Vascular Complications in TAVR: Incidence, Clinical Impact, and Management
Abstract
1. Introduction
2. Materials and Methods
3. Vascular Complications in TAVR
3.1. Incidence and Definition
| Complication | Definition |
|---|---|
| Major vascular complications |
|
| Minor vascular complications |
|
| Percutaneous closure device failure |
|
3.2. Risk Factors
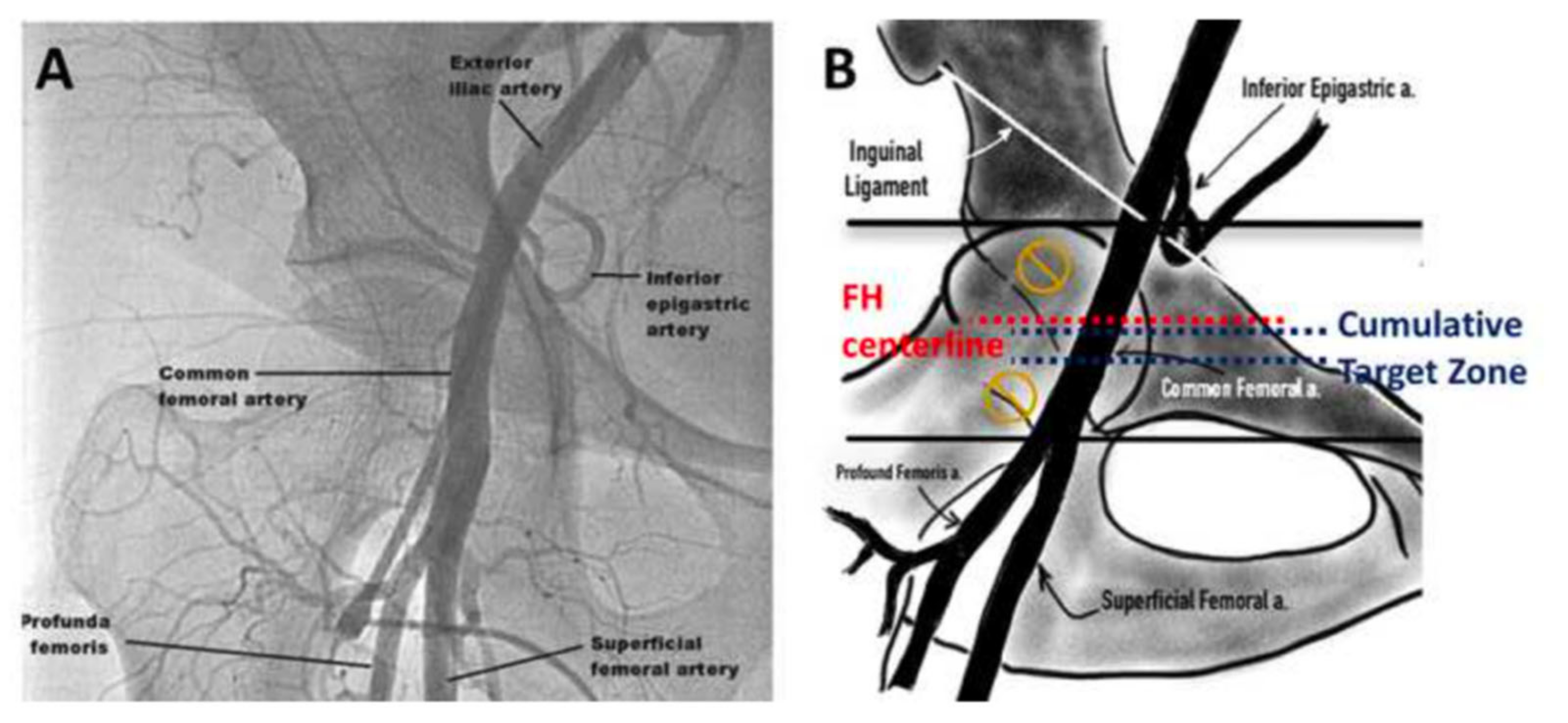
3.3. Access Techniques
3.4. Guidewires, Catheters, and Sheaths
3.4.1. Guidewires
3.4.2. Catheters
3.4.3. Sheaths
3.5. Hemostasis Methods
3.6. Diagnosis and Management of Specific Vascular Complications
3.6.1. Aortic Dissection or Rupture
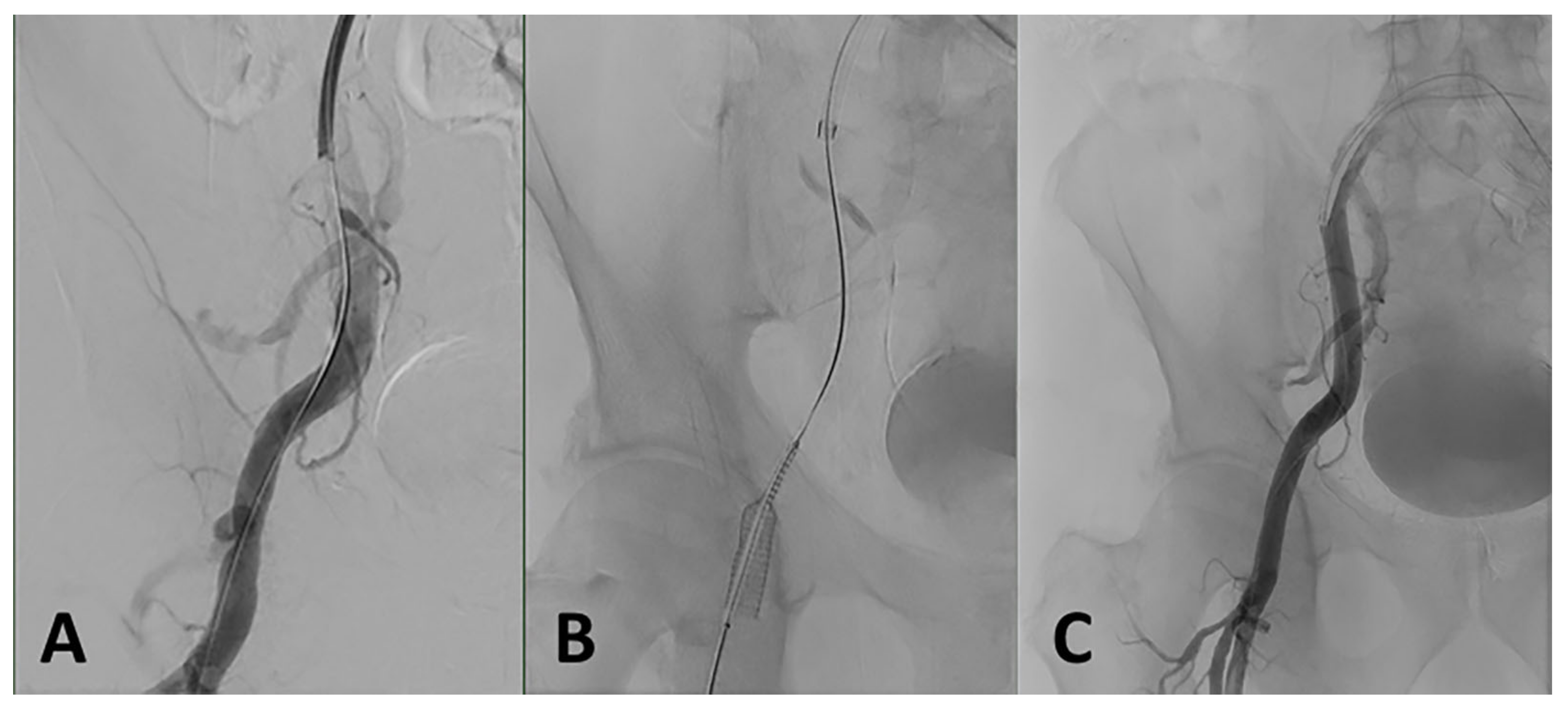
3.6.2. Iliofemoral Dissection or Rupture
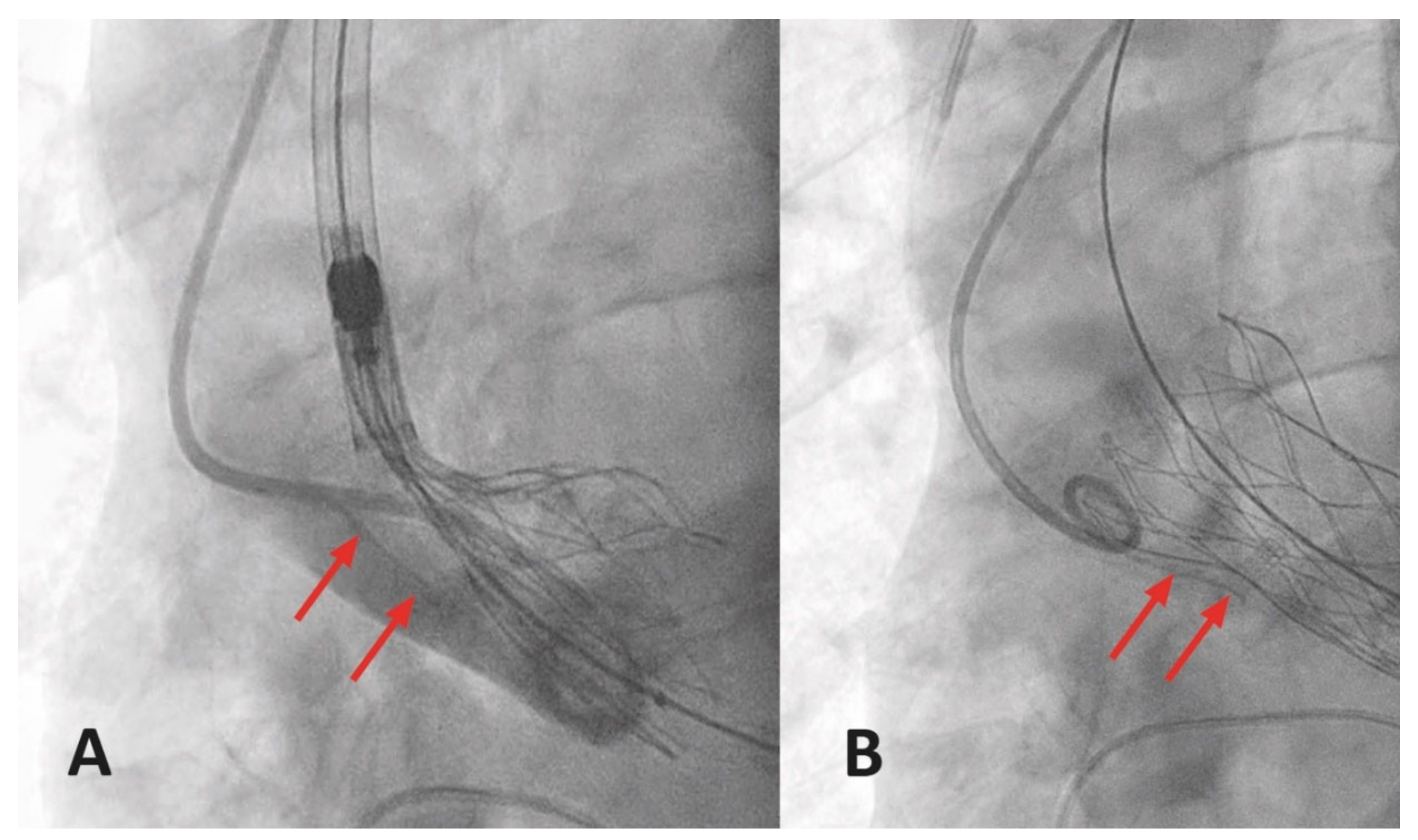
3.6.3. Access Site Bleeding and Hematoma
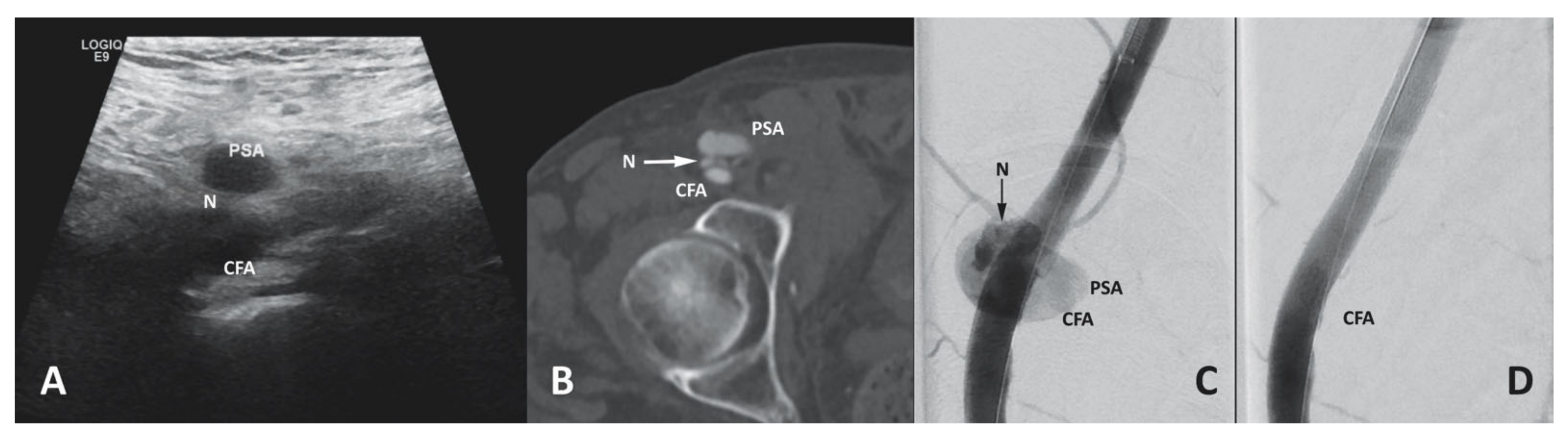
3.6.4. Access Site Pseudoaneurysm
3.6.5. Access Site Infection
3.6.6. Closure Device Failure
3.6.7. Vascular Complications Associated with Non-Transfemoral Access
3.6.8. Prevention Measures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Walther, T.; Hamm, C.W.; Schuler, G.; Berkowitsch, A.; Kotting, J.; Mangner, N.; Mudra, H.; Beckmann, A.; Cremer, J.; Welz, A.; et al. Perioperative Results and Complications in 15,964 Transcatheter Aortic Valve Replacements: Prospective Data from the GARY Registry. J. Am. Coll. Cardiol. 2015, 65, 2173–2180. [Google Scholar] [CrossRef]
- van Wiechen, M.P.; Tchétché, D.; Ooms, J.F.; Hokken, T.W.; Kroon, H.; Ziviello, F.; Ghattas, A.; Siddiqui, S.; Laperche, C.; Spitzer, E.; et al. Suture- or Plug-Based Large-Bore Arteriotomy Closure: A Pilot Randomized Controlled Trial. JACC Cardiovasc. Interv. 2021, 14, 149–157. [Google Scholar] [CrossRef]
- Akodad, M.; Roubille, F.; Marin, G.; Lattuca, B.; Macia, J.C.; Delseny, D.; Gandet, T.; Robert, P.; Schmutz, L.; Piot, C.; et al. Myocardial Injury after Balloon Predilatation Versus Direct Transcatheter Aortic Valve Replacement: Insights from the DIRECTAVI Trial. J. Am. Heart Assoc. 2020, 9, e018405. [Google Scholar] [CrossRef] [PubMed]
- Yong, G.; Walton, T.; Ng, M.; Gurvitch, R.; Worthley, S.; Whitbourn, R.; Jepson, N.; Bhindi, R.; Shang, K.; Sinhal, A. Performance and Safety of Transfemoral TAVI with SAPIEN XT in Australian Patients with Severe Aortic Stenosis at Intermediate Surgical Risk: SOLACE-AU Trial. Heart Lung Circ. 2020, 29, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Cheng, W.; Waksman, R.; Satler, L.F.; Chakravarty, T.; Groh, M.; Abernethy, W.; Russo, M.J.; Heimansohn, D.; Hermiller, J.; et al. Self-expanding intra-annular versus commercially available transcatheter heart valves in high and extreme risk patients with severe aortic stenosis (PORTICO IDE): A randomised, controlled, non-inferiority trial. Lancet 2020, 396, 669–683. [Google Scholar] [CrossRef]
- Waksman, R.; Craig, P.E.; Torguson, R.; Asch, F.M.; Weissman, G.; Ruiz, D.; Gordon, P.; Ehsan, A.; Parikh, P.; Bilfinger, T.; et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients with Symptomatic Severe Bicuspid Aortic Valve Stenosis. JACC Cardiovasc. Interv. 2020, 13, 1019–1027. [Google Scholar] [CrossRef]
- Lanz, J.; Kim, W.K.; Walther, T.; Burgdorf, C.; Möllmann, H.; Linke, A.; Redwood, S.; Thilo, C.; Hilker, M.; Joner, M.; et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: A randomised non-inferiority trial. Lancet 2019, 394, 1619–1628. [Google Scholar] [CrossRef]
- Khan, J.M.; Greenbaum, A.B.; Babaliaros, V.C.; Rogers, T.; Eng, M.H.; Paone, G.; Leshnower, B.G.; Reisman, M.; Satler, L.; Waksman, R.; et al. The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction. JACC Cardiovasc. Interv. 2019, 12, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Toutouzas, K.; Benetos, G.; Voudris, V.; Drakopoulou, M.; Stathogiannis, K.; Latsios, G.; Synetos, A.; Antonopoulos, A.; Kosmas, E.; Iakovou, I.; et al. Pre-Dilatation Versus No Pre-Dilatation for Implantation of a Self-Expanding Valve in All Comers Undergoing TAVR: The DIRECT Trial. JACC Cardiovasc. Interv. 2019, 12, 767–777. [Google Scholar] [CrossRef]
- Barbanti, M.; van Mourik, M.S.; Spence, M.S.; Iacovelli, F.; Martinelli, G.L.; Muir, D.F.; Saia, F.; Bortone, A.S.; Densem, C.G.; van der Kley, F.; et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: The multicentre European FAST-TAVI trial. EuroIntervention 2019, 15, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Beve, M.; Auffret, V.; Belhaj Soulami, R.; Tomasi, J.; Anselmi, A.; Roisne, A.; Boulmier, D.; Bedossa, M.; Leurent, G.; Donal, E.; et al. Comparison of the Transarterial and Transthoracic Approaches in Nontransfemoral Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2019, 123, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.E.; Reardon, M.J.; Rajagopal, V.; Makkar, R.R.; Bajwa, T.K.; Kleiman, N.S.; Linke, A.; Kereiakes, D.J.; Waksman, R.; Thourani, V.H.; et al. Effect of Mechanically Expanded vs Self-Expanding Transcatheter Aortic Valve Replacement on Mortality and Major Adverse Clinical Events in High-Risk Patients with Aortic Stenosis: The REPRISE III Randomized Clinical Trial. JAMA 2018, 319, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, M.; Araki, M.; Ito, T.; Honda, Y.; Tokuda, T.; Ito, Y.; Ueno, H.; Mizutani, K.; Tabata, M.; Higashimori, A.; et al. Ankle-brachial pressure index as a predictor of the 2-year outcome after transcatheter aortic valve replacement: Data from the Japanese OCEAN-TAVI Registry. Heart Vessel. 2018, 33, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Denegri, A.; Nietlispach, F.; Kottwitz, J.; Suetsch, G.; Haager, P.; Rodriguez, H.; Taramasso, M.; Obeid, S.; Maisano, F. Real-world procedural and 30-day outcome using the Portico transcatheter aortic valve prosthesis: A large single center cohort. Int. J. Cardiol. 2018, 253, 40–44. [Google Scholar] [CrossRef]
