Prevalence of Cancer in Acid Sphingomyelinase Deficiency
Abstract
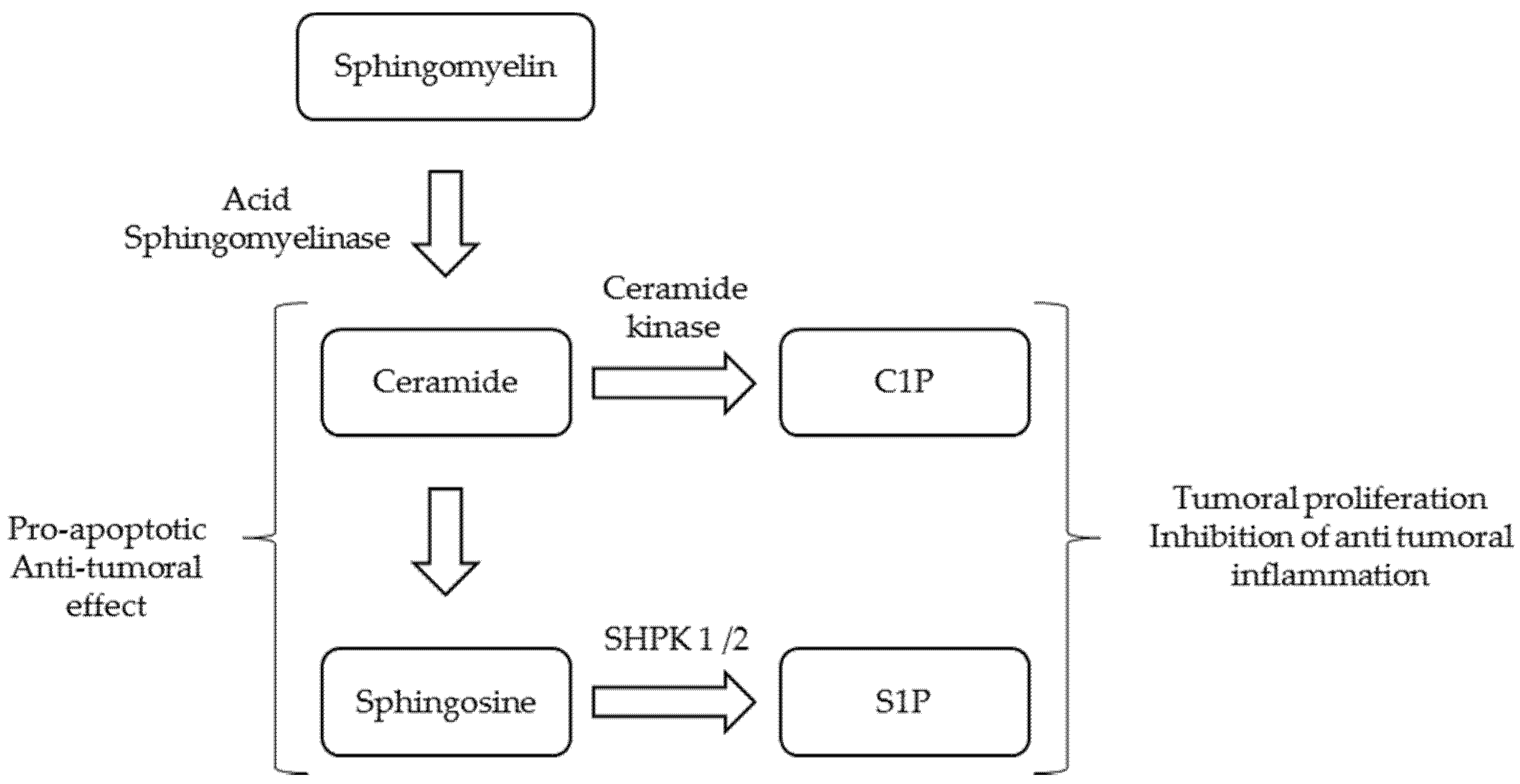
:1. Introduction
2. Patients and Methods
3. Results
3.1. Patients
3.2. Description of Cancers
3.2.1. Lung Cancers (n = 2)
3.2.2. Bladder Cancer (n = 1)
3.2.3. Papillary Thyroid Carcinoma (n = 1)
3.2.4. Breast Cancer (n = 1)
3.3. Conditions Associated with Cancer
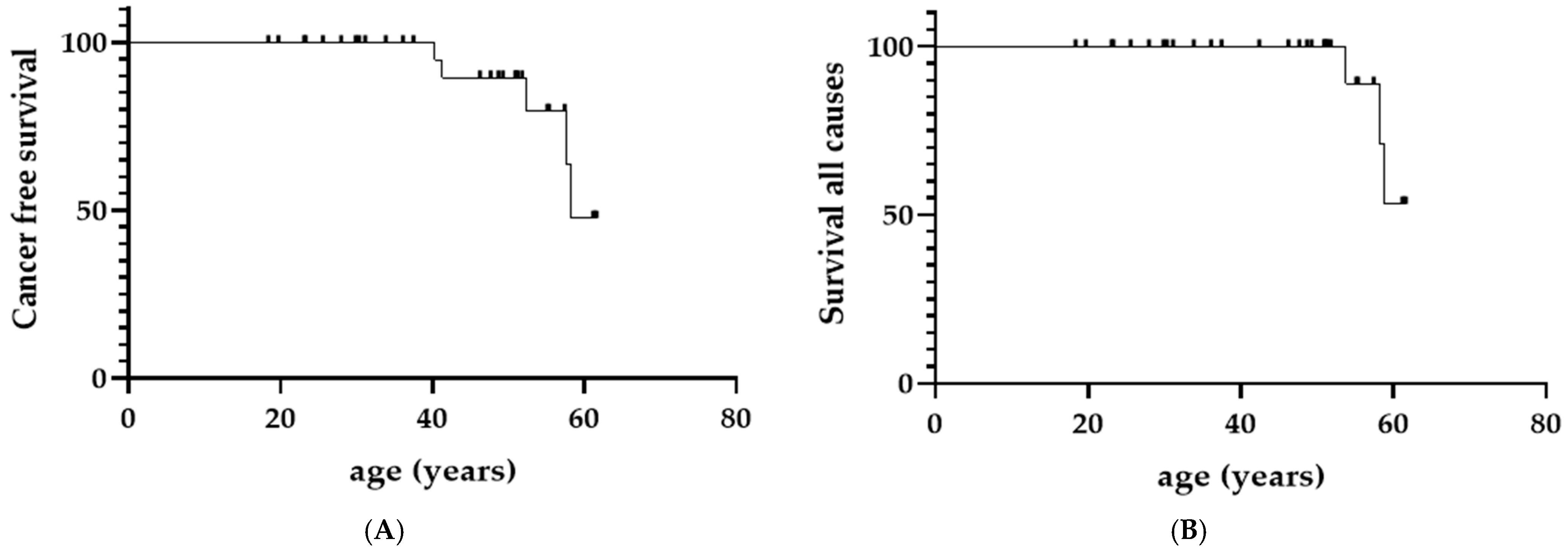
3.4. Survival Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kingma, S.D.; Bodamer, O.A.; Wijburg, F.A. Epidemiology and diagnosis of lysosomal storage disorders; challenges of screening. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Schuchman, E.H.; Desnick, R.J. Types A and B Niemann-Pick disease. Mol. Genet. Metab. 2016, 120, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Lafrasse, C.; Vanier, M.T. Sphingosylphosphorylcholine in Niemann-Pick disease brain: Accumulation in type A but not in type B. Neurochem. Res. 1999, 24, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cassiman, D.; Packman, S.; Bembi, B.; Ben Turkia, H.; Al-Sayed, M.; Schiff, M.; Imrie, J.; Mabe, P.; Takahashi, T.; Mengel, K.E.; et al. Cause of death in patients with chronic visceral and chronic neurovisceral acid sphingomyelinase deficiency (Niemann-Pick disease type B and B variant): Literature review and report of new cases. Mol. Genet. Metab. 2016, 118, 206–213. [Google Scholar] [CrossRef]
- McGovern, M.M.; Wasserstein, M.P.; Bembi, B.; Giugliani, R.; Mengel, K.E.; Vanier, M.T.; Zhang, Q.; Peterschmitt, M.J. Prospective study of the natural history of chronic acid sphingomyelinase deficiency in children and adults: Eleven years of observation. Orphanet J. Rare Dis. 2021, 16, 212. [Google Scholar] [CrossRef]
- Vanier, M.T. Niemann-Pick diseases. Handb. Clin. Neurol. 2013, 113, 1717–1721. [Google Scholar]
