Stent Selection for Primary Angioplasty and Outcomes in the Era of Potent Antiplatelets. Data from the Multicenter Randomized Prague-18 Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. Endpoint Occurrence in Relation to Stent Type
3.3. Endpoint Occurrence in Relation to Stent Type in Patients Treated with Prasugrel vs. Ticagrelor
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabate, M.; Cequier, A.; Iniguez, A.; Serra, A.; Hernandez-Antolin, R.; Mainar, V.; Valgimigli, M.; Tespili, M.; den Heijer, P.; Bethencourt, A.; et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet 2012, 380, 1482–1490. [Google Scholar] [CrossRef]
- Palmerini, T.; Biondi-Zoccai, G.; Della Riva, D.; Mariani, A.; Sabate, M.; Valgimigli, M.; Frati, G.; Kedhi, E.; Smits, P.C.; Kaiser, C.; et al. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: Evidence from a comprehensive network meta-analysis. J. Am. Coll. Cardiol. 2013, 62, 496–504. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Byrne, R.A.; Alfonso, F.; Schneider, S.; Maeng, M.; Wiebe, J.; Kretov, E.; Bradaric, C.; Rai, H.; Cuesta, J.; Rivero, F.; et al. Prospective, randomized trial of bioresorbable scaffolds vs. everolimus-eluting stents in patients undergoing coronary stenting for myocardial infarction: The Intracoronary Scaffold Assessment a Randomized evaluation of Absorb in Myocardial Infarction (ISAR-Absorb MI) trial. Eur. Heart J. 2019, 40, 167–176. [Google Scholar] [PubMed]
- Serruys, P.W.; Chevalier, B.; Dudek, D.; Cequier, A.; Carrie, D.; Iniguez, A.; Dominici, M.; van der Schaaf, R.J.; Haude, M.; Wasungu, L.; et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): An interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet 2015, 385, 43–54. [Google Scholar] [PubMed]
- Ali, Z.A.; Serruys, P.W.; Kimura, T.; Gao, R.; Ellis, S.G.; Kereiakes, D.J.; Onuma, Y.; Simonton, C.; Zhang, Z.; Stone, G.W. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: A systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet 2017, 390, 760–772. [Google Scholar] [CrossRef]
- Motovska, Z.; Hlinomaz, O.; Miklik, R.; Hromadka, M.; Varvarovsky, I.; Dusek, J.; Knot, J.; Jarkovsky, J.; Kala, P.; Rokyta, R.; et al. Prasugrel Versus Ticagrelor in Patients With Acute Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention: Multicenter Randomized PRAGUE-18 Study. Circulation 2016, 134, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Motovska, Z.; Hlinomaz, O.; Kala, P.; Hromadka, M.; Knot, J.; Varvarovsky, I.; Dusek, J.; Jarkovsky, J.; Miklik, R.; Rokyta, R.; et al. 1-Year Outcomes of Patients Undergoing Primary Angioplasty for Myocardial Infarction Treated With Prasugrel Versus Ticagrelor. J. Am. Coll. Cardiol. 2018, 71, 371–381. [Google Scholar] [CrossRef]