- Seeger, J.; Gonska, B.; Rottbauer, W.; Wöhrle, J. New generation devices for transfemoral transcatheter aortic valve replacement are superior compared with last generation devices with respect to VARC-2 outcome. Cardiovasc. Interv. Ther. 2018, 33, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hengstenberg, C.; Chandrasekhar, J.; Sartori, S.; Lefevre, T.; Mikhail, G.; Meneveau, N.; Tron, C.; Jeger, R.; Kupatt, C.; Vogel, B.; et al. Impact of pre-existing or new-onset atrial fibrillation on 30-day clinical outcomes following transcatheter aortic valve replacement: Results from the BRAVO 3 randomized trial. Catheter. Cardiovasc. Interv. 2017, 90, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Popma, J.J.; Reardon, M.J.; Khabbaz, K.; Harrison, J.K.; Hughes, G.C.; Kodali, S.; George, I.; Deeb, G.M.; Chetcuti, S.; Kipperman, R.; et al. Early Clinical Outcomes After Transcatheter Aortic Valve Replacement Using a Novel Self-Expanding Bioprosthesis in Patients with Severe Aortic Stenosis Who Are Suboptimal for Surgery: Results of the Evolut R U.S. Study. JACC Cardiovasc. Interv. 2017, 10, 268–275. [Google Scholar] [CrossRef]
- Takimoto, S.; Saito, N.; Minakata, K.; Shirai, S.; Isotani, A.; Arai, Y.; Hanyu, M.; Komiya, T.; Shimamoto, T.; Goto, T.; et al. Favorable Clinical Outcomes of Transcatheter Aortic Valve Implantation in Japanese Patients—First Report from the Post-Approval K-TAVI Registry. Circ. J. 2016, 81, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lansky, A.J.; Brown, D.; Pena, C.; Pietras, C.G.; Parise, H.; Ng, V.G.; Meller, S.; Abrams, K.J.; Cleman, M.; Margolis, P.; et al. Neurologic Complications of Unprotected Transcatheter Aortic Valve Implantation (from the Neuro-TAVI Trial). Am. J. Cardiol. 2016, 118, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Seeger, J.; Gonska, B.; Rodewald, C.; Rottbauer, W.; Wöhrle, J. Impact of suture mediated femoral access site closure with the Prostar XL compared to the ProGlide system on outcome in transfemoral aortic valve implantation. Int. J. Cardiol. 2016, 223, 564–567. [Google Scholar] [CrossRef]
- Manoharan, G.; Linke, A.; Moellmann, H.; Redwood, S.; Frerker, C.; Kovac, J.; Walther, T. Multicentre clinical study evaluating a novel resheathable annular functioning self-expanding transcatheter aortic valve system: Safety and performance results at 30 days with the Portico system. EuroIntervention 2016, 12, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Wöhrle, J.; Gonska, B.; Rodewald, C.; Seeger, J.; Scharnbeck, D.; Rottbauer, W. Transfemoral aortic valve implantation with the repositionable Lotus valve for treatment of patients with symptomatic severe aortic stenosis: Results from a single-centre experience. EuroIntervention 2016, 12, 760–767. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Wöhrle, J.; Gonska, B.; Rodewald, C.; Seeger, J.; Scharnbeck, D.; Rottbauer, W. Transfemoral Aortic Valve Implantation with the New Edwards Sapien 3 Valve for Treatment of Severe Aortic Stenosis-Impact of Valve Size in a Single Center Experience. PLoS ONE 2016, 11, e0151247. [Google Scholar] [CrossRef]
- Webb, J.G.; Doshi, D.; Mack, M.J.; Makkar, R.; Smith, C.R.; Pichard, A.D.; Kodali, S.; Kapadia, S.; Miller, D.C.; Babaliaros, V.; et al. A Randomized Evaluation of the SAPIEN XT Transcatheter Heart Valve System in Patients with Aortic Stenosis Who Are Not Candidates for Surgery. JACC Cardiovasc. Interv. 2015, 8, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Binder, R.K.; Stortecky, S.; Heg, D.; Tueller, D.; Jeger, R.; Toggweiler, S.; Pedrazzini, G.; Amann, F.W.; Ferrari, E.; Noble, S.; et al. Procedural Results and Clinical Outcomes of Transcatheter Aortic Valve Implantation in Switzerland: An Observational Cohort Study of Sapien 3 Versus Sapien XT Transcatheter Heart Valves. Circ. Cardiovasc. Interv. 2015, 8, e002653. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, G.; Walton, A.S.; Brecker, S.J.; Pasupati, S.; Blackman, D.J.; Qiao, H.; Meredith, I.T. Treatment of Symptomatic Severe Aortic Stenosis with a Novel Resheathable Supra-Annular Self-Expanding Transcatheter Aortic Valve System. JACC Cardiovasc. Interv. 2015, 8, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, Y.; Chakravarty, T.; Jilaihawi, H.; Kashif, M.; Zadikany, R.; Lee, C.; Matar, G.; Cheng, W.; Makkar, R.R. Comparison of Outcomes of Transcatheter Aortic Valve Implantation in Patients ≥ 90 Years versus. Am. J. Cardiol. 2015, 116, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, J.; Bleiziffer, S.; Gerckens, U.; Wenaweser, P.; Brecker, S.; Tamburino, C.; Linke, A. The Incidence and Predictors of Early- and Mid-Term Clinically Relevant Neurological Events after Transcatheter Aortic Valve Replacement in Real-World Patients. J. Am. Coll. Cardiol. 2015, 66, 209–217. [Google Scholar] [CrossRef]
- Wendt, D.; Al-Rashid, F.; Kahlert, P.; Eißmann, M.; El-Chilali, K.; Jánosi, R.A.; Pasa, S.; Tsagakis, K.; Liakopoulos, O.; Erbel, R.; et al. Low Incidence of Paravalvular Leakage with the Balloon-Expandable Sapien 3 Transcatheter Heart Valve. Ann. Thorac. Surg. 2015, 100, 819–825; discussion 825–816. [Google Scholar] [CrossRef] [PubMed]
- Castellant, P.; Didier, R.; Bezon, E.; Couturaud, F.; Eltchaninoff, H.; Iung, B.; Donzeau-Gouge, P.; Chevreul, K.; Fajadet, J.; Leprince, P.; et al. Comparison of Outcome of Transcatheter Aortic Valve Implantation with Versus without Previous Coronary Artery Bypass Grafting (from the FRANCE 2 Registry). Am. J. Cardiol. 2015, 116, 420–425. [Google Scholar] [CrossRef]
