Abstract
(1) Background: Intravesical mitomycin-C (MMC) combined with hyperthermia is increasingly used in non-muscle invasive bladder cancer (NMIBC), especially in the context of a relative BCG shortage. We aim to determine real-world data on the long-term treatment outcomes of adjunct hyperthermic intravesical chemotherapy (HIVEC) with MMC and a COMBAT® bladder recirculation system (BRS); (2) Methods: A prospective observational trial was performed on patients with NMIBC treated with HIVEC using BRS in nine academic institutions in Spain between 2012–2020 (HIVEC-E). Treatment effectiveness (recurrence, progression and overall mortality) was evaluated in patients treated with HIVEC MMC 40mg in the adjuvant setting, with baseline data and a clinical follow-up, that comprise the Full Analysis Set (FAS). Safety, according to the number and severity of adverse effects (AEs), was evaluated in the safety (SAF) population, composed by patients with at least one adjunct HIVEC MMC instillation; (3) Results: The FAS population (n = 502) received a median number of 8.78 ± 3.28 (range 1–20) HIVEC MMC instillations. The median follow-up duration was 24.5 ± 16.5 (range 1–81) months. Its distribution, based on EAU risk stratification, was 297 (59.2%) for intermediate and 205 (40.8%) for high-risk. The figures for five-year recurrence-free and progression-free survival were 50.37% (53.3% for intermediate and 47.14% for high-risk) and 89.83% (94.02% for intermediate and 84.23% for high-risk), respectively. A multivariate analysis identified recurrent tumors (HR 1.83), the duration of adjuvant HIVEC therapy <4 months (HR 1.72) and that high-risk group (HR 1.47) were at an increased risk of recurrence. Independent factors of progression were high-risk (HR 3.89), recurrent tumors (HR 3.32) and the induction of HIVEC therapy without maintenance (HR 2.37). The overall survival was determined by patient age at diagnosis (HR 3.36) and the treatment duration (HR 1.82). The SAF population (n = 592) revealed 406 (68.58%) patients without AEs and 186 (31.42%) with at least one AE: 170 (28.72%) of grade 1–2 and 16 (2.7%) of grade 3–4. The most frequent AEs were dysuria (10%), pain (7.1%), urgency (5.7%), skin rash (4.9%), spasms (3.7%) and hematuria (3.6%); (4) Conclusions: HIVEC using BRS is efficacious and well tolerated. A longer treatment duration, its use in naïve patients and the intermediate-risk disease are independent determinants of success. Furthermore, a monthly maintenance of adjunct MMC HIVEC diminishes the progression rate of NMIBC.
1. Introduction
Bladder cancer is a major urological disease, with more than half a million new cases diagnosed and leading to almost 0.2 million deaths per year worldwide [1]. An increase in bladder cancer incidence but a decrease in mortality has been recently observed in several European countries, possibly related to a better awareness and earlier detection that allows better oncological control [2]. In Spain, despite the efforts launched to limit smoking habit, age-standardized incidence and mortality rates remain at 15.6 and 3.5 per 100.000, respectively [3]. Approximately 75% of patients present as having non-muscle invasive bladder cancer (NMIBC), which, despite being a none life-threatening disease, presents the risk of recurrence and also of progression to a muscle invasive form, most often leading to metastases [4].
Treatments to limit the recurrence and progression of NMIBC include intravesical mitomycin C (MMC) and bacillus Calmette-Guérin (BCG). Maintenance BCG is considered to be the best bladder-sparing treatment for high-risk NMIBC patients and also as a potential alternative for the intermediate risk group [5]. However, BCG administration is by far more toxic than chemotherapy [6,7]. Additionally, recent evidence suggests that maintenance BCG is not a cost-effective alternative for the entire population of patients with intermediate/high risk NMIBC [8]. To make matters worse, shortages in the BCG supply have compromised patient outcomes and left clinicians around the globe without clear effective and reliable alternatives [9,10,11].
Despite conducting instillations with MMC and other chemotherapeutic agents for decades, the length and frequency of the adjuvant chemotherapy regime is controversial and has yet to be established [5]. The Global BCG shortage has not been completely resolved, leading to the need to urgently develop strategies to improve the efficacy of chemotherapy delivery. The concept of device-assisted intravesical therapy to improve the penetration of MMC and other chemotherapeutic agents into the bladder wall is very promising. Two different heating systems, microwave-induced chemo-hyperthermia using radiofrequency (RF) and hyperthermic intravesical chemotherapy (HIVEC) using the bladder recirculation system (BRS), have been increasingly used for both intermediate and high-risk patients during BCG shortage, sometimes without a solid grounding due to the absence of clinical trials [12]. Neither the optimal regime for standard chemotherapy instillations nor the optimal regime for device-assisted chemotherapy have been identified.
We present real-world oncological results of chemo-hyperthermia using Combat BRS from a prospective observational trial conducted in Spain (HIVEC-E). The main objective of the study is to assess the safety and effectiveness results of the therapy in a real world setting and assist in identifying the optimal regime that should be considered when designing new randomized clinical trials using HIVEC.