- Pache, J.; Kastrati, A.; Mehilli, J.; Schühlen, H.; Dotzer, F.; Hausleiter, J.; Fleckenstein, M.; Neumann, F.J.; Sattelberger, U.; Schmitt, C.; et al. Intracoronary stenting and angiographic results: Strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J. Am. Coll. Cardiol. 2003, 41, 1283–1288. [Google Scholar] [CrossRef]
- Wijns, W.; Kolh, P.; Danchin, N.; Di Mario, C.; Falk, V.; Folliguet, T.; Garg, S.; Huber, K.; James, S.; Knuuti, J.; et al. Guidelines on myocardial revascularization. Eur. Heart J. 2010, 31, 2501–2555. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; Jüni, P.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar] [PubMed]
- Pilgrim, T.; Muller, O.; Heg, D.; Roffi, M.; Kurz, D.J.; Moarof, I.; Weilenmann, D.; Kaiser, C.; Tapponnier, M.; Losdat, S.; et al. Biodegradable- Versus Durable-Polymer Drug-Eluting Stents for STEMI: Final 2-Year Outcomes of the BIOSTEMI Trial. JACC Cardiovasc. Interv. 2021, 14, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Smits, P.C.; Hofma, S.; Togni, M.; Vázquez, N.; Valdés, M.; Voudris, V.; Slagboom, T.; Goy, J.J.; Vuillomenet, A.; Serra, A.; et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): A randomised, controlled, non-inferiority trial. Lancet 2013, 381, 651–660. [Google Scholar] [CrossRef]
- Dworeck, C.; Redfors, B.; Völz, S.; Haraldsson, I.; Angerås, O.; Råmunddal, T.; Ioanes, D.; Myredal, A.; Odenstedt, J.; Hirlekar, G.; et al. Radial artery access is associated with lower mortality in patients undergoing primary PCI: A report from the SWEDEHEART registry. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.V.; Kirtane, A.J.; Redfors, B.; Généreux, P.; Ben-Yehuda, O.; Palmerini, T.; Benedetto, U.; Biondi-Zoccai, G.; Smits, P.C.; von Birgelen, C.; et al. Stent-Related Adverse Events >1 Year After Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2020, 75, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Torii, S.; Jinnouchi, H.; Guo, L.; Cornelissen, A.; Kuntz, S.; Cheng, Q.; Fernandez, R.; Paek, K.H.; Harris, K.; et al. Comparison of endothelial barrier functional recovery after implantation of a novel biodegradable polymer sirolimus-eluting stent in comparison to durable and biodegradable polymer everolimus-eluting stents. Cardiovasc. Revasc. Med. 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Rymer, J.A.; Harrison, R.W.; Dai, D.; Roe, M.T.; Messenger, J.C.; Anderson, H.V.; Peterson, E.D.; Wang, T.Y. Trends in Bare-Metal Stent Use in the United States in Patients Aged ≥65 Years (from the CathPCI Registry). Am. J. Cardiol. 2016, 118, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Morice, M.C.; Urban, P.; Greene, S.; Schuler, G.; Chevalier, B. Why are we still using coronary bare-metal stents? J. Am. Coll. Cardiol. 2013, 61, 1122–1123. [Google Scholar] [CrossRef][Green Version]