- Baumbach, A.; Mullen, M.; Brickman, A.M.; Aggarwal, S.K.; Pietras, C.G.; Forrest, J.K.; Hildick-Smith, D.; Meller, S.M.; Gambone, L.; den Heijer, P.; et al. Safety and performance of a novel embolic deflection device in patients undergoing transcatheter aortic valve replacement: Results from the DEFLECT I study. EuroIntervention 2015, 11, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Kodali, S.; Doshi, D.; Fischbein, M.P.; Yeung, A.C.; Tuzcu, E.M.; Rihal, C.S.; Babaliaros, V.; Zajarias, A.; Herrmann, H.C.; et al. Outcomes after transfemoral transcatheter aortic valve replacement: A comparison of the randomized PARTNER (Placement of AoRTic TraNscathetER Valves) trial with the NRCA (Nonrandomized Continued Access) registry. JACC Cardiovasc. Interv. 2014, 7, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Adams, D.H.; Coselli, J.S.; Deeb, G.M.; Kleiman, N.S.; Chetcuti, S.; Yakubov, S.J.; Heimansohn, D.; Hermiller, J., Jr.; Hughes, G.C.; et al. Self-expanding transcatheter aortic valve replacement using alternative access sites in symptomatic patients with severe aortic stenosis deemed extreme risk of surgery. J. Thorac. Cardiovasc. Surg. 2014, 148, 2869–2876. [Google Scholar] [CrossRef]
- Watanabe, Y.; Hayashida, K.; Takayama, M.; Mitsudo, K.; Nanto, S.; Takanashi, S.; Komiya, T.; Kuratani, T.; Tobaru, T.; Goto, T.; et al. First direct comparison of clinical outcomes between European and Asian cohorts in transcatheter aortic valve implantation: The Massy study group vs. the PREVAIL JAPAN trial. J. Cardiol. 2015, 65, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Stabile, E.; Pucciarelli, A.; Cota, L.; Sorropago, G.; Tesorio, T.; Salemme, L.; Popusoi, G.; Ambrosini, V.; Cioppa, A.; Agrusta, M.; et al. SAT-TAVI (single antiplatelet therapy for TAVI) study: A pilot randomized study comparing double to single antiplatelet therapy for transcatheter aortic valve implantation. Int. J. Cardiol. 2014, 174, 624–627. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Mehilli, J.; Frerker, C.; Neumann, F.J.; Kurz, T.; Tölg, R.; Zachow, D.; Guerra, E.; Massberg, S.; Schäfer, U.; et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: The CHOICE randomized clinical trial. JAMA 2014, 311, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J., Jr.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Sawa, Y.; Saito, S.; Kobayashi, J.; Niinami, H.; Kuratani, T.; Maeda, K.; Kanzaki, H.; Komiyama, N.; Tanaka, Y.; Boyle, A.; et al. First clinical trial of a self-expandable transcatheter heart valve in Japan in patients with symptomatic severe aortic stenosis. Circ. J. 2014, 78, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Adams, D.H.; Reardon, M.J.; Yakubov, S.J.; Kleiman, N.S.; Heimansohn, D.; Hermiller, J., Jr.; Hughes, G.C.; Harrison, J.K.; Coselli, J.; et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J. Am. Coll. Cardiol. 2014, 63, 1972–1981. [Google Scholar] [CrossRef] [PubMed]
- Seco, M.; Martinez, G.; Bannon, P.G.; Cartwright, B.L.; Adams, M.; Ng, M.; Wilson, M.K.; Vallely, M.P. Transapical aortic valve implantation—An Australian experience. Heart Lung Circ. 2014, 23, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska-Jelonkiewicz, K.; Michałowska, I.; Witkowski, A.; Dąbrowski, M.; Księżycka-Majczyńska, E.; Chmielak, Z.; Kuśmierski, K.; Hryniewiecki, T.; Demkow, M.; Stępińska, J. Vascular complications after transcatheter aortic valve implantation (TAVI): Risk and long-term results. J. Thromb. Thrombolysis 2014, 37, 490–498. [Google Scholar] [CrossRef]
- Holper, E.M.; Kim, R.J.; Mack, M.; Brown, D.; Brinkman, W.; Herbert, M.; Stewart, W.; Vance, K.; Bowers, B.; Dewey, T. Randomized trial of surgical cutdown versus percutaneous access in transfemoral TAVR. Catheter. Cardiovasc. Interv. 2014, 83, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Webb, J.G.; Svensson, L.G.; Kodali, S.K.; Satler, L.F.; Fearon, W.F.; Davidson, C.J.; Eisenhauer, A.C.; Makkar, R.R.; Bergman, G.W.; et al. Vascular complications after transcatheter aortic valve replacement: Insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J. Am. Coll. Cardiol. 2012, 60, 1043–1052. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Bleiziffer, S.; Ruge, H.; Mazzitelli, D.; Schreiber, C.; Hutter, A.; Krane, M.; Bauernschmitt, R.; Lange, R. Valve implantation on the beating heart: Catheter-assisted surgery for aortic stenosis. Dtsch. Arztebl. Int. 2009, 106, 235–241. [Google Scholar] [CrossRef]
- Hayashida, K.; Lefevre, T.; Chevalier, B.; Hovasse, T.; Romano, M.; Garot, P.; Mylotte, D.; Uribe, J.; Farge, A.; Donzeau-Gouge, P.; et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc. Interv. 2011, 4, 851–858. [Google Scholar] [CrossRef]
- Leon, M.B.; Piazza, N.; Nikolsky, E.; Blackstone, E.H.; Cutlip, D.E.; Kappetein, A.P.; Krucoff, M.W.; Mack, M.; Mehran, R.; Miller, C.; et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: A consensus report from the Valve Academic Research Consortium. Eur. Heart J. 2011, 32, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Head, S.J.; Genereux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur. Heart J. 2012, 33, 2403–2418. [Google Scholar] [CrossRef] [PubMed]
