Pre-Analytical Modification of Serum miRNAs: Diagnostic Reliability of Serum miRNAs in Hemolytic Diseases
Abstract
1. Introduction
2. Materials and Methods
3. Results
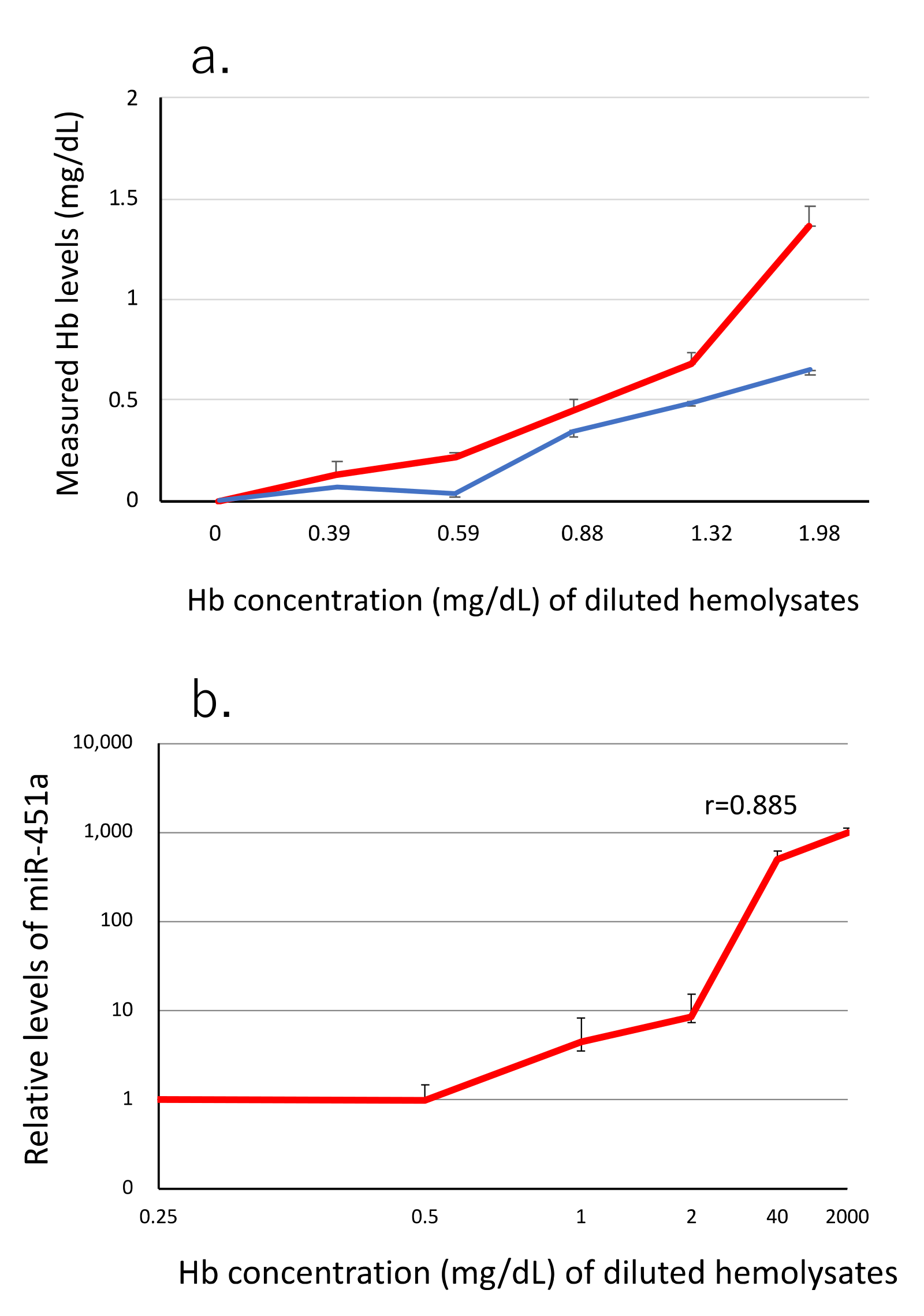
3.1. miR-451a as a Biomarker of Hemolysis
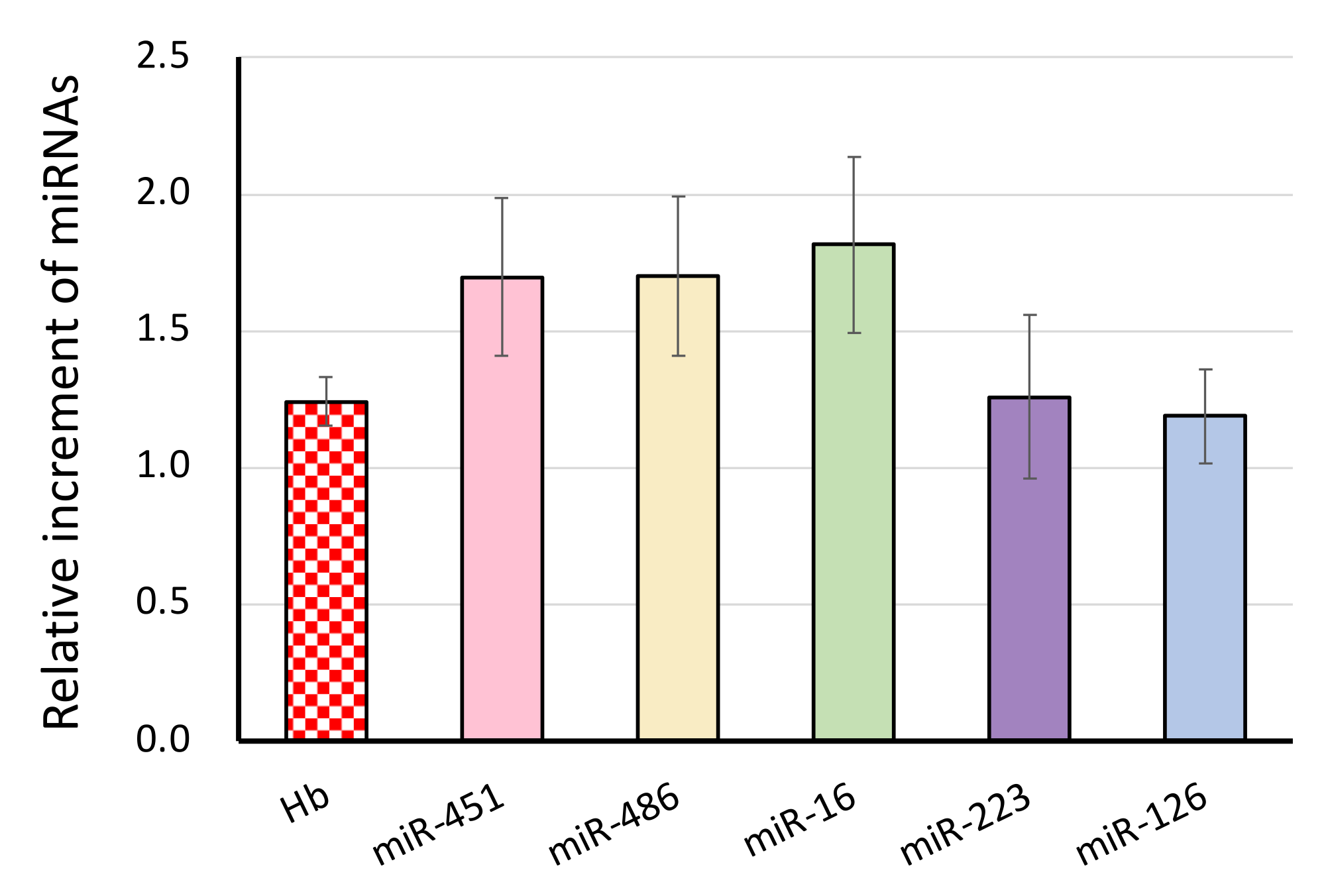
3.2. The Effect of Blood Drawing on the miRNA Signature
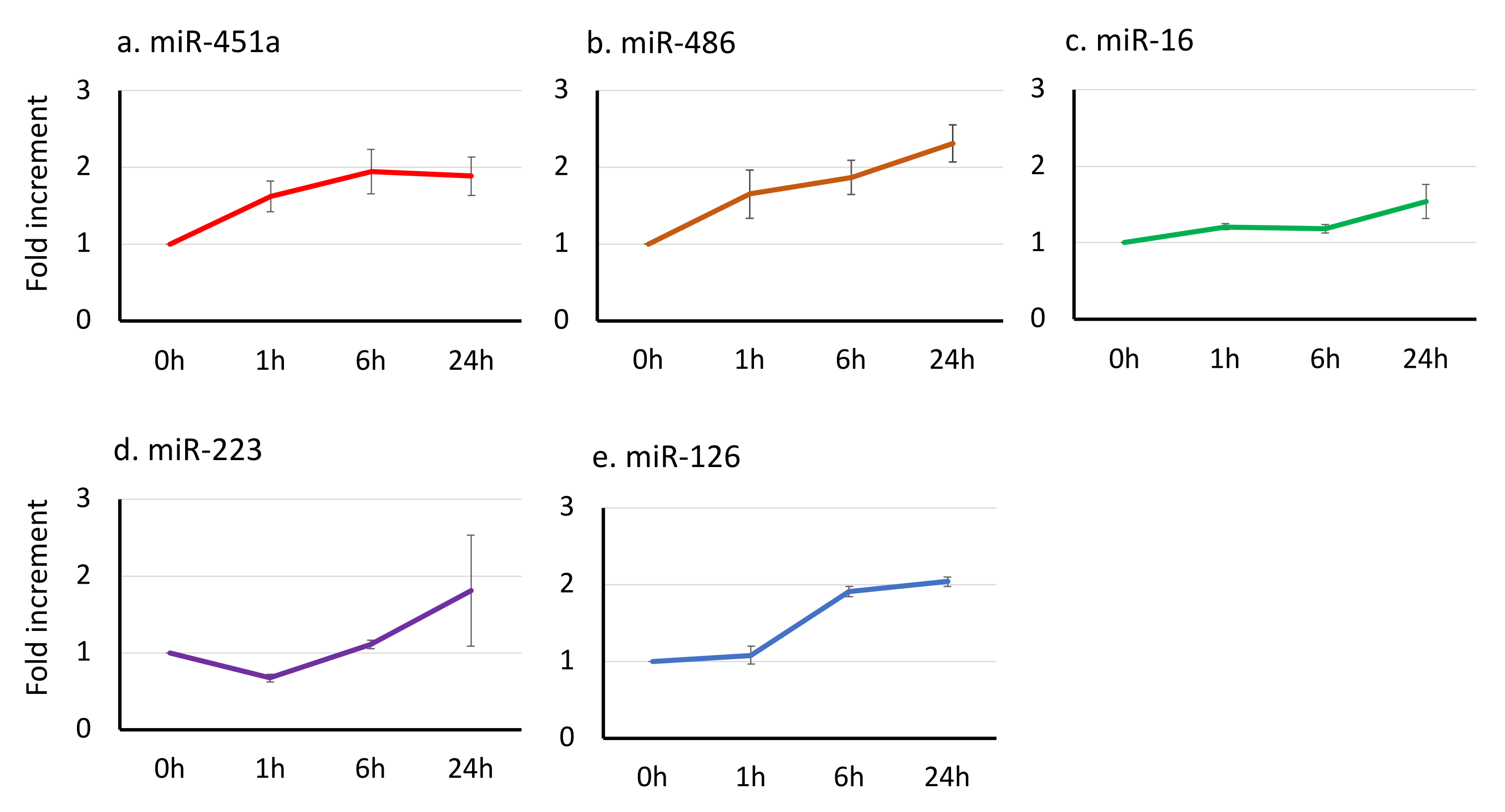
3.3. The Effect of Time-Lag before Serum Separation on the miRNA Signatures
3.4. Biochemical Indexes in Leftover Samples in Clinical Laboratory
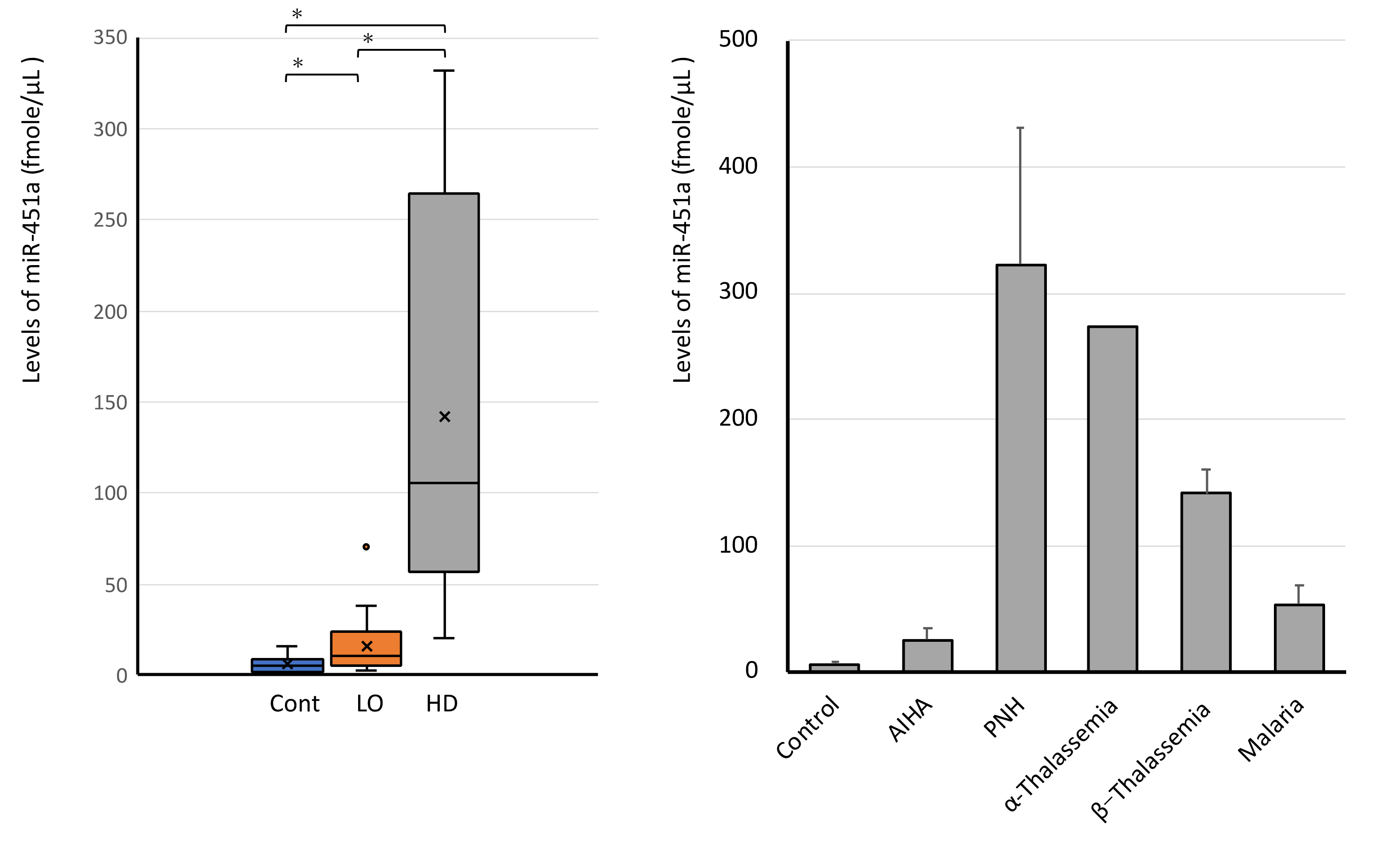
3.5. miR-451a Is Elevated in All Leftover Samples in Clinical Laboratory
3.6. High Levels of Serum miR-451a in Hemolytic Diseases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Genomics, biogenesis mechanism, and function. Cell 2004, 16, 281–297. [Google Scholar] [CrossRef]
- Sayed, D.; Abdullatif, M. MicroRNAs in development and disease. Physiol. Rev. 2011, 91, 827–887. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Kirschner, M.B.; van Zandwijk, N. Circulating microRNAs: Association with disease and potential use as biomarkers. Crit. Rev. Oncol. Hematol. 2010, 80, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, A.; Lee, I.; Hood, L.; Galas, D.; Wang, K. Extracellular microRNA: A new source of biomarkers. Mutat. Res. 2011, 717, 85–90. [Google Scholar] [CrossRef]
- Lichtman, M.A.; Kaushansky, K.; Kipps, T.J.; Prchal, J.T.; Levi, M.M. Williams Hematology, 8th ed.; McGraw Hill Medical: New York, NY, USA, 2011. [Google Scholar]