2. Materials and Methods
2.1. Study Population
A prospective observational multicentre study was performed on consecutive patients with NMIBC, treated with HIVEC using the COMBined Antineoplastic Thermotherapy (COMBAT®) BRS (Combat Medical, Wheathampstead, UK) in nine academic institutions in Spain between 2012 and 2020. The registry, named the HIVEC-epidemiology (HIVEC-E), included consecutive patients with NMIBC, treated with any form of chemotherapeutic regime using the COMBAT BRS device, that increases the temperature of the pharmaceutical agent to 43 °C (± 1 °C) outside the body to enter the bladder through a soft 16F 3-way Foley catheter and recirculate with a constant flow for a period of 1 hour. A closed circuit with a heating system keeps the chemotherapy at a constant temperature. At the end of the procedure, the product is collected in a urine collection bag.
All patients provided their informed consent to participate in the study. Investigators registered the clinical information into an electronic case report form (eCRF) with periodical status updates during follow-up. All patients were treated with a complete transurethral resection of the bladder (TURB) and HIVEC using different agents in the adjuvant (prophylactic) or neoadjuvant (ablative) setting, with or without maintenance, according to the decision made by the investigators and the regular practice in their institution. Histopathologic evidence of muscle-invasive disease was excluded in every case. Patients not included in this trial were treated according to European Guidelines whenever treatment was available.
The Full Analysis Set (FAS) population for the current study focused exclusively on patients receiving adjunct HIVEC MMC 40mg (standard dose) and a clinical follow-up was updated in June 2021. The treatment modalities included in this study were based on weekly induction HIVEC MMC, either as a one-time treatment or followed by monthly maintenance, or as monthly maintenance alone for other patients. Patients receiving a combination treatment with induction BCG and monthly HIVEC MMC maintenance because of BCG shortage during the treatment were excluded. The number of instillations varied according to the criteria of the different centers involved, the patient risk group and treatment tolerance. The Safety (SAF) population was defined as all subjects who received at least one adjunct HIVEC MMC instillation with a post-baseline safety assessment. The statement that a subject had no adverse events (AEs) also constitutes a safety assessment. The number and severity of AEs were evaluated and were, according to Common Terminology Criteria for Adverse Events (CTCAE), defined as Grade 1 (mild toxicity), Grade 2 (moderate), Grade 3 (severe), Grade 4 (life-threatening) and Grade 5 (death). For practical purposes, the mild-moderate and severe-life threatening toxicities were pooled together.
2.2. Study Endpoints
The co-primary endpoints were the evaluation of the effectiveness of adjuvant HIVEC MMC in the FAS population in terms of recurrence-free, progression-free and overall survival. A multivariate analysis was performed to determine prognostic factors, and thus evaluate the likely markers of treatment response. The secondary endpoint was the evaluation of safety of the adjuvant HIVEC MMC in the SAF population.
2.3. Variables Evaluated
Data registered in eCRF included patients’ baseline patient characteristics (date of birth, sex, body mass index (BMI), smoking habit), former tumor history (previous tumors, treatments received), date of inclusion, preoperative tumor characteristics (tumour size, multiplicity), operative data (date of TURB, 2nd TURB, bladder biopsy mapping) and postoperative data (T category, tumor grade, presence of concomitant cis, EAU risk group), treatment schedule (date of HIVEC instillation, tolerance), status at each follow-up visit (date of event, recurrence, progression, cystectomy) and patient death. Cause of death was registered whenever possible but was preferred overall to disease-specific survival as an endpoint in the absence of a mortality committee.
2.4. Statistical Analysis
Mean and standard deviation (SD) or median and interquartile range (IQR) were calculated for quantitative variables and those that were qualitative were described using absolute and relative frequencies. A paired t-test or Wilcoxon rank sum test were used to compare continuous variables. A Cochran–Armitage trend test and Chi-square contingency test or a Fisher exact test were performed to compare the categorical variables. The factors affecting tumor recurrence, progression and overall survival were evaluated using the Kaplan-Meier analysis method and their significance was evaluated by two-sided log-rank test. All patients were updated to June 2021. A univariate analysis using hazard ratios and 95% Wald confidence limits was performed for the variables investigated. All the variables with a significant impact in the univariate analysis were evaluated in a multivariate Cox regression model using a stepwise logistic regression with p = 0.15 entry and p = 0.05 stay criteria. Both the hazard ratio and 95% confidence intervals were calculated for the multivariate models defining disease recurrence, progression and overall survival. A p-value of <0.05 was considered as significant. The statistical analysis was performed using Statistical Analysis System 9.4 (SAS Institute Inc, Cary, NY, USA).
3. Results
Figure 1 shows the flowchart of patients registered in HIVEC-E and the populations of patients included in this analysis. The FAS population (n = 502) included patients receiving adjunct HIVEC MMC with follow-up treatment that allowed for the evaluation of primary endpoints (recurrence-free, progression-free and overall survival).