- Genereux, P.; Head, S.J.; Van Mieghem, N.M.; Kodali, S.; Kirtane, A.J.; Xu, K.; Smith, C.; Serruys, P.W.; Kappetein, A.P.; Leon, M.B. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: A weighted meta-analysis of 3519 patients from 16 studies. J. Am. Coll. Cardiol. 2012, 59, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Gurvitch, R.; Toggweiler, S.; Willson, A.B.; Wijesinghe, N.; Cheung, A.; Wood, D.A.; Ye, J.; Webb, J.G. Outcomes and complications of transcatheter aortic valve replacement using a balloon expandable valve according to the Valve Academic Research Consortium (VARC) guidelines. EuroIntervention 2011, 7, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; Tchetche, D.; Chieffo, A.; Dumonteil, N.; Messika-Zeitoun, D.; van der Boon, R.M.; Vahdat, O.; Buchanan, G.L.; Marcheix, B.; Himbert, D.; et al. Incidence, predictors, and implications of access site complications with transfemoral transcatheter aortic valve implantation. Am. J. Cardiol. 2012, 110, 1361–1367. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Nishimura, R.A.; Grover, F.L.; Brindis, R.G.; Carroll, J.D.; Edwards, F.H.; Peterson, E.D.; Rumsfeld, J.S.; Shahian, D.M.; Thourani, V.H.; et al. Annual Outcomes with Transcatheter Valve Therapy: From the STS/ACC TVT Registry. J. Am. Coll. Cardiol. 2015, 66, 2813–2823. [Google Scholar] [CrossRef]
- Beurtheret, S.; Karam, N.; Resseguier, N.; Houel, R.; Modine, T.; Folliguet, T.; Chamandi, C.; Com, O.; Gelisse, R.; Bille, J.; et al. Femoral versus Nonfemoral Peripheral Access for Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 2728–2739. [Google Scholar] [CrossRef]
- Abdelaziz, H.K.; Megaly, M.; Debski, M.; Rahbi, H.; Kamal, D.; Saad, M.; Wiper, A.; More, R.; Roberts, D.H. Meta-Analysis Comparing Percutaneous to Surgical Access in Trans-Femoral Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 125, 1239–1248. [Google Scholar] [CrossRef]
- Reidy, C.; Sophocles, A.; Ramakrishna, H.; Ghadimi, K.; Patel, P.A.; Augoustides, J.G. Challenges after the first decade of transcatheter aortic valve replacement: Focus on vascular complications, stroke, and paravalvular leak. J. Cardiothorac. Vasc. Anesth. 2013, 27, 184–189. [Google Scholar] [CrossRef]
- Toggweiler, S.; Gurvitch, R.; Leipsic, J.; Wood, D.A.; Willson, A.B.; Binder, R.K.; Cheung, A.; Ye, J.; Webb, J.G. Percutaneous aortic valve replacement: Vascular outcomes with a fully percutaneous procedure. J. Am. Coll. Cardiol. 2012, 59, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Steinvil, A.; Leshem-Rubinow, E.; Halkin, A.; Abramowitz, Y.; Ben-Assa, E.; Shacham, Y.; Bar-Dayan, A.; Keren, G.; Banai, S.; Finkelstein, A. Vascular complications after transcatheter aortic valve implantation and their association with mortality reevaluated by the valve academic research. consortium definitions. Am. J. Cardiol. 2015, 115, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Humphries, K.H.; Toggweiler, S.; Rodes-Cabau, J.; Nombela-Franco, L.; Dumont, E.; Wood, D.A.; Willson, A.B.; Binder, R.K.; Freeman, M.; Lee, M.K.; et al. Sex differences in mortality after transcatheter aortic valve replacement for severe aortic stenosis. J. Am. Coll. Cardiol. 2012, 60, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, M.B.; Herrmann, H.C.; Desai, N.D.; Fox, Z.; Ogbara, J.; Anwaruddin, S.; Jagasia, D.; Bavaria, J.E.; Szeto, W.Y.; Vallabhajosyula, P.; et al. Factors associated with vascular complications in patients undergoing balloon-expandable transfemoral transcatheter aortic valve replacement via open versus percutaneous approaches. Circ. Cardiovasc. Interv. 2014, 7, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.; Bleiziffer, S.; Piazza, N.; Mazzitelli, D.; Hutter, A.; Tassani-Prell, P.; Laborde, J.C.; Bauernschmitt, R. Incidence and treatment of procedural cardiovascular complications associated with trans-arterial and trans-apical interventional aortic valve implantation in 412 consecutive patients. Eur. J. Cardiothorac. Surg. 2011, 40, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Sardar, M.R.; Goldsweig, A.M.; Abbott, J.D.; Sharaf, B.L.; Gordon, P.C.; Ehsan, A.; Aronow, H.D. Vascular complications associated with transcatheter aortic valve replacement. Vasc. Med. 2017, 22, 234–244. [Google Scholar] [CrossRef]
- Mach, M.; Poschner, T.; Hasan, W.; Szalkiewicz, P.; Andreas, M.; Winkler, B.; Geisler, S.; Geisler, D.; Rudziński, P.N.; Watzal, V.; et al. Iliofemoral tortuosity score predicts access and bleeding complications during transfemoral transcatheter aortic valve replacement. Eur. J. Clin. Investig. 2021, 51, e13491. [Google Scholar] [CrossRef]
- Ducrocq, G.; Francis, F.; Serfaty, J.M.; Himbert, D.; Maury, J.M.; Pasi, N.; Marouene, S.; Provenchere, S.; Iung, B.; Castier, Y.; et al. Vascular complications of transfemoral aortic valve implantation with the Edwards SAPIEN prosthesis: Incidence and impact on outcome. EuroIntervention 2010, 5, 666–672. [Google Scholar] [CrossRef]
- Mussardo, M.; Latib, A.; Chieffo, A.; Godino, C.; Ielasi, A.; Cioni, M.; Takagi, K.; Davidavicius, G.; Montorfano, M.; Maisano, F.; et al. Periprocedural and short-term outcomes of transfemoral transcatheter aortic valve implantation with the Sapien XT as compared with the Edwards Sapien valve. JACC Cardiovasc. Interv. 2011, 4, 743–750. [Google Scholar] [CrossRef][Green Version]
- Kim, D.; Orron, D.E.; Skillman, J.J.; Kent, K.C.; Porter, D.H.; Schlam, B.W.; Carrozza, J.; Reis, G.J.; Baim, D.S. Role of superficial femoral artery puncture in the development of pseudoaneurysm and arteriovenous fistula complicating percutaneous transfemoral cardiac catheterization. Catheter. Cardiovasc. Diagn. 1992, 25, 91–97. [Google Scholar] [CrossRef]
