The Emerging Role of Gut Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Current Evidence and Potential Therapeutic Applications
Abstract
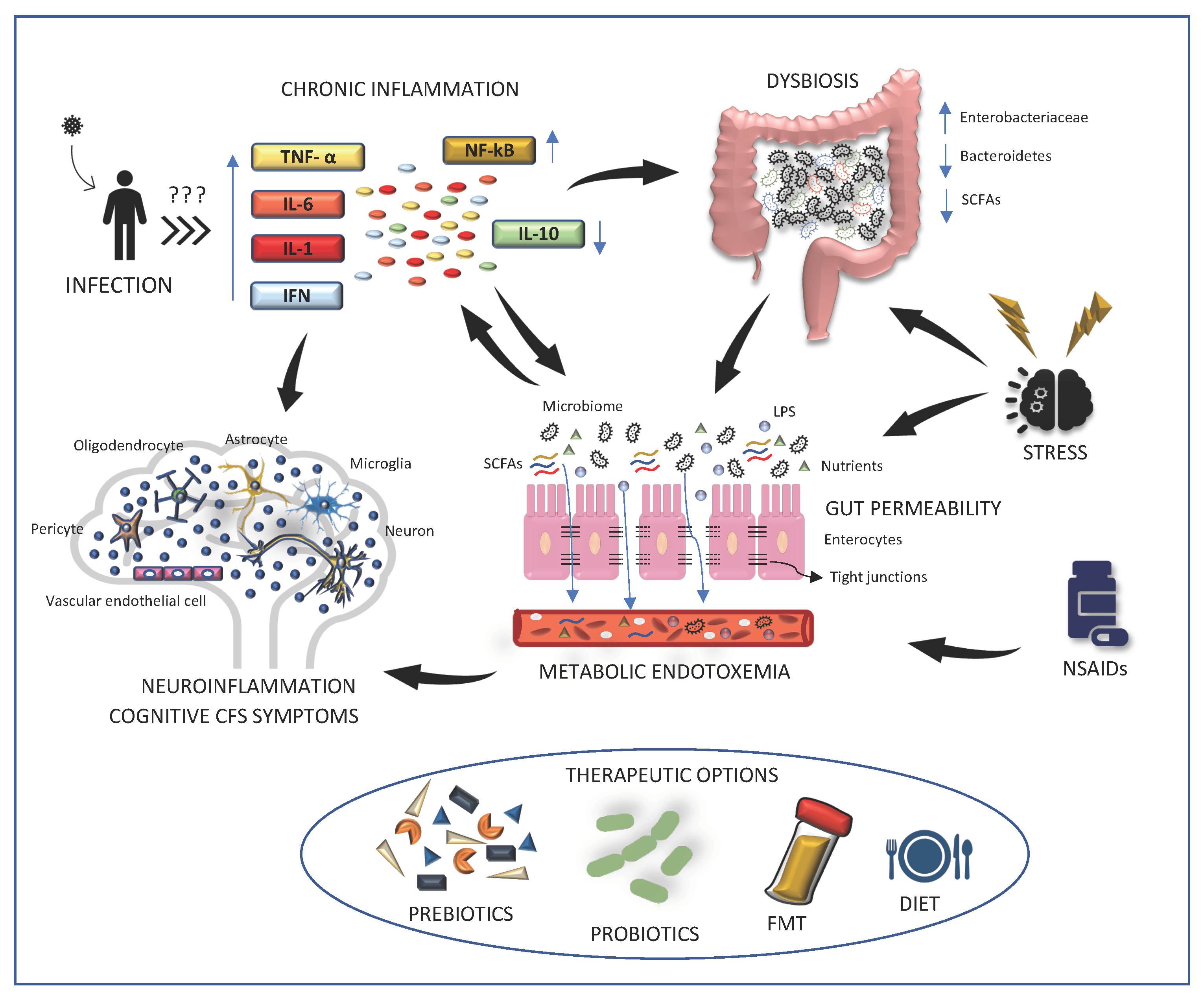
:1. Introduction
2. Main Findings
2.1. Alterations of Human Microbiome in ME/CFS
2.2. Increased Gut Permeability in ME/CFS
2.3. Oxidative Stress and Inflammation in Disease Pathogenesis
2.4. Therapies Aimed at Microbiota May Alleviate ME/CFS Symptoms
2.4.1. Probiotics
2.4.2. Prebiotics
2.4.3. Diet
2.4.4. Fecal Microbiota Transplantation (FMT)
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prins, J.B.; van der Meer, J.W.; Bleijenberg, G. Chronic Fatigue Syndrome. Lancet 2006, 367, S0140–S6736. [Google Scholar] [CrossRef]
- Sharpe, M.C.; Archard, L.C.; Banatvala, J.E.; Borysiewicz, L.K.; Clare, A.W.; David, A.; Edwards, R.H.; Hawton, K.E.; Lambert, H.P.; Lane, R.J. A Report—Chronic Fatigue Syndrome: Guidelines for Research. J. R. Soc. Med. 1991, 84, 118–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carruthers, B.; Jain, A.; de Meirleir, K.; Peterson, D.; Klimas, N.; Lerner, A.; Bested, A.; Pierre, F.; Joshi, P.; Powles, A.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Fukuda, K. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; de Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic Encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Bested, A.; Marshall, L. Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An Evidence-Based Approach to Diagnosis and Management by Clinicians. Rev. Environ. Health 2015, 30, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Mandarano, A.H.; Maya, J.; Giloteaux, L.; Peterson, D.L.; Maynard, M.; Gottschalk, C.G.; Hanson, M.R. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients Exhibit Altered T Cell Metabolism and Cytokine Associations. J. Clin. Investig. 2020, 130, 1491–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorusso, L.; Mikhaylova, S.V.; Capelli, E.; Ferrari, D.; Ngonga, G.K.; Ricevuti, G. Immunological Aspects of Chronic Fatigue Syndrome. Autoimmun. Rev. 2009, 8, 287–291. [Google Scholar] [CrossRef]
- Wirth, K.; Scheibenbogen, C. A Unifying Hypothesis of the Pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Recognitions from the Finding of Autoantibodies against SS2-Adrenergic Receptors. Autoimmun. Rev. 2020, 19, 102527. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Twisk, F.N.M.; Kubera, M.; Ringel, K. Evidence for Inflammation and Activation of Cell-Mediated Immunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Increased Interleukin-1, Tumor Necrosis Factor-α, PMN-Elastase, Lysozyme and Neopterin. J. Affect. Disord. 2012, 136, 933–939. [Google Scholar] [CrossRef]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.; Arcos-Burgos, M.; Lidbury, B. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Magnus, P.; Gunnes, N.; Tveito, K.; Bakken, I.J.; Ghaderi, S.; Stoltenberg, C.; Hornig, M.; Lipkin, W.I.; Trogstad, L.; Håberg, S.E. Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) Is Associated with Pandemic Influenza Infection, but Not with an Adjuvanted Pandemic Influenza Vaccine. Vaccine 2015, 33, 6173–6177. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Szakal, D.; Williams, B.L.; Mishra, N.; Che, X.; Lee, B.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; Levine, S.; Montoya, J.G.; et al. Fecal Metagenomic Profiles in Subgroups of Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Microbiome 2017, 5, 44. [Google Scholar] [CrossRef]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced Diversity and Altered Composition of the Gut Microbiome in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Microbiome 2016, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navaneetharaja, N.; Griffiths, V.; Wileman, T.; Carding, S. A Role for the Intestinal Microbiota and Virome in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)? J. Clin. Med. 2016, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Blomberg, J.; Gottfries, C.-G.; Elfaitouri, A.; Rizwan, M.; Rosén, A. Infection Elicited Autoimmunity and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An Explanatory Model. Front. Immunol. 2018, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, P.F.; Evengard, B.; Jacks, A.; Pedersen, N.L. Twin Analyses of Chronic Fatigue in a Swedish National Sample. Psychol. Med. 2005, 35, 1327–1336. [Google Scholar] [CrossRef]
- Glassford, J.A.G. The Neuroinflammatory Etiopathology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front. Physiol. 2017, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Hickie, I.; Bennett, B.; Lloyd, A.; Heath, A.; Martin, N. Complex Genetic and Environmental Relationships between Psychological Distress, Fatigue and Immune Functioning: A Twin Study. Psychol. Med. 1999, 29, 269–277. [Google Scholar] [CrossRef]
- Van de Putte, E.; van Doornen, L.; Engelbert, R.; Kuis, W.; Kimpen, J.; Uiterwaal, C. Mirrored Symptoms in Mother and Child with Chronic Fatigue Syndrome. Pediatrics 2006, 117, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Albright, F.; Light, K.; Light, A.; Bateman, L.; Cannon-Albright, L.A. Evidence for a Heritable Predisposition to Chronic Fatigue Syndrome. BMC Neurol. 2011, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Dibble, J.J.; McGrath, S.J.; Ponting, C.P. Genetic Risk Factors of ME/CFS: A Critical Review. Hum. Mol. Genet. 2020, 29, R118–R125. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.K.; Daly, J.S.; Thorne, G.M.; McIntosh, K. Chronic Parvovirus B19 Infection Resulting in Chronic Fatigue Syndrome: Case History and Review. Clin. Infect. Dis. 1997, 24, 1048–1051. [Google Scholar] [CrossRef] [Green Version]