- Byron, J.C.H.; Papayannopoulou, T.h. MicroRNAs: Allies or Foes in Erythropoiesis? J. Cell. Physiol 2012, 227, 7–13. [Google Scholar]
- Doty, R.T.; Phelps, S.R.; Shadle, C.; Sanchez-Bonilla, M.; Keel, S.B.; Abkowitz, J.L. Coordinate expression of heme and globin is essential for effective erythropoiesis. J. Clin. Investig. 2015, 125, 4681–4691. [Google Scholar] [CrossRef]
- Rasmussen, K.D.; Simmini, S.; Abreu-Goodger, C.; Bartonicek, N.; Di Giacomo, M.; Bilbao-Cortes, D.; Horos, R.; Von Lindern, M.; Enright, A.J.; O’Carroll, D. The miR-144/451 locus is required for erythroid homeostasis. J. Exp. Med. 2010, 207, 1351–1358. [Google Scholar] [CrossRef]
- Svasti, S.; Masaki, S.; Penglong, T.; Abe, Y.; Winichagoon, P.; Fucharoen, S.; Umemura, T. Expression of microRNA-451 in normal and thalassemic erythropoiesis. Ann. Hematol. 2010, 89, 953–958. [Google Scholar] [CrossRef]
- Kirschner, M.B.; Edelman, J.B.; Steven, S.C.; Vallely, M.P.; van Zandwijk, N.; Reid, R. The impact of hemolysis on cell-free microRNA biomarkers. Front. Genet. 2013, 89, 94. [Google Scholar] [CrossRef]
- Lippi, G.; Giavarina, D.; Gelati, M.; Salvagno, G.L. Reference range of hemolysis index in serum and lithium-heparin plasma measured with two analytical platforms in a population of unselected outpatients. Clin. Chim. Acta 2014, 429, 143–146. [Google Scholar] [CrossRef]
- Lippi, G.; von Meyer, A.; Cadamuro, J.; Simundic, A.-M. Blood sample quality. Diagnosis 2019, 6, 25–31. [Google Scholar] [CrossRef]