- Ellis, S.G.; Bhatt, D.; Kapadia, S.; Lee, D.; Yen, M.; Whitlow, P.L. Correlates and outcomes of retroperitoneal hemorrhage complicating percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2006, 67, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.S.; Yoon, J.; Ko, J.Y.; Kim, J.Y.; Lee, S.H.; Hwang, S.O.; Choe, K.H. Anatomical consideration of the radial artery for transradial coronary procedures: Arterial diameter, branching anomaly and vessel tortuosity. Int. J. Cardiol. 2005, 101, 421–427. [Google Scholar] [CrossRef]
- Seto, A.H.; Abu-Fadel, M.S.; Sparling, J.M.; Zacharias, S.J.; Daly, T.S.; Harrison, A.T.; Suh, W.M.; Vera, J.A.; Aston, C.E.; Winters, R.J.; et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access with Ultrasound Trial). JACC Cardiovasc. Interv. 2010, 3, 751–758. [Google Scholar] [CrossRef]
- Turi, Z.G. Fluoroscopy guided vascular access: Asking the right question, but getting the wrong answer? Catheter. Cardiovasc. Interv. 2009, 74, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, G.; Sawhney, N.; Whisenant, B.; Tsimikas, S.; Turi, Z.G. Common femoral artery anatomy is influenced by demographics and comorbidity: Implications for cardiac and peripheral invasive studies. Catheter. Cardiovasc. Interv. 2001, 53, 289–295. [Google Scholar] [CrossRef]
- Bruschi, G.; de Marco, F.; Botta, L.; Cannata, A.; Oreglia, J.; Colombo, P.; Barosi, A.; Colombo, T.; Nonini, S.; Paino, R.; et al. Direct aortic access for transcatheter self-expanding aortic bioprosthetic valves implantation. Ann. Thorac. Surg. 2012, 94, 497–503. [Google Scholar] [CrossRef]
- Barbanti, M.; Capranzano, P.; Ohno, Y.; Gulino, S.; Sgroi, C.; Immè, S.; Tamburino, C.; Cannata, S.; Patanè, M.; Di Stefano, D.; et al. Comparison of suture-based vascular closure devices in transfemoral transcatheter aortic valve implantation. EuroIntervention 2015, 11, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, Z.; Scholtz, W.; Börgermann, J.; Wiemer, M.; Piper, C.; Vlachojannis, M.; Gummert, J.; Horstkotte, D.; Ensminger, S.; Faber, L.; et al. Impact of closure devices on vascular complication and mortality rates in TAVI procedures. Int. J. Cardiol. 2017, 241, 133–137. [Google Scholar] [CrossRef]
- Hernández-Enriquez, M.; Andrea, R.; Brugaletta, S.; Jiménez-Quevedo, P.; Hernández-García, J.M.; Trillo, R.; Larman, M.; Fernández-Avilés, F.; Vázquez-González, N.; Iñiguez, A.; et al. Puncture versus Surgical Cutdown Complications of Transfemoral Aortic Valve Implantation (from the Spanish TAVI Registry). Am. J. Cardiol. 2016, 118, 578–584. [Google Scholar] [CrossRef]
- Mach, M.; Wilbring, M.; Winkler, B.; Alexiou, K.; Kappert, U.; Delle-Karth, G.; Grabenwöger, M.; Matschke, K. Cut-down outperforms complete percutaneous transcatheter valve implantation. Asian Cardiovasc. Thorac. Ann. 2018, 26, 107–113. [Google Scholar] [CrossRef]
- Nakamura, M.; Chakravarty, T.; Jilaihawi, H.; Doctor, N.; Dohad, S.; Fontana, G.; Cheng, W.; Makkar, R.R. Complete percutaneous approach for arterial access in transfemoral transcatheter aortic valve replacement: A comparison with surgical cut-down and closure. Catheter. Cardiovasc. Interv. 2014, 84, 293–300. [Google Scholar] [CrossRef]
- Kawashima, H.; Watanabe, Y.; Kozuma, K.; Nara, Y.; Hioki, H.; Kataoka, A.; Yamamoto, M.; Takagi, K.; Araki, M.; Tada, N.; et al. Propensity-matched comparison of percutaneous and surgical cut-down approaches in transfemoral transcatheter aortic valve implantation using a balloon-expandable valve. EuroIntervention 2017, 12, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Petronio, A.S.; De Carlo, M.; Bedogni, F.; Maisano, F.; Ettori, F.; Klugmann, S.; Poli, A.; Marzocchi, A.; Santoro, G.; Napodano, M.; et al. 2-year results of CoreValve implantation through the subclavian access: A propensity-matched comparison with the femoral access. J. Am. Coll. Cardiol. 2012, 60, 502–507. [Google Scholar] [CrossRef]
- Mylotte, D.; Sudre, A.; Teiger, E.; Obadia, J.F.; Lee, M.; Spence, M.; Khamis, H.; Al Nooryani, A.; Delhaye, C.; Amr, G.; et al. Transcarotid Transcatheter Aortic Valve Replacement: Feasibility and Safety. JACC Cardiovasc. Interv. 2016, 9, 472–480. [Google Scholar] [CrossRef]
- Debry, N.; Delhaye, C.; Azmoun, A.; Ramadan, R.; Fradi, S.; Brenot, P.; Sudre, A.; Moussa, M.D.; Tchetche, D.; Ghostine, S.; et al. Transcarotid Transcatheter Aortic Valve Replacement: General or Local Anesthesia. JACC Cardiovasc. Interv. 2016, 9, 2113–2120. [Google Scholar] [CrossRef]
- Latsios, G.; Gerckens, U.; Grube, E. Transaortic transcatheter aortic valve implantation: A novel approach for the truly “no-access option” patients. Catheter. Cardiovasc. Interv. 2010, 75, 1129–1136. [Google Scholar] [CrossRef]
- Bapat, V.; Khawaja, M.Z.; Attia, R.; Narayana, A.; Wilson, K.; Macgillivray, K.; Young, C.; Hancock, J.; Redwood, S.; Thomas, M. Transaortic Transcatheter Aortic valve implantation using Edwards Sapien valve: A novel approach. Catheter. Cardiovasc. Interv. 2012, 79, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Fiorina, C.; Bruschi, G.; Testa, L.; De Carlo, M.; De Marco, F.; Coletti, G.; Bonardelli, S.; Adamo, M.; Curello, S.; Scioti, G.; et al. Transaxillary versus transaortic approach for transcatheter aortic valve implantation with CoreValve Revalving System: Insights from multicenter experience. J. Cardiovasc. Surg. 2017, 58, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Romano, M.; Lefevre, T.; Chevalier, B.; Farge, A.; Hovasse, T.; Le Houerou, D.; Morice, M.C. The transaortic approach for transcatheter aortic valve implantation: A valid alternative to the transapical access in patients with no peripheral vascular option. A single center experience. Eur. J. Cardiothorac. Surg. 2013, 44, 692–700. [Google Scholar] [CrossRef]