- Biswas, S.; Duffy, S.J.; Lefkovits, J.; Andrianopoulos, N.; Brennan, A.; Walton, A.; Chan, W.; Noaman, S.; Shaw, J.A.; Dawson, L.; et al. Australian Trends in Procedural Characteristics and Outcomes in Patients Undergoing Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction. Am. J. Cardiol. 2018, 121, 279–288. [Google Scholar] [CrossRef]
- Sakamoto, K.; Sato, R.; Tabata, N.; Ishii, M.; Yamashita, T.; Nagamatsu, S.; Motozato, K.; Yamanaga, K.; Hokimoto, S.; Sueta, D.; et al. Temporal trends in coronary intervention strategies and the impact on one-year clinical events: Data from a Japanese multi-center real-world cohort study. Cardiovasc. Interv. Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Brugaletta, S.; Pernigotti, A.; Flores-Ulmanzor, E.; Ortega-Paz, L.; Cequier, A.; Iniguez, A.; Serra, A.; Jiménez-Quevedo, P.; Mainar, V.; et al. Does Large Vessel Size Justify Use of Bare-Metal Stents in Primary Percutaneous Coronary Intervention? Circ. Cardiovasc. Interv. 2019, 12, e007705. [Google Scholar] [CrossRef]
- Bønaa, K.H.; Mannsverk, J.; Wiseth, R.; Aaberge, L.; Myreng, Y.; Nygård, O.; Nilsen, D.W.; Kløw, N.E.; Uchto, M.; Trovik, T.; et al. Drug-Eluting or Bare-Metal Stents for Coronary Artery Disease. N. Engl. J. Med. 2016, 375, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Raj, L.; Upadyhay, R.; Clavijo, L.C.; Tun, H.; Mehra, A.; Hindoyan, A.; Matthews, R.V.; Shavelle, D.M. Factors Associated With the Use of Bare Metal Stents in Patients With ST Elevation Myocardial Infarction. Cardiovasc. Revasc. Med. 2020, 21, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Pasam, R.T.; Chava, S.; Sharma, A.; Malik, B.A.; Ayzenberg, S.; Frankel, R.; Shani, J.; Gidwani, U. Three to four years outcomes of the absorb bioresorbable vascular scaffold versus second-generation drug-eluting stent: A meta-analysis. Catheter. Cardiovasc. Interv. 2020, 95, 216–223. [Google Scholar] [CrossRef]
- Ke, J.; Zhang, H.; Huang, J.; Lv, P.; Chen, Y.; Xu, K.; Yang, W.; Tu, B. Three-year outcomes of bioresorbable vascular scaffolds versus second-generation drug-eluting stents: Meta-analysis of randomized trials. Medicine 2020, 99, e21554. [Google Scholar] [CrossRef]
- Ke, J.; Zhang, H.; Huang, J.; Lv, P.; Yan, J. Mid-term outcomes of bioresorbable vascular scaffolds vs second-generation drug-eluting stents in patients with acute coronary syndromes: A systematic review and meta-analysis. Medicine 2020, 99, e19458. [Google Scholar] [CrossRef]
- Verdoia, M.; Kedhi, E.; Suryapranata, H.; Galasso, G.; Dudek, D.; De Luca, G. Poly (l-lactic acid) bioresorbable scaffolds versus metallic drug-eluting stents for the treatment of coronary artery disease: A meta-analysis of 11 randomized trials. Catheter. Cardiovasc. Interv 2020, 96, 813–824. [Google Scholar] [CrossRef]