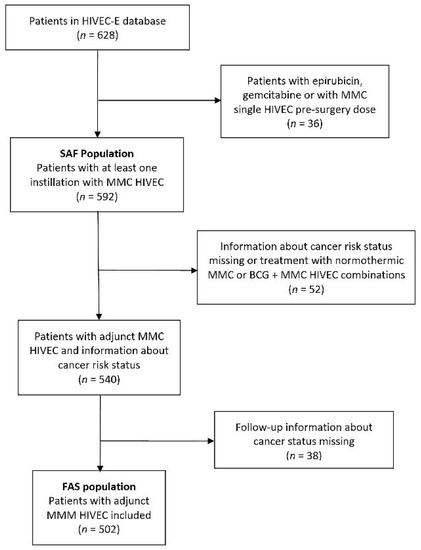
Figure 1.
Flowchart of patients included in the study.
Patient and tumor characteristics are summarized in Table 1. The distribution, based on EAU risk stratification, was 297 (59.2%) for intermediate and 205 (40.8%) for high-risk. Mapping bladder biopsies were performed at the time of TURB in 45 patients and revealed carcinoma in situ (cis) in 22 (4.4%); primary cis in 10 (2%) and cis concomitant to papillary neoplasia in 12 (4.4%).A second-TURB was performed in 122 (24.3%) and revealed persistent NMIBC in 11 (2.2%). According to the previous tumor history and treatments received, a tumor was recurrent in 214 patients (42.6%) and in 62 (12.35%) patients the recurrence rate was higher than 1 episode per year. Regarding previous treatments received, none of the patients had been treated with device-assisted intravesical therapies before their inclusion in HIVEC-E, however, 69 (13.7%) had received MMC in normothermia and 52 (10.4%) received BCG before their inclusion.

Table 1.
Clinico-pathological characteristics of patients, FAS population (n = 502).
A median number of 8.91 ± 3.22 (range 1–20) HIVEC MMC instillations per patient were administered in the FAS population; 4.63 ± 1.68 for patients treated with a weekly induction schedule (n = 68), 8.7 ± 3.15 instillations for those treated with monthly maintenance alone (n = 27), and 9.64 ± 2.85 instillations for patients receiving both the weekly induction and monthly maintenance (n = 407). Globally, 434 (86.45%) patients received some form of a maintenance regime; however, treatment duration lasted for more than 4 months for only 371 patients (73.9%). The median follow-up was at 24.5 ± 16.5 (range 1–81) months. During follow-up, 159 patients (31,7%) suffered disease recurrence, 35 (7%) patients progressed to a muscle invasive disease and 66 (13.5%) died (any cause). A radical cystectomy was performed as a rescue surgery in 22 of the 35 patients with disease progression (62.9%). In no case did a cystectomy present as technically more challenging. Furthermore, a cystectomy was performed in another case without neoplasia due to a retractile bladder after repeated TURB.
Figure 2 shows the Kaplan-Meir curves for the recurrence-free interval, progression-free interval and overall survival for the FAS population, and also the stratification for the EAU intermediate- and high-risk groups evaluated.
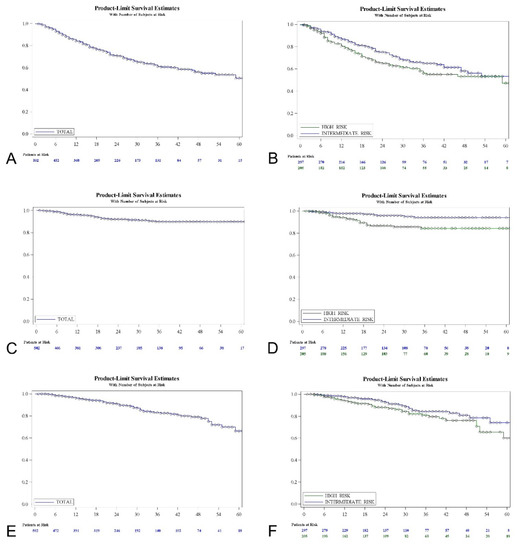
Figure 2.
Recurrence-free survival, FAS population (A) and EAU risk groups (B); progression-free survival, FAS population (C) and EAU risk groups (D); overall survival, FAS population (E) and EAU risk groups (F).
Table 2 shows recurrence, progression and overall mortality at different times with interval limits for the FAS population and a stratification according to the risk groups, with a log-rank test for comparisons. The five-years recurrence-free survival rate was 50.37% for the total series (53.3% intermediate and 47.14% high-risk; log-rank, p = 0.075). Five-years progression-free survival was 89.83% (94.02% intermediate and 84.23% high-risk; log-rank, p = 0.001). The rate of five-years overall survival was 66.35% (74.26% intermediate and 60.12% high-risk; log-rank, p = 0.064). Among the high-risk group, the primary cis population (n = 10) revealed a 50% response rate and an 87.5% progression-free survival at 1 year; a 25% response rate and 65.6% progression-free survival at 2 years, etc. None of these patients died during follow-up due to intensive surveillance and rescue surgery.

Table 2.