- Aoki, R.; Kobayashi, N.; Suzuki, G.; Kuratsune, H.; Shimada, K.; Oka, N.; Takahashi, M.; Yamadera, W.; Iwashita, M.; Tokuno, S.; et al. Human Herpesvirus 6 and 7 Are Biomarkers for Fatigue, Which Distinguish between Physiological Fatigue and Pathological Fatigue. Biochem. Biophys. Res. Commun. 2016, 478, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Niller, H.H.; Wolf, H.; Ay, E.; Minarovits, J. Epigenetic Dysregulation of Epstein-Barr Virus Latency and Development of Autoimmune Disease. Adv. Exp. Med. Biol. 2011, 711, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R. The Role of Parvovirus B19 in the Pathogenesis of Autoimmunity and Autoimmune Disease. J. Clin. Pathol. 2016, 69, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Kerr, J.R.; Bracewell, J.; Laing, I.; Mattey, D.L.; Bernstein, R.M.; Bruce, I.N.; Tyrrell, D.A.J. Chronic Fatigue Syndrome and Arthralgia Following Parvovirus B19 Infection. J. Rheumatol. 2002, 29, 595–602. [Google Scholar]
- Seishima, M.; Mizutani, Y.; Shibuya, Y.; Arakawa, C. Chronic Fatigue Syndrome after Human Parvovirus B19 Infection without Persistent Viremia. Dermatology 2008, 216, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Cameron, B.; Flamand, L.; Juwana, H.; Middeldorp, J.; Naing, Z.; Rawlinson, W.; Ablashi, D.; Lloyd, A. Serological and Virological Investigation of the Role of the Herpesviruses EBV, CMV and HHV-6 in Post-Infective Fatigue Syndrome. J. Med. Virol. 2010, 82, 1684–1688. [Google Scholar] [CrossRef]
- Rasa, S.; Nora-Krukle, Z.; Henning, N.; Eliassen, E.; Shikova, E.; Harrer, T.; Scheibenbogen, C.; Murovska, M.; Prusty, B.K. Chronic Viral Infections in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). J. Transl. Med. 2018, 16, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Soto, N.E.; Straus, S.E. Chronic Fatigue Syndrome and Herpesviruses: The Fading Evidence. Herpes J. IHMF 2000, 7, 46–50. [Google Scholar]
- Levine, P.H.; Jacobson, S.; Pocinki, A.G.; Cheney, P.; Peterson, D.; Connelly, R.R.; Weil, R.; Robinson, S.M.; Ablashi, D.V.; Salahuddin, S.Z. Clinical, Epidemiologic, and Virologic Studies in Four Clusters of the Chronic Fatigue Syndrome. Arch. Intern. Med. 1992, 152, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, J.; Rizwan, M.; Böhlin-Wiener, A.; Elfaitouri, A.; Julin, P.; Zachrisson, O.; Rosén, A.; Gottfries, C.-G. Antibodies to Human Herpesviruses in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Front. Immunol. 2019, 10, 1946. [Google Scholar] [CrossRef] [Green Version]
- Burbelo, P.D.; Bayat, A.; Wagner, J.; Nutman, T.B.; Baraniuk, J.N.; Iadarola, M.J. No Serological Evidence for a Role of HHV-6 Infection in Chronic Fatigue Syndrome. Am. J. Transl. Res. 2012, 4, 443. [Google Scholar]
- Domingues, T.D.; Grabowska, A.D.; Lee, J.-S.; Ameijeiras-Alonso, J.; Westermeier, F.; Scheibenbogen, C.; Cliff, J.M.; Nacul, L.; Lacerda, E.M.; Mouriño, H.; et al. Herpesviruses Serology Distinguishes Different Subgroups of Patients From the United Kingdom Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Biobank. Front. Med. 2021, 8, 959. [Google Scholar] [CrossRef]
- Clauw, D.J. Perspectives on Fatigue from the Study of Chronic Fatigue Syndrome and Related Conditions. PM&R 2010, 2, 414–430. [Google Scholar] [CrossRef]
- Simani, L.; Ramezani, M.; Darazam, I.A.; Sagharichi, M.; Aalipour, M.A.; Ghorbani, F.; Pakdaman, H. Prevalence and Correlates of Chronic Fatigue Syndrome and Post-Traumatic Stress Disorder after the Outbreak of the COVID-19. J. Neurovirol. 2021, 27, 154–159. [Google Scholar] [CrossRef]
- Kennedy, G.; Khan, F.; Hill, A.; Underwood, C.; Belch, J.J.F. Biochemical and Vascular Aspects of Pediatric Chronic Fatigue Syndrome. Arch. Pediatr. Adolesc. Med. 2010, 164, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogino, S.; Nowak, J.A.; Hamada, T.; Phipps, A.I.; Peters, U.; Milner, D.A., Jr.; Giovannucci, E.L.; Nishihara, R.; Giannakis, M.; Garrett, W.S.; et al. Integrative Analysis of Exogenous, Endogenous, Tumour and Immune Factors for Precision Medicine. Gut 2018, 67, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, S.; Rodes, L.; Coussa-Charley, M.; Tomaro-Duchesneau, C.; Tomaro-Duchesneau, C.; Coussa-Charley, M. Rodes Gut Microbiota: Next Frontier in Understanding Human Health and Development of Biotherapeutics. Biol. Targets Ther. 2011, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota–Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/Brain Axis and the Microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Petersen, F.C.; Shekhar, S. Commensal Bacteria: An Emerging Player in Defense against Respiratory Pathogens. Front. Immunol. 2019, 10, 1203. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human Gut Microbiota in Obesity and after Gastric Bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [Green Version]
- Malaguarnera, L. Vitamin D and Microbiota: Two Sides of the Same Coin in the Immunomodulatory Aspects. Int. Immunopharmacol. 2020, 79, 106112. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, S.; Staines, D.; Marshall-Gradisnik, S. Epidemiological Characteristics of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis in Australian Patients. Clin. Epidemiol. 2016, 8, 97. [Google Scholar] [CrossRef] [Green Version]
- Wallis, A.; Ball, M.; McKechnie, S.; Butt, H.; Lewis, D.P.; Bruck, D. Examining Clinical Similarities between Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and d-Lactic Acidosis: A Systematic Review. J. Transl. Med. 2017, 15, 1–22. [Google Scholar] [CrossRef]