- Serruys, P.W.; Chevalier, B.; Sotomi, Y.; Cequier, A.; Carrié, D.; Piek, J.J.; Van Boven, A.J.; Dominici, M.; Dudek, D.; McClean, D.; et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): A 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016, 388, 2479–2491. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Escarcega, R.O.; Baker, N.C.; Benn, H.A.; Gaglia, M.A.; Torguson, R.; Waksman, R. Scaffold Thrombosis After Percutaneous Coronary Intervention With ABSORB Bioresorbable Vascular Scaffold: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Interv. 2016, 9, 12–24. [Google Scholar] [CrossRef]
- Onuma, Y.; Sotomi, Y.; Shiomi, H.; Ozaki, Y.; Namiki, A.; Yasuda, S.; Ueno, T.; Ando, K.; Furuya, J.; Igarashi, K.; et al. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: Insights from the randomised ABSORB Japan trial. EuroIntervention 2016, 12, 1090–1101. [Google Scholar] [CrossRef]
- Tamburino, C.; Latib, A.; van Geuns, R.J.; Sabate, M.; Mehilli, J.; Gori, T.; Achenbach, S.; Alvarez, M.P.; Nef, H.; Lesiak, M.; et al. Contemporary practice and technical aspects in coronary intervention with bioresorbable scaffolds: A European perspective. EuroIntervention 2015, 11, 45–52. [Google Scholar] [CrossRef]
- Byrne, R.A.; Kastrati, A.; Kufner, S.; Massberg, S.; Birkmeier, K.A.; Laugwitz, K.L.; Schulz, S.; Pache, J.; Fusaro, M.; Seyfarth, M.; et al. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: The Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur. Heart J. 2009, 30, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Kelbæk, H.; Taniwaki, M.; Ostojic, M.; Heg, D.; Baumbach, A.; von Birgelen, C.; Roffi, M.; Tüller, D.; Engstrøm, T.; et al. Biolimus-eluting stents with biodegradable polymer versus bare-metal stents in acute myocardial infarction: Two-year clinical results of the COMFORTABLE AMI trial. Circ. Cardiovasc. Interv. 2014, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Dirksen, M.T.; Spaulding, C.; Kelbaek, H.; Schalij, M.; Thuesen, L.; van der Hoeven, B.; Vink, M.A.; Kaiser, C.; Musto, C.; et al. Drug-eluting vs bare-metal stents in primary angioplasty: A pooled patient-level meta-analysis of randomized trials. Arch. Intern. Med. 2012, 172, 611–621; discussion 621–622. [Google Scholar] [CrossRef]
- Chichareon, P.; Modolo, R.; Collet, C.; Tenekecioglu, E.; Vink, M.A.; Oh, P.C.; Ahn, J.M.; Musto, C.; Díaz de la Llera, L.S.; Cho, Y.S.; et al. Efficacy and Safety of Stents in ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 74, 2572–2584. [Google Scholar] [CrossRef] [PubMed]
| (a) | |||||
| Stent | p-Value | ||||
| DES (n = 749) | BMS (n = 296) | BVS (n = 66) | |||
| Type of acute coronary syndrome | |||||
| ST elevations | 694 (92.7%) | 270 (91.2%) | 64 (97.0%) | 0.287 | |