- Lardizabal, J.A.; O’Neill, B.P.; Desai, H.V.; Macon, C.J.; Rodriguez, A.P.; Martinez, C.A.; Alfonso, C.E.; Bilsker, M.S.; Carillo, R.G.; Cohen, M.G.; et al. The transaortic approach for transcatheter aortic valve replacement: Initial clinical experience in the United States. J. Am. Coll. Cardiol. 2013, 61, 2341–2345. [Google Scholar] [CrossRef]
- Thourani, V.H.; Gunter, R.L.; Neravetla, S.; Block, P.; Guyton, R.A.; Kilgo, P.; Lerakis, S.; Devireddy, C.; Leshnower, B.; Mavromatis, K.; et al. Use of transaortic, transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann. Thorac. Surg. 2013, 96, 1349–1357. [Google Scholar] [CrossRef]
- Webb, J.G.; Altwegg, L.; Boone, R.H.; Cheung, A.; Ye, J.; Lichtenstein, S.; Lee, M.; Masson, J.B.; Thompson, C.; Moss, R.; et al. Transcatheter aortic valve implantation: Impact on clinical and valve-related outcomes. Circulation 2009, 119, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; Thielmann, M.; Kempfert, J.; Schroefel, H.; Wimmer-Greinecker, G.; Treede, H.; Wahlers, T.; Wendler, O. PREVAIL TRANSAPICAL: Multicentre trial of transcatheter aortic valve implantation using the newly designed bioprosthesis (SAPIEN-XT) and delivery system (ASCENDRA-II). Eur. J. Cardiothorac. Surg. 2012, 42, 278–283; discussion 283. [Google Scholar] [CrossRef][Green Version]
- Holzhey, D.M.; Hansig, M.; Walther, T.; Seeburger, J.; Misfeld, M.; Linke, A.; Borger, M.A.; Mohr, F.W. Transapical aortic valve implantation—The Leipzig experience. Ann. Cardiothorac. Surg. 2012, 1, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Toppen, W.; Suh, W.; Aksoy, O.; Benharash, P.; Bowles, C.; Shemin, R.J.; Kwon, M. Vascular Complications in the Sapien 3 Era: Continued Role of Transapical Approach to Transcatheter Aortic Valve Replacement. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 144–149. [Google Scholar] [CrossRef]
- Greenbaum, A.B.; Babaliaros, V.C.; Chen, M.Y.; Stine, A.M.; Rogers, T.; O’Neill, W.W.; Paone, G.; Thourani, V.H.; Muhammad, K.I.; Leonardi, R.A.; et al. Transcaval Access and Closure for Transcatheter Aortic Valve Replacement: A Prospective Investigation. J. Am. Coll. Cardiol. 2017, 69, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.J.; How, T.V.; Vallabhaneni, S.R.; Brennan, J.A.; Fisher, R.K.; Naik, J.B.; McWilliams, R.G. Guidewire stiffness: What’s in a name? J. Endovasc. Ther. 2011, 18, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Brodmann, M.; Werner, M.; Brinton, T.J.; Illindala, U.; Lansky, A.; Lansky, M.R.; Lansky, A. Safety and performance of lithoplasty for treatment of calcified peripheral artery lesions. J. Am. Coll. Cardiol. 2017, 70, 908–910. [Google Scholar] [CrossRef]
- Brodmann, M.; Werner, M.; Holden, A.; Tepe, G.; Scheinert, D.; Schwindt, A.; Wolf, F.; Jaff, M.; Lansky, A.; Zeller, T. Primary outcomes and mechanism of action of intravascular lithotripsy in calcified, femoropopliteal lesions: Results of Disrupt PAD II. Catheter. Cardiovasc. Interv. 2019, 93, 335–342. [Google Scholar] [CrossRef]
- Di Mario, C.; Chiriatti, N.; Stolcova, M.; Meucci, F.; Squillantini, G. Lithoplasty- assisted transfemoral aortic valve implantation. Eur. Heart J. 2018. [Google Scholar] [CrossRef]
- Di Mario, C.; Goodwin, M.; Ristalli, F.; Ravani, M.; Meucci, F.; Stolcova, M.; Sardella, G.; Salvi, N.; Bedogni, F.; Berti, S.; et al. A prospective registry of intravascular lithotripsy-enabled vascular access for transfemoral transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2019, 12, 502–504. [Google Scholar] [CrossRef]
- Barbanti, M.; Binder, R.K.; Freeman, M.; Wood, D.A.; Leipsic, J.; Cheung, A.; Ye, J.; Tan, J.; Toggweiler, S.; Yang, T.H.; et al. Impact of low-profile sheaths on vascular complications during transfemoral transcatheter aortic valve replacement. EuroIntervention 2013, 9, 929–935. [Google Scholar] [CrossRef]
- Millan, X.; Azzalini, L.; Khan, R.; Cournoyer, D.; Dorval, J.F.; Ibrahim, R.; Bonan, R.; Asgar, A.W. Efficacy of a balloon-expandable vascular access system in transfemoral TAVI patients. Catheter. Cardiovasc. Interv. 2016, 88, 1145–1152. [Google Scholar] [CrossRef]
- Tchetche, D.; Dumonteil, N.; Sauguet, A.; Descoutures, F.; Luz, A.; Garcia, O.; Soula, P.; Gabiache, Y.; Fournial, G.; Marcheix, B.; et al. Thirty-day outcome and vascular complications after transarterial aortic valve implantation using both Edwards Sapien and Medtronic CoreValve bioprostheses in a mixed population. EuroIntervention 2010, 5, 659–665. [Google Scholar] [CrossRef]
- Thomas, M.; Schymik, G.; Walther, T.; Himbert, D.; Lefevre, T.; Treede, H.; Eggebrecht, H.; Rubino, P.; Michev, I.; Lange, R.; et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010, 122, 62–69. [Google Scholar] [CrossRef]
- Spitzer, S.G.; Wilbring, M.; Alexiou, K.; Stumpf, J.; Kappert, U.; Matschke, K. Surgical cut-down or percutaneous access-which is best for less vascular access complications in transfemoral TAVI? Catheter. Cardiovasc. Interv. 2016, 88, E52–E58. [Google Scholar] [CrossRef]
- Sharp, A.S.; Michev, I.; Maisano, F.; Taramasso, M.; Godino, C.; Latib, A.; Denti, P.; Dorigo, E.; Giacomini, A.; Iaci, G.; et al. A new technique for vascular access management in transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2010, 75, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, P.; Al-Rashid, F.; Plicht, B.; Konorza, T.; Neumann, T.; Thielmann, M.; Wendt, D.; Erbel, R.; Eggebrecht, H. Suture-mediated arterial access site closure after transfemoral aortic valve implantation. Catheter. Cardiovasc. Interv. 2013, 81, E139–E150. [Google Scholar] [CrossRef]