- Leecharoenkiat, K.; Tanaka, Y.; Harada, Y.; Chaichompoo, P.; Sarakul, O.; Abe, Y.; Smith, D.R.; Fucharoen, S.; Svasti, S.; Umemura, T. Plasma microRNA-451 as a novel hemolytic marker for β0-thalassemia/HbE disease. Mol. Med. Rep. 2017, 15, 2495–2502. [Google Scholar] [CrossRef]
- Bruchova, H.; Yoon, D.; Agarwal, A.M.; Mendell, J.; Prchal, J.T. Regulated expression of miRNAs in normal and polycythemia vera erythropoiesis. Exp. Hematol. 2007, 35, 1657–1667. [Google Scholar] [CrossRef]
- Lulli, V.; Romania, P.; Morsilli, O.; Cianciulli, P.; Gabbianelli, M.; Testa, U.; Giuliani, A. Marziali GMicroRNA-486-3p Regulates c-Globin Expression in Human Erythroid Cells by Directly Modulating BCL11A. PLoS ONE 2013, 8, e60436. [Google Scholar] [CrossRef]
- Doss, J.F.; Corcoran, D.L.; Jima, D.D.; Telen, M.J.; Dave, S.S.; Chi, J.-T. A comprehensive joint analysis of the long and short RNA transcriptomes of human erythrocytes. BMC Genom. 2015, 16, 952. [Google Scholar] [CrossRef]
- Zhan, M.; Miller, C.P.; Papayannoupoulou, T.H.; Stamatoyannopoulos, G.; Song, C.Z. MicroRNA expression dynamics during murine and human erythroid differentiation. Exp. Hematol. 2007, 35, 1015–1025. [Google Scholar] [CrossRef]
- Masaki, S.; Ohtsuka, R.; Abe, Y.; Muta, K.; Umemura, T. Expression patterns of microRNAs 155 and 451 during normal human erythropoiesis. Biochem. Biophys. Res. Commun. 2007, 364, 509–514. [Google Scholar] [CrossRef]
- Rasmussen, K.D.; O’Carroll, D. The miR-144/451eGFP allele, a novel tool for resolving the erythroid potential of hematopoietic precursors. Blood 2011, 118, 2988–2992. [Google Scholar] [CrossRef][Green Version]