- Sabourdy, F.; Selves, J.; Astudillo, L.; Laurent, C.; Brousset, P.; Delisle, M.-B.; Therville, N.; Andrieu-Abadie, N.; Ségui, B.; Recher, C.; et al. Is active acid sphingomyelinase required for the antiproliferative response to rituximab? Blood 2011, 117, 3695–3696. [Google Scholar] [CrossRef] [Green Version]
- Kanda, Y. Statistical analysis using freely-available “EZR (Easy R)” software. Rinsho Ketsueki 2015, 56, 2258–2266. [Google Scholar] [CrossRef]
- Vanier, M.T.; Revol, A.; Fichet, M. Sphingomyelinase activities of various human tissues in control subjects and in Niemann-Pick disease — development and evaluation of a microprocedure. Clin. Chim. Acta 1980, 106, 257–267. [Google Scholar] [CrossRef]
- Wasserstein, M.; Dionisi-Vici, C.; Giugliani, R.; Hwu, W.-L.; Lidove, O.; Lukacs, Z.; Mengel, E.; Mistry, P.K.; Schuchman, E.H.; McGovern, M. Recommendations for clinical monitoring of patients with acid sphingomyelinase deficiency (ASMD). Mol. Genet. Metab. 2018, 126, 98–105. [Google Scholar] [CrossRef]
- Jones, S.A.; McGovern, M.; Lidove, O.; Giugliani, R.; Mistry, P.K.; Dionisi-Vici, C.; Munoz-Rojas, M.-V.; Nalysnyk, L.; Schecter, A.D.; Wasserstein, M. Clinical relevance of endpoints in clinical trials for acid sphingomyelinase deficiency enzyme replacement therapy. Mol. Genet. Metab. 2020, 131, 116–123. [Google Scholar] [CrossRef]
- McGovern, M.M.; Wasserstein, M.P.; Giugliani, R.; Bembi, B.; Vanier, M.; Mengel, E.; Brodie, S.E.; Mendelson, D.; Skloot, G.; Desnick, R.J.; et al. A Prospective, Cross-Sectional Survey Study of the Natural History of Niemann-Pick B Disease. Pediatrics 2008, 122, e341–e349. [Google Scholar] [CrossRef] [Green Version]
- Breilyn, M.S.; Zhang, W.; Yu, C.; Wasserstein, M.P. Plasma lyso-sphingomyelin levels are positively associated with clinical severity in acid sphingomyelinase deficiency. Mol. Genet. Metab. Rep. 2021, 28, 100780. [Google Scholar] [CrossRef]