- Corbitt, M.; Campagnolo, N.; Staines, D.; Marshall-Gradisnik, S. A Systematic Review of Probiotic Interventions for Gastrointestinal Symptoms and Irritable Bowel Syndrome in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME). Probiotics Antimicrob. Proteins 2018, 10, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Riedl, A.; Schmidtmann, M.; Stengel, A.; Goebel, M.; Wisser, A.-S.; Klapp, B.F.; Mönnikes, H. Somatic Comorbidities of Irritable Bowel Syndrome: A Systematic Analysis. J. Psychosom. Res. 2008, 64, 573–582. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A. Gut Inflammation in Chronic Fatigue Syndrome. Nutr. Metab. 2010, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Mandarano, A.H.; Giloteaux, L.; Keller, B.A.; Levine, S.M.; Hanson, M.R. Eukaryotes in the Gut Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. PeerJ 2018, 6, e4282. [Google Scholar] [CrossRef] [Green Version]
- Sheedy, J.R.; Wettenhall, R.E.H.; Scanlon, D.; Gooley, P.R.; Lewis, D.P.; McGregor, N.; Stapleton, D.I.; Butt, H.L.; de Meirleir, K.L. Increased D-Lactic Acid Intestinal Bacteria in Patients with Chronic Fatigue Syndrome. In Vivo 2009, 23, 621–628. [Google Scholar] [PubMed]
- Lupo, G.F.D.; Rocchetti, G.; Lucini, L.; Lorusso, L.; Manara, E.; Bertelli, M.; Puglisi, E.; Capelli, E. Potential Role of Microbiome in Chronic Fatigue Syndrome/Myalgic Encephalomyelits (CFS/ME). Sci. Rep. 2021, 11, 7043. [Google Scholar] [CrossRef] [PubMed]
- Frémont, M.; Coomans, D.; Massart, S.; de Meirleir, K. High-Throughput 16S RRNA Gene Sequencing Reveals Alterations of Intestinal Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Anaerobe 2013, 22, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Kitami, T.; Fukuda, S.; Kato, T.; Yamaguti, K.; Nakatomi, Y.; Yamano, E.; Kataoka, Y.; Mizuno, K.; Tsuboi, Y.; Kogo, Y.; et al. Deep Phenotyping of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in Japanese Population. Sci. Rep. 2020, 10, 19933. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Cook, D.; Meyer, J.; Vernon, S.D.; Le, T.; Clevidence, D.; Robertson, C.E.; Schrodi, S.J.; Yale, S.; Frank, D.N. Changes in Gut and Plasma Microbiome Following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). PLoS ONE 2015, 10, e0145453. [Google Scholar] [CrossRef]
- Manichanh, C. Reduced Diversity of Faecal Microbiota in Crohn’s Disease Revealed by a Metagenomic Approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Hevia, A.; Milani, C.; López, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; González, S.; Suárez, A.; Gueimonde, M.; et al. Intestinal Dysbiosis Associated with Systemic Lupus Erythematosus. mBio 2014, 5, e01548-14. [Google Scholar] [CrossRef] [Green Version]
- Gianchecchi, E.; Fierabracci, A. Recent Advances on Microbiota Involvement in the Pathogenesis of Autoimmunity. Int. J. Mol. Sci. 2019, 20, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Giloteaux, L.; Hanson, M.R.; Keller, B.A. A Pair of Identical Twins Discordant for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Differ in Physiological Parameters and Gut Microbiome Composition. Am. J. Med. Case Rep. 2016, 17, 720–729. [Google Scholar] [CrossRef] [Green Version]
- Holmes, G.P. Chronic Fatigue Syndrome: A Working Case Definition. Ann. Intern. Med. 1988, 108, 387–389. [Google Scholar] [CrossRef]
- Balows, A.; Hausler, W.; Herrmann, K.; Isenberg, H.; Shadomy, H. Manual of Clinical Microbiology; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- Maes, M.; Mihaylova, I.; Leunis, J.C. Increased Serum IgA and IgM against LPS of Enterobacteria in Chronic Fatigue Syndrome (CFS): Indication for the Involvement of Gram-Negative Enterobacteria in the Etiology of CFS and for the Presence of an Increased Gut-Intestinal Permeability. J. Affect. Disord. 2007, 99, 237–240. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Oxidative and Nitrosative Stress and Immune-Inflammatory Pathways in Patients with Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Curr. Neuropharmacol. 2014, 12, 168–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Twisk, F.N.M.; Kubera, M.; Ringel, K.; Leunis, J.C.; Geffard, M. Increased IgA Responses to the LPS of Commensal Bacteria Is Associated with Inflammation and Activation of Cell-Mediated Immunity in Chronic Fatigue Syndrome. J. Affect. Disord. 2012, 136, 909–917. [Google Scholar] [CrossRef]
- Maes, M.; Leunis, J.-C. Normalization of Leaky Gut in Chronic Fatigue Syndrome (CFS) Is Accompanied by a Clinical Improvement: Effects of Age, Duration of Illness and the Translocation of LPS from Gram-Negative Bacteria. Neuro Endocrinol. Lett. 2008, 29, 902–910. [Google Scholar]
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Hashem, A.; Abd_Allah, E.F. Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment. Front. Immunol. 2019, 9, 2868. [Google Scholar] [CrossRef]
- Lemke, D.; Klement, R.J.; Schweiger, F.; Schweiger, B.; Spitz, J. Vitamin D Resistance as a Possible Cause of Autoimmune Diseases: A Hypothesis Confirmed by a Therapeutic High-Dose Vitamin D Protocol. Front. Immunol. 2021, 12, 655739. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, M.A.L.; Buchwald, M.D.S. CHRONIC FATIGUE SYNDROME: An Update. Annu. Rev. Med. 1998, 49, 1–13. [Google Scholar] [CrossRef]