- Pase, L.; Layton, J.E.; Kloosterman, W.P.; Carradice, D.; Waterhouse, P.M.; Lieschke, G.J. miR-451 regulates zebrafish erythroid maturation in vivo via its target gata2. Blood 2009, 113, 1794–1804. [Google Scholar] [CrossRef]
- Dore, L.C.; Amigo, J.D.; Dos Santos, C.O.; Zhang, Z.; Gai, X.; Tobias, J.W.; Yu, D.; Klein, A.M.; Dorman, C.; Wu, W.; et al. A GATA-1-regulated microRNA locus essential for erythropoiesis. Proc. Natl. Acad. Sci. USA 2008, 105, 3333–3338. [Google Scholar] [CrossRef]
- Cheloufi, S.; Dos Santos, C.O.; MWChong Hannon, G.J. A Dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 2010, 465, 584–590. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Kroh, E.; Wood, B.; Arroyo, J.D.; Dougherty, K.J.; Miyaji, M.M.; Tait, J.F.; Tewari, M. Blood cell origin of circulating microRNAs: A cautionary note for cancer biomarker studies. Cancer Prev. Res. 2012, 5, 492–497. [Google Scholar] [CrossRef]
- Azzouzi, I.; Schmugge, M.; Speer, O. MicroRNAs as components of regulatory networks controlling erythropoiesis. Eur. J. Haematol. 2012, 89, 1–9. [Google Scholar] [CrossRef]
- Shah, J.S.; Soon, P.S.; Marsh, D.J. Comparison of Methodologies to Detect Low Levels of Hemolysis in Serum for Accurate Assessment of Serum microRNAs. PLoS ONE 2016, 11, e0153200. [Google Scholar] [CrossRef]
- Ortiz-Quintero, B. Cell-free microRNAs in blood and other body fluids, as cancer biomarkers. Cell Prolif. 2016, 49, 281–303. [Google Scholar] [CrossRef]
- Becan-McBride, K. Laboratory Sampling: Does the process affect the outcome? J. Intraven. Nurs. 1999, 22, 137–142. [Google Scholar]