- Ghosh, S.; Juin, S.K.; Majumdar, S. Cancer stem cells and ceramide signaling: The cutting edges of immunotherapy. Mol. Biol. Rep. 2020, 47, 8101–8111. [Google Scholar] [CrossRef]
- Sedić, M.; Grbčić, P.; Pavelić, S.K. Bioactive Sphingolipids as Biomarkers Predictive of Disease Severity and Treatment Response in Cancer: Current Status and Translational Challenges. Anticancer. Res. 2018, 39, 41–56. [Google Scholar] [CrossRef]
- Hait, N.C.; Maiti, A. The Role of Sphingosine-1-Phosphate and Ceramide-1-Phosphate in Inflammation and Cancer. Mediat. Inflamm. 2017, 2017, 4806541. [Google Scholar] [CrossRef]
- Osawa, Y.; Suetsugu, A.; Matsushima-Nishiwaki, R.; Yasuda, I.; Saibara, T.; Moriwaki, H.; Seishima, M.; Kozawa, O. Liver acid sphingomyelinase inhibits growth of metastatic colon cancer. J. Clin. Investig. 2013, 123, 834–843. [Google Scholar] [CrossRef] [Green Version]
- Scheel-Toellner, D.; Wang, K.; Assi, L.; Webb, P.; Craddock, R.; Salmon, M.; Lord, J. Clustering of death receptors in lipid rafts initiates neutrophil spontaneous apoptosis. Biochem. Soc. Trans. 2004, 32, 679–681. [Google Scholar] [CrossRef] [Green Version]
- Bai, A.; Kokkotou, E.; Zheng, Y.; Robson, S.C. Role of acid sphingomyelinase bioactivity in human CD4+ T-cell activation and immune responses. Cell Death Dis. 2015, 6, e1828. [Google Scholar] [CrossRef] [Green Version]
- Herz, J.; Pardo, J.; Kashkar, H.; Schramm, M.; Kuzmenkina, E.; Bos, E.; Wiegmann, K.; Wallich, R.; Peters, P.J.; Herzig, S.; et al. Acid sphingomyelinase is a key regulator of cytotoxic granule secretion by primary T lymphocytes. Nat. Immunol. 2009, 10, 761–768. [Google Scholar] [CrossRef]