- Hayashida, K.; Lefevre, T.; Chevalier, B.; Hovasse, T.; Romano, M.; Garot, P.; Mylotte, D.; Uribe, J.; Farge, A.; Donzeau-Gouge, P.; et al. True percutaneous approach for transfemoral aortic valve implantation using the Prostar XL device: Impact of learning curve on vascular complications. JACC Cardiovasc. Interv. 2012, 5, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Grube, E.; Schuler, G.; Buellesfeld, L.; Gerckens, U.; Linke, A.; Wenaweser, P.; Sauren, B.; Mohr, F.W.; Walther, T.; Zickmann, B.; et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: Device success and 30-day clinical outcome. J. Am. Coll. Cardiol. 2007, 50, 69–76. [Google Scholar] [CrossRef] [PubMed]
- van Wiechen, M.P.; Ligthart, J.M.; Van Mieghem, N.M. Large-bore Vascular Closure: New Devices and Techniques. Interv. Cardiol. 2019, 14, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Frerker, C.; Schewel, D.; Kuck, K.H.; Schafer, U. Ipsilateral arterial access for management of vascular complication in transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2013, 81, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Curran, H.; Chieffo, A.; Buchanan, G.L.; Bernelli, C.; Montorfano, M.; Maisano, F.; Latib, A.; Maccagni, D.; Carlino, M.; Figini, F.; et al. A comparison of the femoral and radial crossover techniques for vascular access management in transcatheter aortic valve implantation: The Milan experience. Catheter. Cardiovasc. Interv. 2014, 83, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Laborde, J.C.; Brecker, S.J.; Roy, D.; Jahangiri, M. Complications at the time of transcatheter aortic valve implantation. Methodist DeBakey Cardiovasc. J. 2012, 8, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, A.; Shirai, S.; Hanyu, M.; Arai, Y.; Kamioka, N.; Hayashi, M. Descending aortic dissection injured by tip of the sheath during transcatheter aortic valve implantation. Cardiovasc. Interv. Ther. 2016, 31, 122–127. [Google Scholar] [CrossRef]
- Hansson, N.C.; Nørgaard, B.L.; Barbanti, M.; Nielssen, N.N.E.; Yang, T.H.; Tamburino, C.; Dvir, D.; Jilaihawi, H.; Blanke, P.; Makkar, R.R.; et al. The impact of calcium volume and distribution in aortic root injury related to balloon-expandable transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2015, 9, 382–392. [Google Scholar] [CrossRef]
- .Barbanti, M.; Yang, T.H.; Rodès Cabau, J.; Tamburino, C.; Wood, D.A.; Jilaihawi, H.; Blanke, P.; Makkar, R.R.; Latib, A.; Colombo, A.; et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013, 128, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Tzamtzis, S.; Viquerat, J.; Yap, J.; Mullen, M.J.; Burriesci, G. Numerical analysis of the radial force produced by the Medtronic-CoreValve and Edwards-SAPIEN after transcatheter aortic valve implantation (TAVI). Med. Eng. Phys. 2013, 35, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hiltrop, N.; Adriaenssens, T.; Dymarkowski, S.; Herijgers, P.; Dubois, C. Aortic Annulus Rupture during TAVI: A Therapeutic Dilemma in the Inoperable Patient. J. Heart Valve Dis. 2015, 24, 436–438. [Google Scholar] [PubMed]
- Hayashida, K.; Bouvier, E.; Lefevre, T.; Hovasse, T.; Morice, M.C.; Chevalier, B.; Romano, M.; Garot, P.; Farge, A.; Donzeau-Gouge, P.; et al. Potential mechanism of annulus rupture during transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2013, 82, E742–E746. [Google Scholar] [CrossRef]
- Eltchaninoff, H.; Prat, A.; Gilard, M.; Leguerrier, A.; Blanchard, D.; Fournial, G.; Iung, B.; Donzeau-Gouge, P.; Tribouilloy, C.; Debrux, J.L.; et al. Transcatheter aortic valve implantation: Early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur. Heart J. 2011, 32, 191–197. [Google Scholar] [CrossRef]
- Tsetis, D. Endovascular treatment of complications of femoral arterial access. Cardiovasc. Interv. Radiol. 2010, 33, 457–468. [Google Scholar] [CrossRef]
- Masson, J.B.; Al Bugami, S.; Webb, J.G. Endovascular balloon occlusion for catheter-induced large artery perforation in the catheterization laboratory. Catheter. Cardiovasc. Interv. 2009, 73, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Dahdouh, Z.; Roule, V.; Grollier, G. Life-threatening iliac artery rupture during transcatheter aortic valve implantation (TAVI): Diagnosis and management. Heart 2013, 99, 1217–1218. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.B.; Kovac, J.; Schuler, G.; Ye, J.; Cheung, A.; Kapadia, S.; Tuzcu, M.E.; Kodali, S.; Leon, M.B.; Webb, J.G. Transcatheter aortic valve implantation: Review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc. Interv. 2009, 2, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; Nuis, R.J.; Piazza, N.; Apostolos, T.; Ligthart, J.; Schultz, C.; de Jaegere, P.P.; Serruys, P.W. Vascular complications with transcatheter aortic valve implantation using the 18 Fr Medtronic CoreValve System: The Rotterdam experience. EuroIntervention 2010, 5, 673–679. [Google Scholar] [CrossRef]
- Yatskar, L.; Selzer, F.; Feit, F.; Cohen, H.A.; Jacobs, A.K.; Williams, D.O.; Slater, J. Access site hematoma requiring blood transfusion predicts mortality in patients undergoing percutaneous coronary intervention: Data from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter. Cardiovasc. Interv. 2007, 69, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Tonnessen, B.H. Iatrogenic injury from vascular access and endovascular procedures. Perspect. Vasc. Surg. Endovasc. Ther. 2011, 23, 128–135. [Google Scholar] [CrossRef]
- Stone, P.A.; Campbell, J.E.; AbuRahma, A.F. Femoral pseudoaneurysms after percutaneous access. J. Vasc. Surg. 2014, 60, 1359–1366. [Google Scholar] [CrossRef]