Recurrence, progression and overall mortality at different times with interval limits for the FAS population (n = 502), and for intermediate (n = 297) and high-risk patients (n = 205).
3.1. Recurrence-Free Survival
Kaplan-Meier analysis revealed that T category (log-rank; p = 0.0004), presence of cis (log-rank; p = 0.0005), primary vs. recurrent tumor (log-rank; p = 0.0004), duration of treatment (log-rank; p = 0.0002), use of maintenance therapy (log-rank; p = 0.0007), previous treatment with MMC (log-rank; p = 0.0201) and previous treatment with BCG (log-rank; p = 0.0052) were predictors of tumor recurrence-free interval. Duration of HIVEC MMC (log-rank, p = 0.0002) seems more determinant than use of maintenance (log-rank, p = 0.0007) in terms of recurrence-free survival (Figure 3).
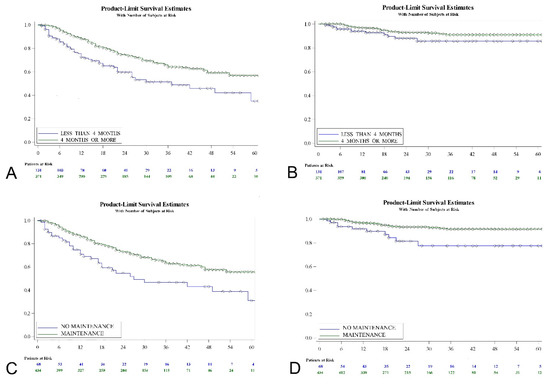
Figure 3.
Recurrence (A) and progression-free survival (B) according to duration of adjunct HIVEC MMC treatment; recurrence (C) and progression-free survival (D) according to whether maintenance schedule was used.
Table 3 shows the corresponding hazard ratios and confidence interval limits for each variable as obtained in the univariate analysis. The risk-group, T category, grade, cis, tumor history, duration of treatment, use of maintenance therapy, former use of MMC and of BCG were entered into the stepwise model for recurrence (p < 0.15). Patient age, sex, smoking habit, tumor multiplicity and tumor size were not related to tumor recurrence. A multivariate analysis revealed previous tumor history (recurrent vs. primary; HR 1.828 (95% CI 1.327–2.518); p = 0.0002), duration of treatment (<4 months vs. ≥4 months; HR 1.724 (95% CI 1.235–2.407); p = 0.0014) and EAU risk-group (high-risk vs. intermediate-risk; HR 1.472 (95% CI 1.071–2.024); p = 0.0171) remained independent factors (p < 0.05) of tumor recurrence using adjunct HIVEC MMC.

Table 3.
Cox regression model to predict tumor recurrence, FAS population (n = 502).
3.2. Progression-Free Survival
Regarding progression to muscle invasive disease, a Kaplan-Meier analysis revealed that the EAU risk-group (log-rank; p = 0.001), T category (log-rank; p = 0.0004), presence of cis (log-rank; p = 0.0007), primary vs. recurrent tumor (log-rank; p = 0.0019), use of maintenance therapy (log-rank; p = 0.0016), previous treatment with MMC (log-rank; p = 0.0117) and previous treatment with BCG (log-rank; p = 0.0097) were predictive factors. The use of maintenance (log-rank; p = 0.0016) seems more determinant than the duration of the treatment (log-rank; p = 0.065) in terms of progression-free survival (Figure 3). Table 4 shows the univariate Cox regression analysis with hazard ratios for the variables evaluated.

Table 4.
Cox regression model to predict tumor progression, FAS population (n = 502).
Patient sex, smoking habit, tumor multiplicity and tumor size did not appear related to tumor progression to the invasive disease. Conversely, patient age, EAU risk-group, T category, tumor grade, cis, tumor history, duration of treatment, use of maintenance therapy, former use of MMC and of BCG were entered into the stepwise model as likely determinant factors (p < 0.15). A multivariate analysis revealed that the EAU risk-group (high-risk vs. intermediate-risk; HR 3.891 (95% CI 1.886–8); p = 0.0002), previous tumor history (recurrent vs. primary; HR 3.32 (95% CI 1.613–6.833); p = 0.0011) and treatment schedule using maintenance (w/o vs. with maintenance; HR 2.374 (95% CI 1.125–5.01); p = 0.0233) independently predict progression to muscle invasive disease in patients receiving adjunct HIVEC with MMC in the present study.
3.3. Overall Survival
A Kaplan-Meier analysis revealed that patient age (log-rank; p < 0.0001), tumor grade (log-rank; p = 0.046) and duration of treatment (log-rank; p = 0.0087) were predictive factors of mortality. Table 5 shows the univariate Cox regression analysis. The sex of the patient, smoking habit, presence of cis, previous history, tumor multiplicity, tumor size, previous treatment with MMC and with BCG are not related to mortality. However, patient age, EAU risk-group, T category, grade, concomitant cis, tumor history, duration of treatment, use of maintenance therapy, former use of MMC and of BCG were entered into the stepwise model as likely determinant factors (p < 0.15). A multivariate analysis revealed that patient age (older than 70 vs. 70 or less; HR 3.356 (95% CI 1.884–5.976); p < 0.0001) and treatment duration (<4 months vs. ≥4 months; HR 1.824 (95% CI 1.095–3.039); p = 0.0211) independently predict the survival (p < 0.05) of patients with NMIBC treated with adjunct HIVEC MMC.