- Wu, C.-H.; Lin, F.-C.; Chen, S.-J.; Chen, Y.-L.; Chung, W.-J.; Cheng, C.-I. Optimized Collection Protocol for Plasma MicroRNA Measurement in Patients with Cardiovascular Disease. BioMed Res. Int. 2016, 2016, 2901938. [Google Scholar] [CrossRef]
- Marzi, M.J.; Montani, F.; Carletti, R.M.; Dezi, F.; Dama, E.; Bonizzi, G.; Sandri, M.T.; Rampinelli, C.; Bellomi, M.; Maisonneuve, P.; et al. Optimization and Standardization of Circulating MicroRNA Detection for Clinical Application: The miR-Test Case. Clin. Chem. 2016, 62, 743–754. [Google Scholar] [CrossRef]
- de Ronde, M.J.; Ruijter, J.M.; Moerland, P.D.; Creemers, E.E.; Pinto-Sietsma, S.-J. Study design and qPCR data analysis guidelines for reliable circulating miRNA biomarker experiments: A review. Clin. Chem. 2018, 64, 1308–1318. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Simundic, A.M.; Bölenius, K.; Cadamuro, J.; Church, S.; Cornes, M.P.; van Dongen-Lases, E.C.; Eker, P.; Erdeljanovic, T.; Grankvist, K.; Guimaraes, J.T.; et al. Joint EFLM-COLABIOCLI Recommendation for venous blood sampling. Clin. Chem. Lab. Med. 2018, 56, 2015–2038. [Google Scholar] [CrossRef] [PubMed]
- JCCLS Standard Phlebotomy Guideline; GP4-A3; Japanese Committee for Clinical Laboratory Standards: Tokyo, Japan, 2018.
- Ntelios, D.; Meditskou, S.; Efthimiadis, G.; Pitsis, A.; Nikolakaki, E.; Girtovitis, F.; Parcharidou, D.; Zegkos, T.; Kouidou, S.; Karvounis, H.; et al. Elevated plasma levels of miR-29a are associated with hemolysis in patients with hypertrophic cardiomyopathy. Clin. Chim. Acta 2017, 471, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Lee, E.J.; Jiang, J.; Sarkar, A.; Yang, L.; Elton, T.S.; Chen, C. Real-time PCR quantification of precursor and mature microRNA. Methods 2008, 44, 31–38. [Google Scholar] [CrossRef]
