaziri et al., 2016 | 169 (169) | 26.3 ± 4.6 years | Inclusion: ≥ 18 years; women; healthy—no history of mental illness and internal diseases such as hyper/hypothyroidism, parathyroid, renal, diabetes, and heart diseases; no addiction to any kind of narcotic drugs; living with a husband; a singleton live fetus; without any pregnancy complications such as preeclampsia, gestational diabetes, ruptured membranes, and suspicion of preterm birth; no previous cesarean sections; gestational age of 26–28 weeks based on ultrasound results; Edinburgh Postnatal Depression Scale (EPDS) baseline scores of 0–13 Exclusion: not providing blood sample at the onset of the study; less than 8 weeks consumption of vitamin D3 supplementations; irregular consumption of vitamin D3 supplementations |
| [37] | Choukri et al., 2018 | 150 (150) | 24.2 ± 6.0 years | Inclusion: 18–40 years; women; not currently pregnant or breastfeeding; access to the Internet; willing to provide a repeated blood sample Exclusion: current/planned vitamin D supplementation (including as part of a multivitamin supplement); chronic liver and kidney disease; arteriosclerosis or cardiac function impairment; sarcoidosis and other possible granulomatous diseases; medication, including anticonvulsants, glucocorticoids, and barbiturates that might affect vitamin D metabolism; overseas travel during the study period |
| [38] | Jorde and Kubiak, 2018 | 408 (191) | 52.0 ± 8.8 years | Inclusion: age 40–80 years; vitamin D insufficiency (serum 25(OH)D < 42 nmol/L) Exclusion: granulomatous diseases; diabetes; renal stones in the last 5 years; serious diseases making the subject unfit for participation; vitamin D supplementation of > 20 μg per day; use of solarium on a regular basis; planned holiday in tropical areas during the study period; women of childbearing potential without use of acceptable contraception |
| [39] | Krokosz et al., 2018 | 20 (0) | 40.7 ± 7.1 years | Inclusion: male; aged 31–50 years; experienced marathon and ultramarathon runners taking part in a 100 km track run Exclusion: not specified |
| [40] | de Koning et al., 2019 | 155 (89) | 68.4 ± 5.3 years * | Inclusion: age 60–80 years; presence of depressive symptoms (Center of Epidemiological Studies–Depression scale (CES–D) score of ≥16); ≥1 functional limitation (e.g., difficulties with walking, climbing stairs, or dressing oneself); serum 25(OH)D concentration 15–50 nmol/L in winter or 15–70 nmol/L in summer Exclusion: current major depressive disorder diagnosis; life-threatening illness; current antidepressant medication; vitamin D supplementation of >10 μg per day; calcium supplementation of >1000 mg per day |
| [41] | Gugger et al., 2019 | 200 (134) | 78 (71–92) years | Inclusion: age ≥ 70 years; pre-frail (low-trauma fall in the previous 12 months); Mini-Mental State Examination (MMSE) score of ≥27; a normal clock test Exclusion: unwillingness to stop additional vitamin D supplementation during the trial; insufficient mobility to come to the study center |
| [42] | Lipowski et al., 2019 | 52 (52) | 69.8 ± 4.7 years | Inclusion: women; participating in a Nordic walking training program; baseline 25(OH)D3 concentration above 50 nmol/L Exclusion: uncontrolled hypertension (diastolic blood pressure over 100 mmHg); history of cardiac arrhythmia; cardio-respiratory disorders; orthopedic problems |
| Ref. | Authors, Year | Vitamin D Measure | Vitamin D Supplementation Dose and Regimen | Mental Health Outcome | Psychological Measure |
|---|---|---|---|---|---|
| [29] | Vieth et al., 2004 | 25(OH)D blood level 1,25(OH)2D blood level | 15 µg vs. 100 µg/day for at least 2–6 months or > 6 months |
|
|
| [30] | Shipowick et al., 2009 | 25(OH)D blood level | 125 µg/day for 2 months | Depression | Beck Depression Inventory–Second Edition (BDI-II) |
| [31] | Bertone-Johnson et al., 2011 | No biochemical assessment —supplement use questionnaire | None vs. <10 µg vs. 10–20 µg vs. > 20 µg/day for 3 years based on the questionnaire retrospective data | Depressive symptoms | Burnam 8-item scale for depressive symptomsCurrent antidepressant medication use |