- Du Preez, S.; Corbitt, M.; Cabanas, H.; Eaton, N.; Staines, D.; Marshall-Gradisnik, S. A Systematic Review of Enteric Dysbiosis in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. Syst. Rev. 2018, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Alhasson, F.; Das, S.; Seth, R.; Dattaroy, D.; Chandrashekaran, V.; Ryan, C.N.; Chan, L.S.; Testerman, T.; Burch, J.; Hofseth, L.J.; et al. Altered Gut Microbiome in a Mouse Model of Gulf War Illness Causes Neuroinflammation and Intestinal Injury via Leaky Gut and TLR4 Activation. PLoS ONE 2017, 12, e0172914. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef]
- Lucas, K.; Maes, M. Role of the Toll like Receptor (TLR) Radical Cycle in Chronic Inflammation: Possible Treatments Targeting the TLR4 Pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Munford, R. Endotoxemia-Menace, Marker, or Mistake? J. Leukoc. Biol. 2016, 100, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Leunis, J.C.; Berk, M. Increased IgA and IgM Responses against Gut Commensals in Chronic Depression: Further Evidence for Increased Bacterial Translocation or Leaky Gut. J. Affect. Disord. 2012, 141, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Mihaylova, I.; Kubera, M.; Leunis, J. An IgM-Mediated Immune Response Directed against Nitro-Bovine Serum Albumin (Nitro-BSA) in Chronic Fatigue Syndrome (CFS) and Major Depression: Evidence That Nitrosative Stress Is Another Factor Underpinning the Comorbidity between Major Depression and CFS. Neuro Endocrinol. Lett. 2008, 29, 313–319. [Google Scholar] [PubMed]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The Impact of Inflammation on Cognitive Function in Older Adults: Implications for Healthcare Practice and Research. J. Neurosci. Nurs. 2012, 44, 206–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorelick, P.B. Role of Inflammation in Cognitive Impairment: Results of Observational Epidemiological Studies and Clinical Trials. Ann. N.Y. Acad. Sci. 2010, 1207, 155–162. [Google Scholar] [CrossRef]
- Slyepchenko, A.; Maes, M.; Jacka, F.N.; Köhler, C.A.; Barichello, T.; McIntyre, R.S.; Berk, M.; Grande, I.; Foster, J.A.; Vieta, E.; et al. Gut Microbiota, Bacterial Translocation, and Interactions with Diet: Pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychother. Psychosom. 2016, 86, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Maes, M.; Berk, M.; Puri, B.K. Myalgic Encephalomyelitis or Chronic Fatigue Syndrome: How Could the Illness Develop? Metab. Brain Dis. 2019, 34, 385–415. [Google Scholar] [CrossRef] [Green Version]
- Sotzny, F.; Blanco, J.; Capelli, E.; Castro-Marrero, J.; Steiner, S.; Murovska, M.; Scheibenbogen, C. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome—Evidence for an Autoimmune Disease. Autoimmun. Rev. 2018, 17, 601–609. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.; Sanz, Y.; Maes, M. The Role of Microbiota and Intestinal Permeability in the Pathophysiology of Autoimmune and Neuroimmune Processes with an Emphasis on Inflammatory Bowel Disease Type 1 Diabetes and Chronic Fatigue Syndrome. Curr. Pharm. Des. 2016, 22, 6058–6075. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Galecki, P.; Maes, M. The Emerging Role of Autoimmunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/Cfs). Mol. Neurobiol. 2014, 49, 741–756. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sido, B.; Hack, V.; Hochlehnert, A.; Lipps, H.; Herfarth, C.; Dröge, W. Impairment of Intestinal Glutathione Synthesis in Patients with Inflammatory Bowel Disease. Gut 1998, 42, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Puri, B.K.; Walker, A.J.; Maes, M.; Carvalho, A.F.; Walder, K.; Mazza, C.; Berk, M. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: From Pathophysiological Insights to Novel Therapeutic Opportunities. Pharmacol. Res. 2019, 148, 104450. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Nojima, J.; Motoki, Y.; Yamaguti, K.; Nakatomi, Y.; Okawa, N.; Fujiwara, K.; Watanabe, Y.; Kuratsune, H. A Potential Biomarker for Fatigue: Oxidative Stress and Anti-Oxidative Activity. Biol. Psychol. 2016, 118, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Mihaylova, I.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Increased 8-Hydroxy-Deoxyguanosine, a Marker of Oxidative Damage to DNA, in Major Depression and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Neuro Endocrinol. Lett. 2009, 30, 715–722. [Google Scholar]
- Monro, J.A.; Puri, B.K. A Molecular Neurobiological Approach to Understanding the Aetiology of Chronic Fatigue Syndrome (Myalgic Encephalomyelitis or Systemic Exertion Intolerance Disease) with Treatment Implications. Mol. Neurobiol. 2018, 55, 7377–7388. [Google Scholar] [CrossRef] [Green Version]
- Ivashkin, V.; Poluektov, Y.; Kogan, E.; Shifrin, O.; Sheptulin, A.; Kovaleva, A.; Kurbatova, A.; Krasnov, G.; Poluektova, E. Disruption of the Pro-Inflammatory, Anti-Inflammatory Cytokines and Tight Junction Proteins Expression, Associated with Changes of the Composition of the Gut Microbiota in Patients with Irritable Bowel Syndrome. PLoS ONE 2021, 16, e0252930. [Google Scholar] [CrossRef]
- Raijmakers, R.P.H.; Roerink, M.E.; Jansen, A.F.M.; Keijmel, S.P.; Gacesa, R.; Li, Y.; Joosten, L.A.B.; van der Meer, J.W.M.; Netea, M.G.; Bleeker-Rovers, C.P.; et al. Multi-Omics Examination of Q Fever Fatigue Syndrome Identifies Similarities with Chronic Fatigue Syndrome. J. Transl. Med. 2020, 18, 448. [Google Scholar] [CrossRef]
- Maes, M. A New Case Definition of Neuro-Inflammatory and Oxidative Fatigue (NIOF), a Neuroprogressive Disorder, Formerly Known as Chronic Fatigue Syndrome or Myalgic Encephalomyelitis: Results of Multivariate Pattern Recognition Methods and External Validation by Neuro-Immune Biomarkers. Neuro Endocrinol. Lett. 2015, 36, 320–329. [Google Scholar]