Table 5.
Cox regression model to predict tumor overall survival, FAS population (n = 502).
3.4. Tolerability and Safety
The frequency and severity of AEs was assessed in the SAF population (n = 592), that included patients with at least one HIVEC MMC instillation on any schedule. Globally, 406 patients (68.58%) did not suffer any AEs while 186 (31.42%) registered at least one. A single AE was registered for 130 cases, 2 AES per patient for 36, 3 AES per patient for 16 and 4 AES per patient for 4. In total, the number of AES registered was 266 for 186 patients. The AE severity was evaluated as grade 1–2 in 170 patients (28.72%) and grade 3–4 in the remaining 16 (2.7%). No case revealed toxicity grade 5. Table 6 shows the distribution of severity of AEs according to each particular AE. The most frequent AEs were dysuria (9.9%), bladder pain (7.1%), urgency (5.7%), skin rash (4.9%), spasms (3.7%) and hematuria (3.55%).

Table 6.
Frequency and severity of adverse events (AEs) for FAS population (n = 592) receiving at least one instillation of HIVEC MMC.
4. Discussion
Hyperthermia-based therapy for NMIBC is gaining traction, especially since the shortage of BCG has severely affected clinical practice in disease management worldwide. The problem of BCG shortage, and also of MMC more recently, appears to have been exacerbated in the COVID-19 pandemic, so finding a solution to the challenges in the optimalization of intravesical chemotherapy instillations is of supreme importance. In general, device-assisted therapies have gained popularity and, despite clinical evidence still not being mature, they constitute an attractive alternative to improve the efficacy of intravesical chemotherapy by enhancing cell membrane permeability to facilitate a higher penetration of the drug into the bladder and also for the direct toxic effect of heat [13,14,15]. Additionally, the release of the heat shock protein from cancer cells by chemo-hyperthermia could activate the adaptive T-cell response [16,17]. This presumed synergistic effect of hyperthermia and chemotherapy was demonstrated in vitro for several chemotherapeutic agents including MMC, epirubicin and gemcitabine [18].
The most common application of chemo-hyperthermia is as an adjuvant treatment (prophylactic) after complete TURB, with the intention to reduce the chance of tumor recurrence and progression. However, a neoadjuvant (ablative) approach can also be used in cases with a residual tumor after TURB and also for carcinoma in situ [19]. Different hyperthermia systems are available to heat the bladder, including microwave induced heating using an intravesical radiofrequency-emitting antenna incorporated in a catheter, conductive-based heating outside the bladder using a recirculating fluid system and an external radiofrequency energy source. The COMBAT ® BRS device uses a conductive aluminum heat exchanger that heats and controls the temperature at 43 °C. The first in vivo studies were conducted in the porcine model [20]. The preliminary clinical data, obtained using this system support, have shown satisfactory results both in the neoadjuvant and the adjunct setting [21,22]. Prospective trials have been specifically conducted in different populations that are currently under analysis. Highly interesting results have recently confirmed that HIVEC MMC is valuable in the high-risk NMIBC population [23,24] and also in cases of BCG failure [25,26]. The present study, based on a real-world analysis, provides additional prognostic information on the value of adjunct HIVEC MMC, both in intermediate and high-risk groups, and aims to provide a rationale for selecting specific populations that could benefit from this approach.
It is difficult to provide an indirect comparison of efficacy in the absence of control arms but the progression-free data we provide for HIVEC using BRS seem preferable to the results provided by the long-term experience with RF-induced hyperthermia combined with intravesical chemotherapy in the recent publication of Brummelhuis et al [27]. Similarly, the progression-free rate we report may also be equivalent to the results provided by long-term BCG maintenance [7,28]. However, a strict randomized comparison is necessary. A randomized study (HYMN trial) comparing RF-induced chemo-hyperthermia using MMC (6-weekly induction instillations, followed by maintenance instillations at 6-week intervals for the first year and at 8-week intervals for the second year) and BCG (induction and maintenance for one year) in patients with recurrent intermediate- and high-risk NMIBC following induction and/or maintenance BCG revealed no differences in complete response at both 3 months and in disease-free survival between the two groups [29]. The subgroup analyses in this trial have shown that patients with CIS had a lower disease-free survival with chemo-hyperthermia. Hopefully, trials with conductive chemo-hyperthermia will provide additional insight on the issue. In our experience, patients without cis, who are treated with adjunct HIVEC MMC, fare better than those with cis, both in terms of recurrence/tumor response and progression. However, in a multivariate analysis detection of cis, it is not found to be an independent predictor itself.