- Kresowik, T.F.; Khoury, M.D.; Miller, B.V.; Winniford, M.D.; Shamma, A.R.; Sharp, W.J.; Blecha, M.B.; Corson, J.D. A prospective study of the incidence and natural history of femoral vascular complications after percutaneous transluminal coronary angioplasty. J. Vasc. Surg. 1991, 13, 328–333; discussion 333–325. [Google Scholar] [CrossRef]
- Toursarkissian, B.; Allen, B.T.; Petrinec, D.; Thompson, R.W.; Rubin, B.G.; Reilly, J.M.; Anderson, C.B.; Flye, M.W.; Sicard, G.A. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J. Vasc. Surg. 1997, 25, 803–808; discussion 808–809. [Google Scholar] [CrossRef]
- Lonn, L.; Olmarker, A.; Geterud, K.; Risberg, B. Prospective randomized study comparing ultrasound-guided thrombin injection to compression in the treatment of femoral pseudoaneurysms. J. Endovasc. Ther. 2004, 11, 570–576. [Google Scholar] [CrossRef]
- Stone, P.; Lohan, J.A.; Copeland, S.E.; Hamrick, R.E., Jr.; Tiley, E.H., 3rd; Flaherty, S.K. Iatrogenic pseudoaneurysms: Comparison of treatment modalities, including duplex-guided thrombin injection. West Va. Med. J. 2003, 99, 230–232. [Google Scholar]
- Dvir, D.; Assali, A.; Porat, E.; Kornowski, R. Distal left anterior descending coronary artery obstruction: A rare complication of transapical aortic valve implantation. J. Invasive Cardiol. 2011, 23, E281–E283. [Google Scholar]
- Nielsen, H.H.; Klaaborg, K.E.; Nissen, H.; Terp, K.; Mortensen, P.E.; Kjeldsen, B.J.; Jakobsen, C.J.; Andersen, H.R.; Egeblad, H.; Krusell, L.R.; et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: The STACCATO trial. EuroIntervention 2012, 8, 383–389. [Google Scholar] [CrossRef]
- Himbert, D.; Descoutures, F.; Al-Attar, N.; Iung, B.; Ducrocq, G.; Detaint, D.; Brochet, E.; Messika-Zeitoun, D.; Francis, F.; Ibrahim, H.; et al. Results of transfemoral or transapical aortic valve implantation following a uniform assessment in high-risk patients with aortic stenosis. J. Am. Coll. Cardiol. 2009, 54, 303–311. [Google Scholar] [CrossRef]
- Schafer, U.; Deuschl, F.; Schofer, N.; Frerker, C.; Schmidt, T.; Kuck, K.H.; Kreidel, F.; Schirmer, J.; Mizote, I.; Reichenspurner, H.; et al. Safety and efficacy of the percutaneous transaxillary access for transcatheter aortic valve implantation using various transcatheter heart valves in 100 consecutive patients. Int. J. Cardiol. 2017, 232, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Etienne, P.Y.; Papadatos, S.; El Khoury, E.; Pieters, D.; Price, J.; Glineur, D. Transaortic transcatheter aortic valve implantation with the Edwards SAPIEN valve: Feasibility, technical considerations, and clinical advantages. Ann. Thorac. Surg. 2011, 92, 746–748. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Mack, M.J.; Kaul, S.; Agnihotri, A.; Alexander, K.P.; Bailey, S.R.; Calhoon, J.H.; Carabello, B.A.; Desai, M.Y.; Edwards, F.H.; et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement: Developed in collabration with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J. Thorac. Cardiovasc. Surg. 2012, 144, e29–e84. [Google Scholar] [CrossRef]
- Achenbach, S.; Delgado, V.; Hausleiter, J.; Schoenhagen, P.; Min, J.K.; Leipsic, J.A. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J. Cardiovasc. Comput. Tomogr. 2012, 6, 366–380. [Google Scholar] [CrossRef] [PubMed]
| Risk Factors | |
|---|---|
| Non-modifiable |
|
| Modifiable |
|
| Location | Management |
|---|---|
| Aortic complications | |
| Aortic rupture | Open surgical repair Aortic occlusion balloon and cardiopulmonary bypass to stabilize |
| Aortic dissection | Surgical and endovascular repair Medical management |
| Iliofemoral complications | |
| Arterial perforation | Immediate reversal of anticoagulation Prolonged balloon angioplasty or, less commonly, covered stent-graft implantation from a contralateral or ipsilateral CFA access |
| Arterial dissection | Flow-limiting, prolonged balloon angioplasty or covered stent-graft implantation from a contralateral or ipsilateral CFA access |
| Arterial stenosis, thrombosis, and occlusion | Thrombectomy or balloon angioplasty |
| Pseudoaneurysm | Size <3.0–3.5 cm: observation Size >3.0–3.5 cm or expanding: thrombin injection |
| Hematoma | Conservative, manual compression, prolonged balloon angioplasty from contralateral CFA access |
| Recommendations for assessment of access route by CT before TAVR |
|
| Recommendations for assessment of the aorta before TAVR |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mach, M.; Okutucu, S.; Kerbel, T.; Arjomand, A.; Fatihoglu, S.G.; Werner, P.; Simon, P.; Andreas, M. Vascular Complications in TAVR: Incidence, Clinical Impact, and Management. J. Clin. Med. 2021, 10, 5046. https://doi.org/10.3390/jcm10215046
Mach M, Okutucu S, Kerbel T, Arjomand A, Fatihoglu SG, Werner P, Simon P, Andreas M. Vascular Complications in TAVR: Incidence, Clinical Impact, and Management. Journal of Clinical Medicine. 2021; 10(21):5046. https://doi.org/10.3390/jcm10215046
Chicago/Turabian StyleMach, Markus, Sercan Okutucu, Tillmann Kerbel, Aref Arjomand, Sefik Gorkem Fatihoglu, Paul Werner, Paul Simon, and Martin Andreas. 2021. "Vascular Complications in TAVR: Incidence, Clinical Impact, and Management" Journal of Clinical Medicine 10, no. 21: 5046. https://doi.org/10.3390/jcm10215046
APA StyleMach, M., Okutucu, S., Kerbel, T., Arjomand, A., Fatihoglu, S. G., Werner, P., Simon, P., & Andreas, M. (2021). Vascular Complications in TAVR: Incidence, Clinical Impact, and Management. Journal of Clinical Medicine, 10(21), 5046. https://doi.org/10.3390/jcm10215046