- Borton, M.A.; Sabag-Daigle, A.; Wu, J.; Solden, L.M.; O’Banion, B.S.; Daly, R.A.; Wolfe, R.A.; Gonzalez, J.F.; Wysocki, V.H.; Ahmer, B.M.M.; et al. Chemical and Pathogen-Induced Inflammation Disrupt the Murine Intestinal Microbiome. Microbiome 2017, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.G.; Lee, D.S.; Son, C.G. Oxidative Stress Is a Convincing Contributor to Idiopathic Chronic Fatigue. Sci. Rep. 2018, 8, 12890. [Google Scholar] [CrossRef]
- Maes, M.; Twisk, F. Why Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) May Kill You: Disorders in the Inflammatory and Oxidative and Nitrosative Stress (IO&NS) Pathways May Explain Cardiovascular Disorders in ME/CFS. Neuro Endocrinol. Lett. 2009, 30, 677–693. [Google Scholar]
- Logan, A.C.; Rao, A.V.; Irani, D. Chronic Fatigue Syndrome: Lactic Acid Bacteria May Be of Therapeutic Value. Med. Hypotheses 2003, 60, 915–923. [Google Scholar] [CrossRef]
- Sullivan, Å.; Nord, C.E.; Evengård, B. Effect of Supplement with Lactic-Acid Producing Bacteria on Fatigue and Physical Activity in Patients with Chronic Fatigue Syndrome. Nutr. J. 2009, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campagnolo, N.; Johnston, S.; Collatz, A.; Staines, D.; Marshall-Gradisnik, S. Dietary and Nutrition Interventions for the Therapeutic Treatment of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: A Systematic Review. J. Hum. Nutr. Diet. 2017, 30, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hod, K.; Sperber, A.D.; Ron, Y.; Boaz, M.; Dickman, R.; Berliner, S.; Halpern, Z.; Maharshak, N.; Dekel, R. A Double-Blind, Placebo-Controlled Study to Assess the Effect of a Probiotic Mixture on Symptoms and Inflammatory Markers in Women with Diarrhea-Predominant IBS. Neurogastroenterol. Motil. 2017, 29, e13037. [Google Scholar] [CrossRef]
- Ishaque, S.M.; Khosruzzaman, S.M.; Ahmed, D.S.; Sah, M.P. A Randomized Placebo-Controlled Clinical Trial of a Multi-Strain Probiotic Formulation (Bio-Kult®) in the Management of Diarrhea-Predominant Irritable Bowel Syndrome. BMC Gastroenterol. 2018, 18, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francavilla, R.; Piccolo, M.; Francavilla, A.; Polimeno, L.; Semeraro, F.; Cristofori, F.; Castellaneta, S.; Barone, M.; Indrio, F.; Gobbetti, M.; et al. Clinical and Microbiological Effect of a Multispecies Probiotic Supplementation in Celiac Patients with Persistent IBS-Type Symptoms: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Clin. Gastroenterol. 2019, 53, E117–E125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leventogiannis, K.; Gkolfakis, P.; Spithakis, G.; Tsatali, A.; Pistiki, A.; Sioulas, A.; Giamarellos-Bourboulis, E.J.; Triantafyllou, K. Effect of a Preparation of Four Probiotics on Symptoms of Patients with Irritable Bowel Syndrome: Association with Intestinal Bacterial Overgrowth. Probiotics Antimicrob. Proteins 2019, 11, 627–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.H.; Jang, Y.S.; Kang, D.; Chang, D.K.; Min, Y.W. Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, E.D.; Antony, J.M.; Crowley, D.C.; Piano, A.; Bhardwaj, R.; Tompkins, T.A.; Evans, M. Efficacy of Lactobacillus Paracasei Ha-196 and Bifidobacterium Longum R0175 in Alleviating Symptoms of Irritable Bowel Syndrome (IBS): A Randomized, Placebo-Controlled Study. Nutrients 2020, 12, 1159. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-Zúñiga, V.; Llop, E.; Suárez, C.; Álvarez, B.; Abreu, L.; Espadaler, J.; Serra, J. I. 31, a New Combination of Probiotics, Improves Irritable Bowel Syndrome-Related Quality of Life. World J. Gastroenterol. 2014, 20, 8709–8716. [Google Scholar] [CrossRef]
- Skrzydło-Radomańska, B.; Prozorow-Król, B.; Cichoż-Lach, H.; Majsiak, E.; Bierła, J.B.; Kosikowski, W.; Szczerbiński, M.; Gantzel, J.; Cukrowska, B. The Effectiveness of Synbiotic Preparation Containing Lactobacillus and Bifidobacterium Probiotic Strains and Short Chain Fructooligosaccharides in Patients with Diarrhea Predominant Irritable Bowel Syndrome—a Randomized Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, 1999. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.; Hall, G.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.; Martin, F.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium Longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459. [Google Scholar] [CrossRef]
- Yuan, F.; Ni, H.; Asche, C.; Kim, M.; Walayat, S.; Ren, J. Efficacy of Bifidobacterium Infantis 35624 in Patients with Irritable Bowel Syndrome: A Meta-Analysis. Curr. Med. Res. Opin. 2017, 33, 1191–1197. [Google Scholar] [CrossRef]
- Andresen, V.; Gschossmann, J.; Layer, P. Heat-Inactivated Bifidobacterium Bifidum MIMBb75 (SYN-HI-001) in the Treatment of Irritable Bowel Syndrome: A Multicentre, Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Lancet Gastroenterol. Hepatol. 2020, 5, 658–666. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, W.R.; Wang, X.H.; Li, G.Q.; Xu, L.Q.; Cui, X.; Liu, Y.; Zuo, X.L. Clostridium Butyricum Alleviates Intestinal Low-Grade Inflamm TNBS-Induced Irritable Bowel Syndrome in Mice by Regulating Functional Status of Lamina Propria Dendritic Cells. World J. Gastroenterol. 2019, 25, 5469–5482. [Google Scholar] [CrossRef]