The current study provides real world long-term data on COMBAT BRS adjunct HIVEC MMC. In an overt clinical practice setting, both a 50.37% recurrence-free (53.3% intermediate- and 47.14% high-risk) and 89.83% (94.02% intermediate- and 84.23% high-risk) progression-free survival rate at 5 years have been observed. This treatment is generally well tolerated, with 68.58% of patients suffering no AE, and a serious toxicity is presented in only 2.7%. No case presented life-threatening toxicity. Furthermore,, this treatment did not compromise the oncological outcomes of cystectomy in cases with disease progression after HIVEC. The safety data we confirm corresponds with other recently reported experiences from other countries [30,31].
The global tolerability of HIVEC-MMC seems to be much better than that of RF-induced hyperthermia in which a rate of 94.2% patients experience at least one AE [27]. Furthermore, the frequency of cutaneous contact allergic reactions observed with HIVEC MMC, including vesicular dermatitis of the hands and feet and/or dermatitis of the genitals, or even more widespread eruptions, is notably lower than the 15.4% rate recently reported for RF-induced hyperthermia [27].
The prospective nature of this study and the consecutive inclusion of patients reduces the risks of a selection bias, information bias and the underreporting of side effects, and brings uniformity both in the chemotherapy dosage and the technique of instillation with the BRS. However, the lack of a control group (MMC in normothermia or BCG) is a very serious limitation in our study. Additionally, the heterogeneous population included as the adjunct HIVEC MMC implies some variation in the maintenance scheme and treatment duration. Nevertheless, this has allowed us to discover the very interesting finding that monthly adjunct MMC HIVEC maintenance diminishes both the recurrence and progression rate of NMIBC compared to patients in which only a weekly induction regime has been applied. Use of a maintenance schedule is an independent factor to protect for its progression to a muscle invasive disease. However, the optimal regime for maintenance therapy, especially for the intermediate-risk group, is not well defined.
Also, we confirm that for a longer treatment duration, both primary tumors and intermediate-risk disease have more favorable results both in recurrence and progression. It seems of paramount importance to define that adjunct COMBAT BRS treatment with MMC with a duration longer than 4 months is an independent prognostic factor, not only to prevent tumor recurrence but also in terms of mortality; and this finding appears critical for the design of comparative trials in the future. The long-term experience with RF-induced hyperthermia also confirms that a long-term maintenance confers better results [27].
The use of device-assisted intravesical therapy in patients in which former therapies, and more specifically BCG, have failed is an area of current and intensive investigation [32]. An additional finding in this COMBAT BRS adjunct MMC study is that the primary untreated patients benefit most from chemo-hyperthermia, a finding that is in consonance with the fact that nonprimary NMIBC also has poorer results for BCG [33]. Finally, patients at an intermediate-risk have a better response in terms of recurrence and progression than their high-risk counterparts; this finding should be analyzed and taken into account when clinical trials are designed for specific populations.
5. Conclusions
HIVEC through MMC using COMBAT BRS is an efficacious and well-tolerated alternative for patients with intermediate- and high-risk NMIBC. A maintenance schedule should be recommended as the duration of treatment is the most important independent prognostic factor. Additionally, HIVEC MMC in primary tumors and intermediate-risk patients offers the best treatment results, and this could be taken into account when the use of long-term BCG requires optimization due to shortages.
Author Contributions
Conceptualization, A.P., F.G.-R., C.G., A.G.-D., I.G.-V., J.M.d.l.M., F.J.D.-G., J.F.d.Á., V.G., J.M., A.S.-E., J.L., J.L.P., F.D., M.A., Á.P., J.C., A.R., L.M.-P., J.C.A.; investigation, A.P., F.G.-R., C.G., A.G.-D., I.G.-V., J.M.d.l.M., F.J.D.-G., J.F.d.Á., V.G., J.M., A.S.-E., J.L., J.L.P., F.D., M.A., Á.P., J.C., A.R., L.M.-P., J.C.A.; data curation, A.P., F.G.-R., C.G., A.G.-D., I.G.-V., J.M.d.l.M., F.J.D.-G., J.F.d.Á., V.G., J.M., A.S.-E., J.L., J.L.P., F.D., M.A., Á.P., J.C., A.R., L.M.-P., J.C.A.; writing—original draft preparation, J.C.A.; writing—review and editing, A.P., F.G.-R., C.G., A.G.-D., I.G.-V., J.M.d.l.M., F.J.D.-G., J.F.d.Á., V.G., J.M., A.S.-E., J.L., J.L.P., F.D., M.A., Á.P., J.C., A.R., L.M.-P., J.C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by COMBAT Medical, S.L. (Madrid, Spain).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Hospital Universitario de Getafe (protocol code A07/15 and date of approval 3 June, 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Full data will be provided upon a reasonable request to the corresponding author.