- Khan, J.; Lieberman, J.A.; Lockwood, C.M. Variability in, variability out: Best practice recommendations to standardize pre-analytical variables in the detection of circulating and tissue microRNAs. Clin. Chem. Lab. Med. 2017, 55, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Blondal, T.; Nielsen, S.J.; Baker, A.; Andreasen, D.; Mouritzen, P.; Teilum, M.W.; Dahlsveen, I.K. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- McCall, M.N.; Kim, M.S.; Adil, M.; Patil, A.H.; Lu, Y.; Mitchell, C.J.; Leal-Rojas, P.; Xu, J.; Kumar, M.; Dawson, V.L.; et al. Toward the human cellular microRNAome. Genome Res. 2017, 27, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Brook, A.C.; Jenkins, R.H.; Clayton, A.; Kift-Morgan, A.; Raby, A.C.; Shephard, A.P.; Mariotti, B.; Cuff, S.M.; Bazzoni, F.; Bowen, T.; et al. Neutrophil-derived miR-223 as local biomarker of bacterial peritonitis. Sci. Rep. 2019, 9, 10136. [Google Scholar] [CrossRef] [PubMed]
- Neudecker, V.; Brodsky, K.S.; Clambey, E.T.; Schmidt, E.P.; Packard, T.A.; Davenport, B.; Standiford, T.J.; Weng, T.; Fletcher, A.A.; Barthel, L.; et al. Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Sci. Transl. Med. 2017, 9, eaah5360. [Google Scholar] [CrossRef]
- de Kerckhove, M.; Tanaka, K.; Umehara, T.; Okamoto, M.; Kanematsu, S.; Hayashi, H.; Yano, H.; Nishiura, S.; Tooyama, S.; Matsubayashi, Y.; et al. Targeting miR-223 in neutrophils enhances the clearance of Staphylococcus aureus in infected wounds. EMBO Mol. Med. 2018, 10, e9024. [Google Scholar] [CrossRef]