- Basturk, A.; Artan, R.; Yilmaz, A. Efficacy of Synbiotic, Probiotic, and Prebiotic Treatments for Irritable Bowel Syndrome in Children: A Randomized Controlled Trial. Turk. J. Gastroenterol. 2020, 27, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Jeong, J.J.; Woo, K.H.; Han, M.J.; Kim, D.H. Lactobacillus Sakei OK67 Ameliorates High-Fat Diet-Induced Blood Glucose Intolerance and Obesity in Mice by Inhibiting Gut Microbiota Lipopolysaccharide Production and Inducing Colon Tight Junction Protein Expression. Nutr. Res. 2016, 36, 337–348. [Google Scholar] [CrossRef]
- Venturini, L.; Bacchi, S.; Capelli, E.; Lorusso, L.; Ricevuti, G.; Cusa, C. Modification of Immunological Parameters, Oxidative Stress Markers, Mood Symptoms, and Well-Being Status in CFS Patients after Probiotic Intake: Observations from a Pilot Study. Oxid. Med. Cell. Longev. 2019, 2019, 1684198. [Google Scholar] [CrossRef] [Green Version]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Bifidobacterium Infantis 35624 Modulates Host Inflammatory Processes beyond the Gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [Green Version]
- Caswell, A.; Daniels, J. Anxiety and Depression in Chronic Fatigue Syndrome: Prevalence and Effect on Treatment. A Systematic Review, Meta-Analysis and Meta-Regression; British Association of Behavioural and Cognitive Psychotherapy: Glasgow, UK, 2018. [Google Scholar]
- Karakula-Juchnowicz, H.; Rog, J.; Juchnowicz, D.; Łoniewski, I.; Skonieczna-Ydecka, K.; Krukow, P.; Futyma-Jedrzejewska, M.; Kaczmarczyk, M. The Study Evaluating the Effect of Probiotic Supplementation on the Mental Status, Inflammation, and Intestinal Barrier in Major Depressive Disorder Patients Using Gluten-Free or Gluten-Containing Diet (SANGUT Study): A 12-Week, Randomized, Double-Blind, and Placebo-Controlled Clinical Study Protocol. Nutr. J. 2019, 18, 50. [Google Scholar] [CrossRef] [Green Version]
- Griffith, J.; Zarrouf, F. A Systematic Review of Chronic Fatigue Syndrome: Don’t Assume It’s Depression. Prim. Care Companion J. Clin. Psychiatry 2008, 10, 120–128. [Google Scholar] [CrossRef]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A Randomized, Double-Blind, Placebo-Controlled Pilot Study of a Probiotic in Emotional Symptoms of Chronic Fatigue Syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudry, G.; Hamilton, M.K.; Chichlowski, M.; Wickramasinghe, S.; Barile, D.; Kalanetra, K.M.; Mills, D.A.; Raybould, H.E. Bovine Milk Oligosaccharides Decrease Gut Permeability and Improve Inflammation and Microbial Dysbiosis in Diet-Induced Obese Mice. J. Dairy Sci. 2017, 100, 2471–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.; Wang, Y.; Chen, X.; Xiong, W.; Tang, Y.; Lin, L. Spirulina Platensis Alleviates Chronic Inflammation with Modulation of Gut Microbiota and Intestinal Permeability in Rats Fed a High-Fat Diet. J. Cell. Mol. Med. 2020, 24, 8603–8613. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, T.; Meng, Y.; Hu, M.; Shu, L.; Jiang, H.; Gao, R.; Ma, J.; Wang, C.; Zhou, X. FOS/GOS Attenuates High-Fat Diet Induced Bone Loss via Reversing Microbiota Dysbiosis, High Intestinal Permeability and Systemic Inflammation in Mice. Metab. Clin. Exp. 2021, 119, 154767. [Google Scholar] [CrossRef]
- Cani, P.; Possemiers, S.; van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, L.; et al. Changes in Gut Microbiota Control Inflammation in Obese Mice through a Mechanism Involving GLP-2-Driven Improvement of Gut Permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Nettleton, J.E.; Klancic, T.; Schick, A.; Choo, A.C.; Shearer, J.; Borgland, S.L.; Chleilat, F.; Mayengbam, S.; Reimer, R.A. Low-Dose Stevia (Rebaudioside A) Consumption Perturbs Gut Microbiota and the Mesolimbic Dopamine Reward System. Nutrients 2019, 11, 1248. [Google Scholar] [CrossRef] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingbeil, E.; De, C.B.; Serre, L. Microbiota Modulation by Eating Patterns and Diet Composition: Impact on Food Intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1254–R1260. [Google Scholar] [CrossRef] [Green Version]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; de Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2021, 13, 7. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Hausken, T. Diet in Irritable Bowel Syndrome (IBS): Interaction with Gut Microbiota and Gut Hormones. Nutrients 2019, 11, 1824. [Google Scholar] [CrossRef] [Green Version]
- Varjú, P.; Farkas, N.; Hegyi, P.; Garami, A.; Szabó, I.; Illés, A.; Solymár, M.; Vincze, Á.; Balaskó, M.; Pár, G.; et al. Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols (FODMAP) Diet Improves Symptoms in Adults Suffering from Irritable Bowel Syndrome (IBS) Compared to Standard IBS Diet: A Meta-Analysis of Clinical Studies. PLoS ONE 2017, 12, e0182942. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, R.; Andreozzi, P.; Zito, F.P.; Passananti, V.; de Carlo, G.; Sarnelli, G. Irritable Bowel Syndrome and Food Interaction. World J. Gastroenterol. 2014, 8837–8845. [Google Scholar]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut Microbiome and Serum Metabolome Alterations in Obesity and after Weight-Loss Intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Suskind, D.L.; Lee, D.; Kim, Y.M.; Wahbeh, G.; Singh, N.; Braly, K.; Nuding, M.; Nicora, C.D.; Purvine, S.O.; Lipton, M.S.; et al. The Specific Carbohydrate Diet and Diet Modification as Induction Therapy for Pediatric Crohn’s Disease: A Randomized Diet Controlled Trial. Nutrients 2020, 12, 3749. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional Psychiatry: The Present State of the Evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Snelson, M.; Clarke, R.E.; Nguyen, T.; Penfold, S.A.; Forbes, J.M.; Tan, S.M.; Coughlan, M.T. Long Term High Protein Diet Feeding Alters the Microbiome and Increases Intestinal Permeability, Systemic Inflammation and Kidney Injury in Mice. Mol. Nutr. Food Res. 2021, 65, e2000851. [Google Scholar] [CrossRef] [PubMed]