| LBBB | 7 (0.9%) | 10 (3.4%) | 1 (1.5%) | 0.019 | |
| RBBB | 16 (2.1%) | 4 (1.4%) | 1 (1.5%) | 0.858 | |
| Without ST elevations | 40 (5.3%) | 14 (4.7%) | 1 (1.5%) | 0.452 | |
| Basic characteristics | |||||
| Gender—male | 574 (76.6%) | 223 (75.3%) | 45 (68.2%) | 0.292 | |
| Age | 61.7 (42.9; 78.1) | 62.7 (46.7; 81.5) | 56.9 (40.8; 71.9) | <0.001 | |
| BMI | 27.8 (22.3; 36.1) | 28.3 (22.7; 36.3) | 26.4 (21.2; 35.9) | 0.022 | |
| Laboratory results | |||||
| Hemoglobin | 144.0 (120; 167.0) | 144.0 (118.0;170.0) | 144.0 (118.0;170.0) | 0.510 | |
| Urea | 5.2 (3.1; 9.0) | 5.4 (3.4; 9.7) | 4.9 (2.7; 8.4) | 0.011 | |
| Creatinine | 82.0 (55.0; 124.0) | 85.0 (54.0; 136.0) | 73.0 (47.0; 106.0) | <0.001 | |
| Risk factors and comorbidities | |||||
| Hyperlipidemia | 269 (35.9%) | 93 (31.4%) | 15 (22.7%) | 0.052 | |
| Obesity | 155 (20.7%) | 53 (17.9%) | 6 (9.1%) | 0.050 | |
| Arterial hypertension | 369 (49.3%) | 164 (55.4%) | 33 (50.0%) | 0.198 | |
| Smoking | 485 (64.8%) | 179 (60.5%) | 52 (78.8%) | 0.016 | |
| Diabetes mellitus | 157 (21.0%) | 62 (20.9%) | 9 (13.6%) | 0.387 | |
| Condition after MI | 52 (6.9%) | 21 (7.1%) | 3 (4.5%) | 0.824 | |
| Condition after PCI | 47 (6.3%) | 16 (5.4%) | 3 (4.5%) | 0.840 | |
| Condition after CABG | 7 (0.9%) | 4 (1.4%) | 1 (1.5%) | 0.506 | |
| Chronic heart failure | 7 (0.9%) | 3 (1.0%) | 0 (0.0%) | 0.999 | |
| Chronic renal failure | 8 (1.1%) | 6 (2.0%) | 0 (0.0%) | 0.380 | |
| Bleeding | 2 (0.3%) | 1 (0.3%) | 0 (0.0%) | 0.999 | |
| Peripheral artery disease | 29 (3.9%) | 4 (1.4%) | 1 (1.5%) | 0.078 | |
| Killip class | 1 | 667 (89.1%) | 253 (85.5%) | 64 (97.0%) | 0.041 |
| 2 | 50 (6.7%) | 19 (6.4%) | 2 (3.0%) | ||
| 3 | 11 (1.5%) | 4 (1.4%) | 0 (0.0%) | ||
| 4 | 21 (2.8%) | 20 (6.8%) | 0 (0.0%) | ||
| (b) | |||||
| Parameter | Option | Stent | p-Value | ||
| DES (n = 749) | BMS (n = 296) | BVS (n = 66) | |||
| Coronarography and primary PCI | |||||
| TIMI flow in culprit artery after pPCI | 3 | 723 (67.3%) | 286 (26.6%) | 66 (6.1%) | 0.378 |
| <3 | 26 (72.2%) | 10 (27.8%) | 0 (0.0%) | ||
| Number of diseased coronary arteries | 1 | 381 (68.6%) | 141 (25.4%) | 33 (5.9%) | 0.643 |
| >1 | 368 (66.2%) | 155 (27.9%) | 33 (5.9%) | ||
| Left main stenosis ≥50% | Yes | 17 (50.0%) | 16 (47.1%) | 1 (2.9%) | 0.036 |
| Left main stenosis as culprit lesion | Yes | 4 (36.4%) | 7 (63.6%) | 0 (0.0%) | 0.035 |
| LAD | Yes | 332 (74.3%) | 86 (19.2%) | 29 (6.5%) | <0.001 |
| LAD and Diagonal artery | Yes | 43 (70.5%) | 15 (24.6%) | 3 (4.9%) | 0.948 |
| LCx | Yes | 80 (65.6%) | 37 (30.3%) | 5 (4.1%) | 0.521 |
| LCx and OM | Yes | 48 (63.2%) | 26 (34.2%) | 2 (2.6%) | 0.207 |
| RCA | Yes | 288 (62.9%) | 142 (31.0%) | 28 (6.1%) | 0.018 |
| Result of pPCI | Optimal | 732 (67.7%) | 284 (26.3%) | 65 (6.0%) | 0.257 |