Acknowledgments
The authors acknowledge Silvia Martinez for her administrative and technical support and also Juan Dorado (PeRTICA Statistical Solutions) for his eCRF support and statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teoh, J.Y.; Huang, J.; Ko, W.Y.; Lok, V.; Choi, P.; Ng, C.F.; Sengupta, S.; Mostafid, H.; Kamat, A.M.; Black, P.C.; et al. Global Trends of Bladder Cancer Incidence and Mortality, and Their Associations with Tobacco Use and Gross Domestic Product Per Capita. Eur. Urol. 2020, 78, 893–906. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angulo, J.C.; Lopez, J.I.; Flores, N.; Toledo, J.D. The value of tumour spread, grading and growth pattern as morphological predictive parameters in bladder carcinoma. A critical revision of the 1987 TNM classification. J. Cancer Res. Clin. Oncol. 1993, 119, 578–593. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2021. [Google Scholar] [CrossRef]
- Huncharek, M.; Kupelnick, B. The influence of intravesical therapy on progression of superficial transitional cell carcinoma of the bladder: A metaanalytic comparison of chemotherapy versus bacilli Calmette-Guerin immunotherapy. Am. J. Clin. Oncol. 2004, 27, 522–528. [Google Scholar] [CrossRef]
- Gontero, P.; Sylvester, R.; Pisano, F.; Joniau, S.; Vander Eeckt, K.; Serretta, V.; Larré, S.; Di Stasi, S.; Van Rhijn, B.; Witjes, A.J.; et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guérin: Results of a retrospective multicenter study of 2451 patients. Eur. Urol. 2015, 67, 74–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.; Wymer, K.M.; Borah, B.J.; Saigal, C.S.; Litwin, M.S.; Packiam, V.T.; Thompson, R.H.; Tollefson, M.K.; Karnes, R.J.; Boorjian, S.A. Cost-Effectiveness of Maintenance bacillus Calmette-Guérin for Intermediate and High Risk Nonmuscle Invasive Bladder Cancer. J. Urol. 2020, 204, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Teoh, J.Y.; Mostafid, H. Treatment options and results of adjuvant treatment in nonmuscle-invasive bladder cancer (NMIBC) during the Bacillus Calmette-Guérin shortage. Curr. Opin. Urol. 2020, 30, 365–369. [Google Scholar] [CrossRef]
- Witjes, J.A.; Palou, J.; Soloway, M.; Lamm, D.; Kamat, A.M.; Brausi, M.; Persad, R.; Buckley, R.; Colombel, M.; Böhle, A. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guerin (BCG): Results of an international individual patient data survey (IPDS). BJU Int. 2013, 112, 742–750. [Google Scholar] [CrossRef]
- Slovacek, H.; Zhuo, J.; Taylor, J.M. Approaches to Non-Muscle-Invasive Bladder Cancer. Curr. Oncol. Rep. 2021, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Liem, E.I.M.L.; Crezee, H.; de la Rosette, J.J.; de Reijke, T.M. Chemohyperthermia in non-muscle-invasive bladder cancer: An overview of the literature and recommendations. Int. J. Hyperth. 2016, 32, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Zargar, H.; Aning, J.; Ischia, J.; So, A.; Black, P. Optimizing intravesical mitomycin C therapy in non-muscle-invasive bladder cancer. Nat. Rev. Urol. 2014, 11, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Kowal, C.D.; Kennedy, K.A.; Sartorelli, A.C. Enhancement by hyperthermia of the in vitro cytotoxicity of mitomycin C toward hypoxic tumor cells. Cancer Res. 1981, 41, 1096–1099. [Google Scholar] [PubMed]
- Chiancone, F.; Fabiano, M.; Fedelini, M.; Meccariello, C.; Carrino, M.; Fedelini, P. Outcomes and complications of Hyperthermic IntraVesical using mitomycin C or epirubicin for patients with non-muscle invasive bladder cancer after bacillus Calmette-Guérin treatment failure. Cent. Eur. J. Urol. 2020, 73, 287–294. [Google Scholar]
- Rampersaud, E.N.; Vujaskovic, Z.; Inman, B.A. Hyperthermia as a treatment for bladder cancer. Oncology 2010, 24, 1149–1155. [Google Scholar]
- Multhoff, G.; Habl, G.; Combs, S.E. Rationale of hyperthermia for radio(chemo)therapy and immune responses in patients with bladder cancer: Biological concepts, clinical data, interdisciplinary treatment decisions and biological tumour imaging. Int. J. Hyperth. 2016, 32, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, A.G.; Verhaegh, G.; Jansen, C.F.J.; Schalken, J.A.; Witjes, J.A. Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: An in vitro study. J. Urol. 2005, 173, 1375–1380. [Google Scholar] [CrossRef]
- Sousa Escandón, A.; León Mata, J.; Sousa González, D.; Alvarez Casal, M.; Rodríguez, S.; Piñeiro Vazquez, S. Neoadjuvant chemohyperthermia: Our experience after 10 years. Arch. Esp. Urol. 2018, 71, 438–446. [Google Scholar]