- Do, M.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [Green Version]
- Nilholm, C.; Roth, B.; Ohlsson, B. A Dietary Intervention with Reduction of Starch and Sucrose Leads to Reduced Gastrointestinal and Extra-Intestinal Symptoms in IBS Patients. Nutrients 2019, 11, 1662. [Google Scholar] [CrossRef] [Green Version]
- Ott, B.; Skurk, T.; Hastreiter, L.; Lagkouvardos, I.; Fischer, S.; Büttner, J.; Kellerer, T.; Clavel, T.; Rychlik, M.; Haller, D.; et al. Effect of Caloric Restriction on Gut Permeability, Inflammation Markers, and Fecal Microbiota in Obese Women. Sci. Rep. 2017, 7, 11955. [Google Scholar] [CrossRef]
- Kaliannan, K.; Wang, B.; Li, X.Y.; Kim, K.J.; Kang, J.X. A Host-Microbiome Interaction Mediates the Opposing Effects of Omega-6 and Omega-3 Fatty Acids on Metabolic Endotoxemia. Sci. Rep. 2015, 5, 11276. [Google Scholar] [CrossRef] [Green Version]
- Puri, B.K. The Use of Eicosapentaenoic Acid in the Treatment of Chronic Fatigue Syndrome. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 399–401. [Google Scholar] [CrossRef]
- Puri, B.K. Long-Chain Polyunsaturated Fatty Acids and the Pathophysiology of Myalgic Encephalomyelitis (Chronic Fatigue Syndrome). J. Clin. Pathol. 2007, 60, 122–124. [Google Scholar] [CrossRef]
- Wang, J.H.; Bose, S.; Kim, G.C.; Hong, S.U.; Kim, J.H.; Kim, J.E.; Kim, H. Flos Lonicera Ameliorates Obesity and Associated Endotoxemia in Rats through Modulation of Gut Permeability and Intestinal Microbiota. PLoS ONE 2014, 9, e86117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Li, Q.; Zang, Y.; Zhao, Y.; Liu, N.; Wang, Y.; Xu, X.; Liu, L.; Mei, Q. Apple Polysaccharide Inhibits Microbial Dysbiosis and Chronic Inflammation and Modulates Gut Permeability in HFD-Fed Rats. Int. J. Biol. Macromol. 2017, 99, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Usui, I.; Nawaz, A.; Igarashi, Y.; Okabe, K.; Furusawa, Y.; Watanabe, S.; Yamamoto, S.; Sasahara, M.; Watanabe, Y.; et al. Bofutsushosan Improves Gut Barrier Function with a Bloom of Akkermansia Muciniphila and Improves Glucose Metabolism in Mice with Diet-Induced Obesity. Sci. Rep. 2020, 10, 5544. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, Y.; Zhang, Y.; Li, S.; Sun, Y.; Chen, Z.; Teng, L.; Wang, D. Antifatigue Potential Activity of Sarcodon Imbricatus in Acute Excise-Treated and Chronic Fatigue Syndrome in Mice via Regulation of Nrf2-Mediated Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 9140896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.A.; Kim, Y.S.; Kim, S.K.; Baek, S.H.; Kim, H.K.; Lee, H.S. Antioxidant and Antifatigue Effect of a Standardized Fraction (HemoHIM) from Angelica Gigas, Cnidium Officinale, and Paeonia Lactiflora. Pharm. Biol. 2021, 59, 391–400. [Google Scholar] [CrossRef]
- Tan, P.; Li, X.; Shen, J.; Feng, Q. Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease: An Update. Front. Pharmacol. 2020, 11, 574533. [Google Scholar] [CrossRef]
- Gupta, A.; Khanna, S. Fecal Microbiota Transplantation. JAMA 2017, 318, 102. [Google Scholar] [CrossRef] [PubMed]
- Rodiño-Janeiro, B.K.; Vicario, M.; Alonso-Cotoner, C.; Pascua-García, R.; Santos, J. A Review of Microbiota and Irritable Bowel Syndrome: Future in Therapies. Adv. Ther. 2018, 35, 289–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium Difficile Infections. Am. J. Gastroenterol. 2013, 108, 478–498. [Google Scholar] [CrossRef] [PubMed]
- Malnick, S.D.H.; Fisher, D.; Somin, M.; Neuman, M.G. Treating the Metabolic Syndrome by Fecal Transplantation—Current Status. Biology 2021, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.H.; Cho, Y.S. Fecal Microbiota Transplantation: Current Applications, Effectiveness, and Future Perspectives. Clin. Endosc. 2016, 49, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.E.; Garborg, K.; Bretthauer, M.; Skudal, H.; Øines, M.N.; Wiig, H.; Rose, Ø.; Seip, B.; Lamont, J.T.; Midtvedt, T.; et al. Fecal Microbiota Transplantation for Primary Clostridium Difficile Infection. N. Engl. J. Med. 2018, 378, 2535–2536. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Wu, J.; Jin, D.; Wang, B.; Cao, H. Fecal Microbiota Transplantation in Cancer Management: Current Status and Perspectives. Int. J. Cancer 2019, 145, 2021–2031. [Google Scholar] [CrossRef] [Green Version]
- Evrensel, A.; Ceylan, M.E. Fecal Microbiota Transplantation and Its Usage in Neuropsychiatric Disorders. Clin. Psychopharmacol. Neurosci. 2016, 14, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.Q.; Cao, H.L.; Wang, W.Q.; Wang, S.; Cao, X.C.; Yan, F.; Wang, B.M. Fecal Microbiota Transplantation Broadening Its Application beyond Intestinal Disorders. World J. Gastroenterol. 2015, 21, 102–111. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.; Kim, H.; Choi, H.; Hyun, D.; et al. Transfer of a Healthy Microbiota Reduces Amyloid and Tau Pathology in an Alzheimer’s Disease Animal Model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Wang, Y.; Wiesnoski, D.H.; Helmink, B.A.; Gopalakrishnan, V.; Choi, K.; DuPont, H.L.; Jiang, Z.D.; Abu-Sbeih, H.; Sanchez, C.A.; Chang, C.C.; et al. Fecal Microbiota Transplantation for Refractory Immune Checkpoint Inhibitor-Associated Colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between Intestinal Microbiota and Ulcerative Colitis: Mechanisms and Clinical Application of Probiotics and Fecal Microbiota Transplantation. World J. Gastroenterol. 2018, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Borody, T. Bacteriotherapy for Chronic Fatigue Syndrome: A Long-Term Follow up Study. In Proceedings of the 1995 CFS National Consensus Conference; 1995. [Google Scholar]