| Suboptimal or unsuccessful | 17 (56.7%) | 12 (40.0%) | 1 (3.3%) | ||
| Stent | p-Value | BMS * | BVS * | |||||
|---|---|---|---|---|---|---|---|---|
| DES | BMS | BVS | HR (95% CI) | p | HR (95% CI) | p | ||
| 7 days | ||||||||
| PE (Death/Re-MI/Stroke/Severe bleeding/Revasc) | 19 (2.5%) | 19 (6.3%) | 2 (3.0%) | 0.011 | 2.70 (1.42–5.15) | 0.002 | 1.25 (0.29–5.39) | 0.763 |
| 30 days | ||||||||
| CV death | 12 (1.6%) | 9 (3.0%) | 1 (1.5%) | 0.303 | 1.92 (0.80–4.55) | 0.139 | 0.94 (0.12–7.23) | 0.953 |
| Re-MI | 9 (1.2%) | 3 (1.0%) | 1 (1.5%) | 0.791 | 0.85 (0.23–3.14) | 0.808 | 1.26 (0.16–10.01) | 0.822 |
| Stroke | 2 (0.3%) | 1 (0.3%) | 0 (0.0%) | 0.999 | 1.27 (0.11–14.10) | 0.841 | – | – |
| CV death/Re-MI/Stroke | 19 (2.5%) | 13 (4.4%) | 2 (3.0%) | 0.281 | 1.75 (0.86–3.55) | 0.119 | 1.20 (0.27–5.15) | 0.807 |
| Death | 14 (1.9%) | 12 (4.1%) | 1 (1.5%) | 0.101 | 2.20 (1.02–4.76) | 0.045 | 0.81 (0.11–6.13) | 0.835 |
| Stent thrombosis | 6 (0.8%) | 2 (0.7%) | 1 (1.5%) | 0.587 | 0.84 (0.17–4.19) | 0.838 | 1.89 (0.22–15.75) | 0.553 |
| Bleeding | 40 (5.3%) | 24 (8.1%) | 3 (4.5%) | 0.218 | 1.57 (0.94–2.61) | 0.079 | 0.85 (0.26–2.77) | 0.799 |
| TIMI—severe | 3 (0.4%) | 4 (1.4%) | 0 (0.0%) | 0.232 | 3.43 (0.76–15.33) | 0.106 | – | – |
| BARC—severe | 7 (0.9%) | 6 (2.0%) | 0 (0.0%) | 0.346 | 2.21 (0.74–6.58) | 0.154 | – | – |
| 365 days | ||||||||
| CV death | 20 (2.7%) | 15 (5.1%) | 1 (1.5%) | 0.119 | 1.93 (0.98–3.76) | 0.054 | 0.56 (0.07–4.18) | 0.573 |
| Re-MI | 20 (2.7%) | 8 (2.7%) | 1 (1.5%) | 0.999 | 1.03 (0.45–2.34) | 0.935 | 0.56 (0.07–4.19) | 0.575 |
| Stroke | 6 (0.8%) | 3 (1.0%) | 1 (1.5%) | 0.523 | 1.29 (0.32–5.18) | 0.713 | 1.85 (0.22–15.42) | 0.566 |
| CV death/Re-MI/Stroke | 39 (5.2%) | 25 (8.4%) | 3 (4.5%) | 0.150 | 1.66 (1.01–2.74) | 0.047 | 0.86 (0.26–2.80) | 0.810 |
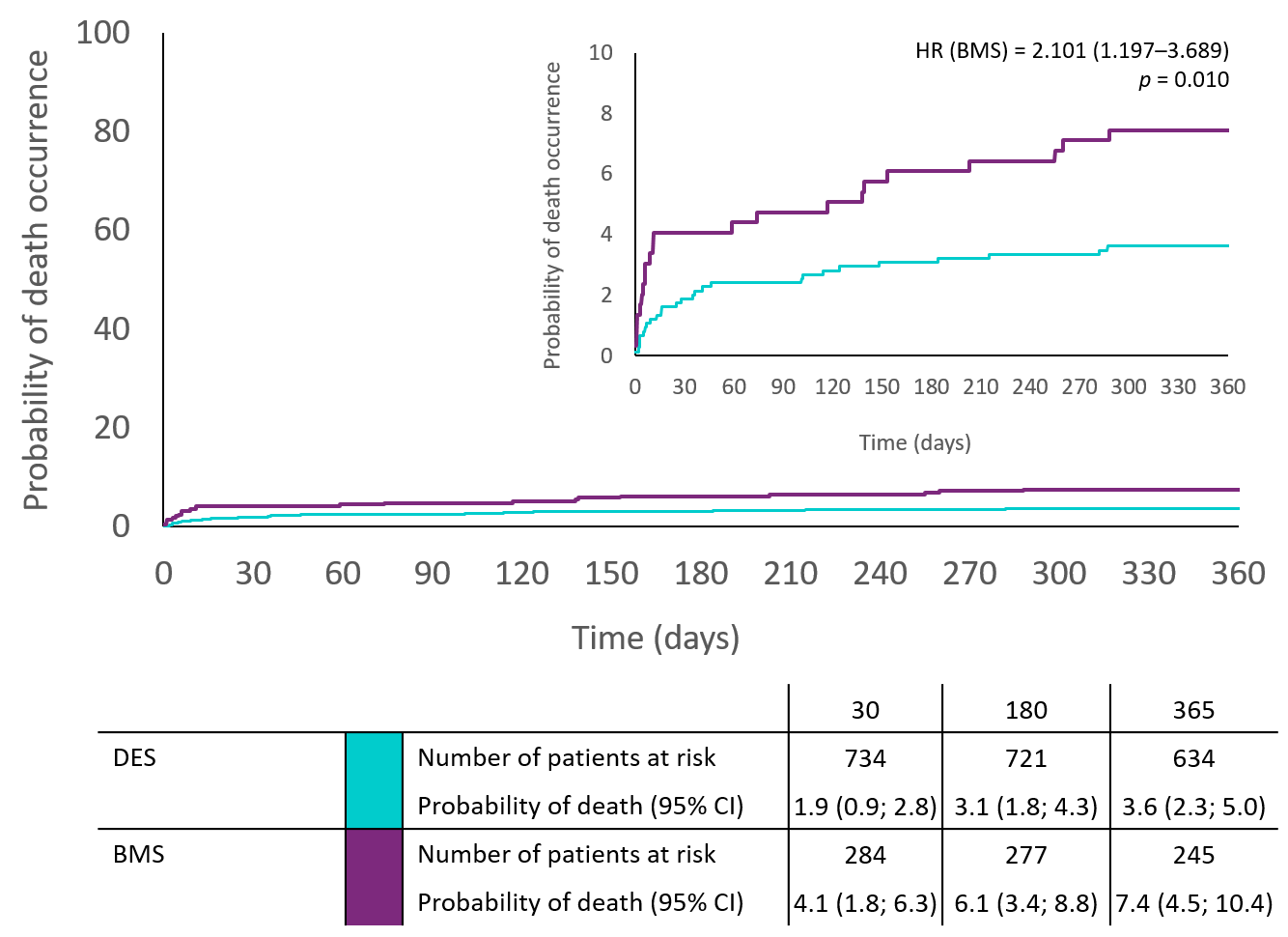
| Death | 27 (3.6%) | 22 (7.4%) | 1 (1.5%) | 0.018 | 2.10 (1.19-3.69) | 0.010 | 0.41 (0.05–3.05) | 0.388 |
| Stent thrombosis | 10 (1.3%) | 3 (1.0%) | 1 (1.5%) | 0.812 | 0.77 (0.21–2.79) | 0.690 | 1.13 (0.14–8.82) | 0.907 |
| Bleeding | 78 (10.4%) | 32 (10.8%) | 10 (15.2%) | 0.461 | 1.08 (0.71–1.62) | 0.715 | 1.45 (0.75–2.80) | 0.268 |
| TIMI—severe | 4 (0.5%) | 4 (1.4%) | 2 (3.0%) | 0.051 | 2.58 (0.64–10.32) | 0.180 | 5.63 (1.03–30.73) | 0.046 |
| BARC—severe | 12 (1.6%) | 6 (2.0%) | 2 (3.0%) | 0.453 | 1.29 (0.48–3.44) | 0.609 | 1.87 (0.41–8.36) | 0.412 |
| Stent | p-Value | BMS * | BVS * | |||||
|---|---|---|---|---|---|---|---|---|
| DES | BMS | BVS | HR (95% CI) | p | HR (95% CI) | p | ||
| Patients Randomized to Prasugrel | ||||||||
| 7 days | ||||||||
| PE (Death/Re-MI/Stroke/Severe bleeding/Revasc) | 10 (2.6%) | 9 (6.3%) | 2 (4.7%) | 0.104 | 2.74 (1.09–6.92) | 0.032 | 1.98 (0.42–9.19) | 0.380 |
| 30 days | ||||||||