- Tan, W.P.; Chang, A.; Brousell, S.C.; Grimberg, D.C.; Fantony, J.J.; Longo, T.A.; Etienne, W.; Spasojevic, I.; Maccarini, P.; Inman, B.A. Safety and efficacy of intravesical chemotherapy and hyperthermia in the bladder: Results of a porcine study. Int. J. Hyperth. 2020, 37, 854–860. [Google Scholar] [CrossRef]
- Sousa, A.; Inman, B.A.; Piñeiro, I.; Monserrat, V.; Pérez, A.; Aparici, V.; Gómez, I.; Neira, P.; Uribarri, C. A clinical trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) for treating intermediate and high-risk non-muscle invasive bladder cancer. Int. J. Hyperth. 2014, 30, 166–170. [Google Scholar] [CrossRef]
- Sousa, A.; Piñeiro, I.; Rodríguez, S.; Aparici, V.; Monserrat, V.; Neira, P.; Carro, E.; Murias, C.; Uribarri, C. Recirculant hyperthermic IntraVEsical chemotherapy (HIVEC) in intermediate-high-risk non-muscle-invasive bladder cancer. Int. J. Hyperth. 2016, 32, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Ekin, R.G.; Akarken, I.; Cakmak, O.; Tarhan, H.; Celik, O.; Ilbey, Y.O.; Divrik, R.T.; Zorlu, F. Results of Intravesical Chemo-Hyperthermia in High-risk Non-muscle Invasive Bladder Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 3241–3245. [Google Scholar] [CrossRef] [Green Version]
- González-Padilla, D.A.; González-Díaz, A.; Miranda-Utrera, N.; De la Rosa Kehrmann, F.; Villacampa, F.; Guerrero-Ramos, F. HIVEC HR: Chemohyperthermia with mitomycin C vs. BCG for high-risk non-muscle invasive bladder cancer. Preliminary results from a randomized controlled trial. Eur. Urol. Suppl. 2019, 18, e768–e770. [Google Scholar] [CrossRef]
- de Jong, J.J.; Hendricksen, K.; Rosier, M.; Mostafid, H.; Boormans, J.L. Hyperthermic Intravesical Chemotherapy for BCG Unresponsive Non-Muscle Invasive Bladder Cancer Patients. Bladder Cancer 2018, 4, 395–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pijpers, O.M.; Hendricksen, K.; Mostafid, H.; de Jong, F.C.; Rosier, M.; Mayor, N.; de Jong, J.J.; Boormans, J.L. Long-term efficacy of hyperthermic intravesical chemotherapy for BCG-unresponsive non-muscle invasive bladder cancer. Urol. Oncol. 2021. [Google Scholar] [CrossRef]
- Brummelhuis, I.S.G.; Wimper, Y.; Witjes-van Os, H.G.J.M.; Arends, T.J.H.; van der Heijden, A.G.; Witjes, J.A. Long-Term Experience with Radiofrequency-Induced Hyperthermia Combined with Intravesical Chemotherapy for Non-Muscle Invasive Bladder Cancer. Cancers 2021, 13, 377. [Google Scholar] [CrossRef]
- Cambier, S.; Sylvester, R.J.; Collette, L.; Gontero, P.; Brausi, M.A.; van Andel, G.; Kirkels, W.J.; Silva, F.C.; Oosterlinck, W.; Prescott, S.; et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guérin. Eur. Urol. 2016, 69, 60–69. [Google Scholar] [CrossRef]
- Tan, W.S.; Panchal, A.; Buckley, L.; Devall, A.J.; Loubière, L.S.; Pope, A.M.; Feneley, M.R.; Cresswell, J.; Issa, R.; Mostafid, H.; et al. Radiofrequency-induced Thermo-chemotherapy Effect Versus a Second Course of Bacillus Calmette-Guérin or Institutional Standard in Patients with Recurrence of Non-muscle-invasive Bladder Cancer Following Induction or Maintenance Bacillus Calmette-Guérin Therapy (HYMN): A Phase III, Open-label, Randomised Controlled Trial. Eur. Urol. 2019, 75, 63–71. [Google Scholar] [PubMed]
- Thomsen, J.A.; Dominiak, H.N.; Lindgren, M.S.; Jensen, J.B. Adverse events of hyperthermic intravesical chemotherapy for non-muscle invasive bladder cancer patients. Scand. J. Urol. 2021, 55, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Marquette, T.; Walz, J.; Rybikowski, S.; Maubon, T.; Branger, N.; Fakhfakh, S.; Verde, K.L.; Dermeche, S.; Gravis, G.; Pignot, G. Safety of Hyperthermic IntraVEsical Chemotherapy (HIVEC) for BCG Unresponsive Non-Muscle Invasive Bladder Cancer Patients. Prog. Urol. 2020, 30, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Hendricksen, K. Device-assisted intravesical therapy for non-muscle invasive bladder cancer. Transl. Androl. Urol. 2019, 8, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, S.S.; Van Rhijn, B.W.G.; Finelli, A.; van der Kwast, T.; Evans, A.; Hanna, S.; Vajpeyi, R.; Fleshner, N.E.; Jewett, M.A.S.; Zlotta, A.R. Nonprimary pT1 nonmuscle invasive bladder cancer treated with bacillus Calmette-Guerin is associated with higher risk of progression compared to primary T1 tumors. J. Urol. 2010, 184, 81–86. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).