- Borody, T.J.; Nowak, A.; Finlayson, S. The GI Microbiome and Its Role in Chronic Fatigue Syndrome: A Summary of BacteriotherapyThe GI Microbiome and Its Role in Chronic Fatigue Syndrome: A Summary of Bacteriotherapy. ACNEM J. 2012, 31, 3–8. [Google Scholar]
- Schmulson, M.; Bashashati, M. Fecal Microbiota Transfer for Bowel Disorders: Efficacy or Hype? Curr. Opin. Pharmacol. 2018, 43, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.; Ianiro, G.; Allegretti, J.; Bibbò, S.; Gasbarrini, A.; Scaldaferri, F.; Cammarota, G. Fecal Transplantation for Ulcerative Colitis: Current Evidence and Future Applications. Expert Opin. Biol. Ther. 2020, 20, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F.; Quigley, E. Manipulation of the Microbiota for Treatment of IBS and IBD-Challenges and Controversies. Gastroenterology 2014, 146, 1554–1563. [Google Scholar] [CrossRef]
- Imdad, A.; Nicholson, M.; Tanner-Smith, E.; Zackular, Z.; Gomez-Duarte, O.; Beaulieu, D.; Acra, S. Fecal Transplantation for Treatment of Inflammatory Bowel Disease. Cochrane Database Syst. Rev. 2018, 11, CD012774. [Google Scholar] [CrossRef]
- Aroniadis, O.C.; Brandt, L.J. Fecal Microbiota Transplantation: Past, Present and Future. Curr. Opin. Gastroenterol. 2013, 29, 79–84. [Google Scholar] [CrossRef]
- Levy, A.N.; Allegretti, J.R. Insights into the Role of Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease. Ther. Adv. Gastroenterol. 2019, 12, 1756284819836893. [Google Scholar] [CrossRef] [Green Version]
| Reference | Journal | Participants | Classification Criteria | Analysis Performed | Results |
|---|---|---|---|---|---|
| Giloteaux et al., 2016 [71] | Am Jour Case Rep | A pair of 34 year old monozygotic male twins, 1 ME/CFS and 1 control | Fukuda (1994) [4] | Two-day CPET; stool biochemical and molecular analysis; 16S RNA sequencing | ↓ Microbial diversity ↓ Faecalibacterium and Bifidobacterium |
| Shukla et al., 2015 [66] | PLOS One | 10 ME/CFS and 10 matched healthy controls | Fukuda (1994) [4] | Maximal exercise challenge, stool examination before and 15 min, 48 h, 72 h after exercise. PCR and 16S rRNA sequence | ↑ Abundance changes of major bacterial phyla (after exercise) ↓ Bacterial clearance (after exercise) |
| Kitami et al., 2020 [65] | Sci Rep | 48 ME/CFS and 52 controls | Fukuda (1994) [4] and International Consensus Criteria (2011) [5] | Stool microbiome analysis by DNA extraction and 16S rRNA sequencing | ↑ Coprobacillus, Eggerthella and Blautia |
| Mandarano et al., 2018 [61] | PeerJ | 49 ME/CFS and 39 healthy controls | Fukuda (2004) [4] | 18S rRNA sequencing in stool samples | ↓ Eukaryotic diversity (nonsignificant) ↑ Basidiomycota/Ascomycota ratio (nonsignificant) |
| Nagy-Szakal et al., 2017 [13] | Microbiome | 50 ME/CFS and 50 matched healthy controls | Fukuda (2004) [4] and/or Canadian Criteria (2003) [3] | Fecal bacterial metagenomics (shotgun metagenomic sequences) | ↑ Dysbiosis ↑ Alistipes (in ME/CFS with IBS), Bacteroides (in ME/CFS without IBS) ↓ Faecalibacterium (in ME/CFS with IBS), Bacteroides vulgatus (in ME/CFS without IBS) |
| Lupo et al., 2021 [63] | Sci Rep | 35 ME/CFS and 70 healthy controls (35 had relatives with ME/CFS and 35 not) | Fukuda (2004) [4] | Fecal bacterial analysis by 16S rRNA Illumina sequencing | ↓ Anaerostipes (Lachnospiraceae) ↑ Bacteroides and Phascolarctobacterium |
| Giloteaux et al., 2016 [14] | Microbiome | 49 ME/CFS and 39 healthy controls | Fukuda (2004) [4] | 16S rRNA sequencing from stool | ↓ Diversity ↓ Firmicutes phylum ↑ Pro-inflammatory species (Proteobacteria species) |
| Frémont et al., 2013 [64] | Anaerobe | 43 ME/CFS and 36 healthy controls | Fukuda (1994) [4] | High-throughput 16S rRNA sequencing from stool samples | ↑ Lactonifactor and Alistipes ↓ Several Firmicutes populations |
| Sheedy et al., 2009 [62] | In Vivo | 108 ME/CFS and 177 healthy controls | Holmes (1988) [72]/Fukuda (1994) [4]/Canadian Definition Criteria (2003) [3] | Fecal sample collection and identification of facultative anaerobic organisms using standard criteria [73] | ↑ Dlactic acid producing Enterococcus and Streptococcus spp. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varesi, A.; Deumer, U.-S.; Ananth, S.; Ricevuti, G. The Emerging Role of Gut Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Current Evidence and Potential Therapeutic Applications. J. Clin. Med. 2021, 10, 5077. https://doi.org/10.3390/jcm10215077
Varesi A, Deumer U-S, Ananth S, Ricevuti G. The Emerging Role of Gut Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Current Evidence and Potential Therapeutic Applications. Journal of Clinical Medicine. 2021; 10(21):5077. https://doi.org/10.3390/jcm10215077
Chicago/Turabian StyleVaresi, Angelica, Undine-Sophie Deumer, Sanjana Ananth, and Giovanni Ricevuti. 2021. "The Emerging Role of Gut Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Current Evidence and Potential Therapeutic Applications" Journal of Clinical Medicine 10, no. 21: 5077. https://doi.org/10.3390/jcm10215077
APA StyleVaresi, A., Deumer, U.-S., Ananth, S., & Ricevuti, G. (2021). The Emerging Role of Gut Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Current Evidence and Potential Therapeutic Applications. Journal of Clinical Medicine, 10(21), 5077. https://doi.org/10.3390/jcm10215077