- Laffont, B.; Corduan, A.; Plé, H.; Duchez, A.C.; Cloutier, N.; Boilard, E.; Provost, P. Activated platelets can deliver mRNA regulatory Ago2-microRNA complexes to endothelial cells via microparticles. Blood 2013, 122, 253–261. [Google Scholar] [CrossRef]
- Vermeersch, P.; Frans, G.; von Meyer, A.; Costelloe, S.; Lippi, G.; Simundic, A.-M. How to meet ISO15189:2012 pre-analytical requirements in clinical laboratories? A consensus document by the EFLM WG-PRE. Clin. Chem. Lab. Med. 2021, 59, 1047–1061. [Google Scholar] [CrossRef]
- Miller, L.H.; Baruch, D.I.; Marsh, K.; Doumbo, O.K. The pathogenic basis of malaria. Nature 2002, 415, 673–679. [Google Scholar] [CrossRef]
- Finotti, A.; Allegretti, M.; Gasparello, J.; Giacomini, P.; Spandidos, D.A.; Spoto, G.; Gambari, R. Liquid biopsy and PCR-free ultrasensitive system in oncology (Review). Int. J. Oncol. 2018, 53, 1395–1434. [Google Scholar] [CrossRef]
- Isobe, A.; Iwabuchi, Y.; Yajima, M.; Sakasegawa, S.I.; Yamaguchi, Y.; Seimiya, M.; Umemura, T.; Osawa, S. A high-sensitivity and rapid enzymatic method using a biochemical automated analyzer to detect inorganic pyrophosphate generated by nucleic acid sequence-based amplification. Clin. Chim. Acta 2020, 511, 298–305. [Google Scholar] [CrossRef]

| Hemolysis Index | Average ± SEM (n = 116) | Range | Normal Range |
|---|---|---|---|
| Hb | 4.37 ± 0.19 | 0.77–12.3 | <5 (mg/dL) |
| LD | 162.8 ± 3.80 | 75–325 | 120–220 (U/L) |