| CV death | 6 (1.6%) | 5 (3.5%) | 0 (0.0%) | 0.280 | 2.30 (0.70–7.55) | 0.167 | – | – |
| Re-MI | 5 (1.3%) | 1 (0.7%) | 1 (2.3%) | 0.649 | 0.54 (0.06–4.68) | 0.583 | 1.81(0.21–15.55) | 0.586 |
| Stroke | 2 (0.5%) | 1 (0.7%) | 0 (0.0%) | 0.999 | 1.38 (0.12–15.22) | 0.792 | – | – |
| CV death/Re-MI/Stroke | 11 (2.8%) | 7 (4.9%) | 1 (2.3%) | 0.427 | 1.75 (0.67–4.51) | 0.246 | 0.82 (0.10–6.39) | 0.854 |
| Death | 7 (1.8%) | 6 (4.2%) | 0 (0.0%) | 0.203 | 2.37 (0.79–7.07) | 0.120 | – | – |
| In stent thrombosis | 2 (0.5%) | 1 (0.7%) | 1 (2.3%) | 0.314 | 1.36 (0.12–15.08) | 0.798 | 4.53(0.41–50.05) | 0.217 |
| Bleeding | 23 (5.9%) | 10 (7.0%) | 3 (7.0%) | 0.810 | 1.22 (0.58–2.56) | 0.597 | 1.20 (0.36–4.00) | 0.763 |
| TIMI—severe | 2 (0.5%) | 2 (1.4%) | 0 (0.0%) | 0.483 | 2.77 (0.39–19.73) | 0.307 | – | – |
| BARC—severe | 5 (1.3%) | 2 (1.4%) | 0 (0.0%) | 0.999 | 1.11 (0.21–5.73) | 0.898 | – | – |
| 365 days (biased by high switch rate to clopidogrel) | ||||||||
| CV death | 11 (2.8%) | 9 (6.3%) | 0 (0.0%) | 0.081 | 2.28 (0.94–5.51) | 0.066 | – | – |
| Re-MI | 12 (3.1%) | 3 (2.1%) | 1 (2.3%) | 0.913 | 0.69 (0.19–2.46) | 0.575 | 0.74 (0.09–5.70) | 0.774 |
| Stroke | 4 (1.0%) | 2 (1.4%) | 1 (2.3%) | 0.425 | 1.40 (0.25–7.67) | 0.694 | 2.19 (0.24–19.59) | 0.483 |
| CV death/Re-MI/Stroke | 23 (5.9%) | 13 (9.2%) | 2 (4.7%) | 0.398 | 1.58 (0.80–3.12) | 0.186 | 0.77 (0.18–3.28) | 0.728 |
| Death | 15 (3.9%) | 13 (9.2%) | 0 (0.0%) | 0.018 | 2.42 (1.15–5.09) | 0.019 | – | – |
| In stent thrombosis | 4 (1.0%) | 2 (1.4%) | 1 (2.3%) | 0.425 | 1.39 (0.25–7.63) | 0.699 | 2.23 (0.25–20.02) | 0.471 |
| Bleeding | 40 (10.3%) | 12 (8.5%) | 9 (20.9%) | 0.075 | 0.84 (0.44–1.61) | 0.611 | 2.069 (1.00–4.26) | 0.049 |
| TIMI—severe | 2 (0.5%) | 2 (1.4%) | 2 (4.7%) | 0.035 | 2.80 (0.39–19.88) | 0.303 | 8.90 (1.25–63.18) | 0.029 |
| BARC—severe | 7 (1.8%) | 2 (1.4%) | 2 (4.7%) | 0.325 | 0.79 (0.16–3.83) | 0.777 | 2.52 (0.52–12.15) | 0.248 |
| Patients Randomized to Ticagrelor | ||||||||
| 7 days | ||||||||
| PE (Death/Re-MI/Stroke/Severe bleeding/Revasc) | 9 (2.5%) | 10 (6.6%) | 0 (0.0%) | 0.080 | 2.65 (1.07–6.52) | 0.034 | – | – |
| 30 days | ||||||||
| CV death | 6 (1.7%) | 4 (2.6%) | 1 (4.3%) | 0.343 | 1.58 (0.44–5.60) | 0.478 | 2.61 (0.31–21.68) | 0.374 |
| Re-MI | 4 (1.1%) | 2 (1.3%) | 0 (0.0%) | 0.999 | 1.19 (0.21–6.50) | 0.839 | – | – |
| Stroke | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – | – | – | – | – |
| CV death/Re-MI/Stroke | 8 (2.2%) | 6 (3.9%) | 1 (4.3%) | 0.345 | 1.78 (0.62–5.15) | 0.282 | 1.95 (0.24–15.66) | 0.526 |
| Death | 7 (1.9%) | 6 (3.9%) | 1 (4.3%) | 0.265 | 2.04 (0.68–6.07) | 0.199 | 2.23 (0.27–18.19) | 0.451 |
| In stent thrombosis | 4 (1.1%) | 1 (0.6%) | 0 (0.0%) | 0.999 | 0.58 (0.06–5.26) | 0.636 | – | – |
| Bleeding | 17 (4.7%) | 14 (9.1%) | 0 (0.0%) | 0.090 | 2.01 (0.99–4.09) | 0.052 | – | – |