| [32] | Sanders et al., 2011 | 25(OH)D blood level | 12,500 µg/year (as one dose) vs. placebo for 3–5 years |
|
|
| [33] | Kjaergaard et al., 2012 | 25(OH)D blood level | 500 µg/week vs. placebo for 6 months | Depressive symptoms |
|
| [34] | Cheema and Chaudhry, 2016 | 25(OH)D blood level | 1500 µg/week for 2 months (followed by maintenance regimen of 10 µg and 600 mg of calcium) | Mental component | Short Form Health Survey (SF-12) |
| [35] | Patil et al., 2016 | 25(OH)D blood level | 20 µg/day vs. placebo for 24 months |
|
|
| [36] | Vaziri et al., 2016 | 25(OH)D blood level | 50 µg/day vs. placebo from 26–28 weeks of gestation until childbirth | Depression | Edinburgh Postnatal Depression Scale (EPDS) |
| [37] | Choukri et al., 2018 | 25(OH)D blood level | 1250 µg/month vs. placebo for 6 months |
|
|
| [38] | Jorde and Kubiak, 2018 | 25(OH)D blood level | 500 µg/week (following bolus dose of 2500 µg/day) vs. placebo for 4 months | Depression | Beck Depression Inventory–Second Edition (BDI-II) |
| [39] | Krokosz et al., 2018 | 25(OH)D blood level | 250 µg/day vs. placebo for 2 weeks preceding the race | Mood states | University of Wales Institute of Science and Technology (UWIST) Mood Adjective Check List (UMACL) with Polish adaptation |
| [40] | de Koning et al., 2019 | 25(OH)D blood level | 30 µg/day vs. placebo for 12 months |
|
|
| [41] | Gugger et al., 2019 | 25(OH)D blood level | 600 µg vs. 1500 µg vs. 600 µg + 300 µg of calcifediol/month for 12 months |
|
|
| [42] | Lipowski et al., 2019 | 25(OH)D blood level | 100 µg/day for 12 weeks |
|
|
| Ref. | Authors, Year | Concluded Association between Vitamin D Supplementation and Mental Health in Adults | Quality b | |
|---|---|---|---|---|
| Studied Outcome | Supporting/Inconclusive/Not Supporting a | |||
| [29] | Vieth et al., 2004 | Well-being | Supporting | 4 |
| [30] | Shipowick et al., 2009 | Depressive symptoms | Supporting | 5 |
| [31] | Bertone-Johnson et al., 2011 | Depressive symptoms | Supporting, but less effective for supplementation than for food sources | 8 |
| [32] | Sanders et al., 2011 | Mental well-being | Not supporting | 7 |
| [33] | Kjaergaard et al., 2012 | Depressive symptoms | Not supporting | 5 |
| [34] | Cheema and Chaudhry, 2016 | Mental component | Not supporting | 5 |
| [35] | Patil et al., 2016 | Quality of life, fear of falling, mental well-being | Not supporting | 9 |
| [36] | Vaziri et al., 2016 | Perinatal depression | Supporting | 8 |
| [37] | Choukri et al., 2018 | Depression, anxiety, flourishing, mood | Not supporting | 7 |
| [38] | Jorde and Kubiak, 2018 | Depressive symptoms | Not supporting | 8 |
| [39] | Krokosz et al., 2018 | Mood | Inconclusive | 3 |
| [40] | de Koning et al., 2019 | Depressive symptoms, health related quality of life | Not supporting | 9 |
| [41] | Gugger et al., 2019 | Mental health | Inconclusive | 4 |
| [42] | Lipowski et al., 2019 | Depressive symptoms | Supporting while combined with physical activity | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzek, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Głąbska, D. Association between Vitamin D Supplementation and Mental Health in Healthy Adults: A Systematic Review. J. Clin. Med. 2021, 10, 5156. https://doi.org/10.3390/jcm10215156
Guzek D, Kołota A, Lachowicz K, Skolmowska D, Stachoń M, Głąbska D. Association between Vitamin D Supplementation and Mental Health in Healthy Adults: A Systematic Review. Journal of Clinical Medicine. 2021; 10(21):5156. https://doi.org/10.3390/jcm10215156
Chicago/Turabian StyleGuzek, Dominika, Aleksandra Kołota, Katarzyna Lachowicz, Dominika Skolmowska, Małgorzata Stachoń, and Dominika Głąbska. 2021. "Association between Vitamin D Supplementation and Mental Health in Healthy Adults: A Systematic Review" Journal of Clinical Medicine 10, no. 21: 5156. https://doi.org/10.3390/jcm10215156
APA StyleGuzek, D., Kołota, A., Lachowicz, K., Skolmowska, D., Stachoń, M., & Głąbska, D. (2021). Association between Vitamin D Supplementation and Mental Health in Healthy Adults: A Systematic Review. Journal of Clinical Medicine, 10(21), 5156. https://doi.org/10.3390/jcm10215156