| AST | 22.9 ± 1.52 | 8–160 | 10–35 (U/L) |
| K | 4.2 ± 0.05 | 3.0–5.4 | 3.5–4.5 (mmol/L) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, Y.; Shibuta, T.; Hatano, M.; Sato, K.; Koga, M.; Ishibashi, A.; Harada, T.; Hisatomi, T.; Shimura, H.; Fukushima, N.; et al. Pre-Analytical Modification of Serum miRNAs: Diagnostic Reliability of Serum miRNAs in Hemolytic Diseases. J. Clin. Med. 2021, 10, 5045. https://doi.org/10.3390/jcm10215045
Takada Y, Shibuta T, Hatano M, Sato K, Koga M, Ishibashi A, Harada T, Hisatomi T, Shimura H, Fukushima N, et al. Pre-Analytical Modification of Serum miRNAs: Diagnostic Reliability of Serum miRNAs in Hemolytic Diseases. Journal of Clinical Medicine. 2021; 10(21):5045. https://doi.org/10.3390/jcm10215045
Chicago/Turabian StyleTakada, Yukichi, Tatsuki Shibuta, Mayu Hatano, Kenichi Sato, Mari Koga, Ayaka Ishibashi, Tetsuhiro Harada, Takashi Hisatomi, Hanae Shimura, Noriyasu Fukushima, and et al. 2021. "Pre-Analytical Modification of Serum miRNAs: Diagnostic Reliability of Serum miRNAs in Hemolytic Diseases" Journal of Clinical Medicine 10, no. 21: 5045. https://doi.org/10.3390/jcm10215045
APA StyleTakada, Y., Shibuta, T., Hatano, M., Sato, K., Koga, M., Ishibashi, A., Harada, T., Hisatomi, T., Shimura, H., Fukushima, N., Leecharoenkiat, K., Chamnanchanunt, S., Svasti, S., Fucharoen, S., & Umemura, T. (2021). Pre-Analytical Modification of Serum miRNAs: Diagnostic Reliability of Serum miRNAs in Hemolytic Diseases. Journal of Clinical Medicine, 10(21), 5045. https://doi.org/10.3390/jcm10215045