| TIMI—severe | 1 (0.3%) | 2 (1.3%) | 0 (0.0%) | 0.310 | 4.76 (0.43–52.56) | 0.202 | – | – |
| BARC—severe | 2 (0.6%) | 4 (2.6%) | 0 (0.0%) | 0.144 | 4.77 (0.87–26.08) | 0.071 | – | – |
| 365 days (biased by high switch rate to clopidogrel) | ||||||||
| CV death | 9 (2.5%) | 6 (3.9%) | 1 (4.3%) | 0.420 | 1.58 (0.56–4.44) | 0.384 | 1.74 (0.22–13.79) | 0.596 |
| Re-MI | 8 (2.2%) | 5 (3.2%) | 0 (0.0%) | 0.742 | 1.50 (0.49–4.59) | 0.475 | – | – |
| Stroke | 2 (0.6%) | 1 (0.6%) | 0 (0.0%) | 0.999 | 1.19 (0.10–13.16) | 0.885 | – | – |
| CV death/Re-MI/Stroke | 16 (4.4%) | 12(7.8%) | 1 (4.3%) | 0.294 | 1.80 (0.85–3.80) | 0.124 | 0.98 (0.13–7.38) | 0.984 |
| Death | 12 (3.3%) | 9 (5.8%) | 1 (4.3%) | 0.315 | 1.78 (0.75–4.24) | 0.188 | 1.31 (0.17–10.09) | 0.794 |
| In stent thrombosis | 6 (1.7%) | 1 (0.6%) | 0 (0.0%) | 0.765 | 0.39 (0.04–3.27) | 0.388 | – | – |
| Bleeding | 38 (10.5%) | 20 (13.0%) | 1 (4.3%) | 0.496 | 1.29 (0.75–2.22) | 0.351 | 0.39 (0.05–2.89) | 0.363 |
| TIMI—severe | 2 (0.6%) | 2 (1.3%) | 0 (0.0%) | 0.653 | 2.38 (0.33–16.91) | 0.385 | – | – |
| BARC—severe | 5 (1.4%) | 4 (2.6%) | 0 (0.0%) | 0.638 | 1.91 (0.51–7.12) | 0.333 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlinomaz, O.; Motovska, Z.; Knot, J.; Miklik, R.; Sabbah, M.; Hromadka, M.; Varvarovsky, I.; Dusek, J.; Svoboda, M.; Tousek, F.; et al. Stent Selection for Primary Angioplasty and Outcomes in the Era of Potent Antiplatelets. Data from the Multicenter Randomized Prague-18 Trial. J. Clin. Med. 2021, 10, 5103. https://doi.org/10.3390/jcm10215103
Hlinomaz O, Motovska Z, Knot J, Miklik R, Sabbah M, Hromadka M, Varvarovsky I, Dusek J, Svoboda M, Tousek F, et al. Stent Selection for Primary Angioplasty and Outcomes in the Era of Potent Antiplatelets. Data from the Multicenter Randomized Prague-18 Trial. Journal of Clinical Medicine. 2021; 10(21):5103. https://doi.org/10.3390/jcm10215103
Chicago/Turabian StyleHlinomaz, Ota, Zuzana Motovska, Jiri Knot, Roman Miklik, Mahmoud Sabbah, Milan Hromadka, Ivo Varvarovsky, Jaroslav Dusek, Michal Svoboda, Frantisek Tousek, and et al. 2021. "Stent Selection for Primary Angioplasty and Outcomes in the Era of Potent Antiplatelets. Data from the Multicenter Randomized Prague-18 Trial" Journal of Clinical Medicine 10, no. 21: 5103. https://doi.org/10.3390/jcm10215103
APA StyleHlinomaz, O., Motovska, Z., Knot, J., Miklik, R., Sabbah, M., Hromadka, M., Varvarovsky, I., Dusek, J., Svoboda, M., Tousek, F., Majtan, B., Simek, S., Branny, M., & Jarkovský, J. (2021). Stent Selection for Primary Angioplasty and Outcomes in the Era of Potent Antiplatelets. Data from the Multicenter Randomized Prague-18 Trial. Journal of Clinical Medicine, 10(21), 5103. https://doi.org/10.3390/jcm10215103