- Bai, A.; Moss, A.; Kokkotou, E.; Usheva, A.; Sun, X.; Cheifetz, A.; Zheng, Y.; Longhi, M.S.; Gao, W.; Wu, Y.; et al. CD39 and CD161 Modulate Th17 Responses in Crohn’s Disease. J. Immunol. 2014, 193, 3366–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider-Schaulies, J.; Beyersdorf, N. CD4+ Foxp3+ regulatory T cell-mediated immunomodulation by anti-depressants inhibiting acid sphingomyelinase. Biol. Chem. 2018, 399, 1175–1182. [Google Scholar] [CrossRef]
- Nair, S.; Branagan, A.; Liu, J.; Boddupalli, C.S.; Mistry, P.K.; Dhodapkar, M.V. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N. Engl. J. Med. 2016, 374, 555–561. [Google Scholar] [CrossRef]
- Nair, S.; Boddupalli, C.S.; Verma, R.; Liu, J.; Yang, R.; Pastores, G.M.; Mistry, P.K.; Dhodapkar, M.V. Type II NKT-TFH cells against Gaucher lipids regulate B-cell immunity and inflammation. Blood 2015, 125, 1256–1271. [Google Scholar] [CrossRef] [Green Version]
- Melum, E.; Jiang, X.; Baker, K.D.; Macedo, M.F.; Fritsch, J.; Dowds, C.M.; Wang, J.; Pharo, A.; Kaser, A.; Tan, C.; et al. Control of CD1d-restricted antigen presentation and inflammation by sphingomyelin. Nat. Immunol. 2019, 20, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Wasserstein, M.P.; Diaz, G.A.; Lachmann, R.H.; Jouvin, M.-H.; Nandy, I.; Ji, A.J.; Puga, A.C. Olipudase alfa for treatment of acid sphingomyelinase deficiency (ASMD): Safety and efficacy in adults treated for 30 months. J. Inherit. Metab. Dis. 2018, 41, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurberg, B.L.; Diaz, G.A.; Lachmann, R.H.; Schiano, T.; Wasserstein, M.P.; Ji, A.J.; Zaher, A.; Peterschmitt, M.J. Long-term efficacy of olipudase alfa in adults with acid sphingomyelinase deficiency (ASMD): Further clearance of hepatic sphingomyelin is associated with additional improvements in pro- and anti-atherogenic lipid profiles after 42 months of treatment. Mol. Genet. Metab. 2020, 131, 245–252. [Google Scholar] [CrossRef]
| Data Available, n | ||
|---|---|---|
| Total, n | 31 | |
| Males, n | 19 | |
| Females, n | 12 | |
| Peripheral leucocyte ASM activity (Median, IQ) | 0.36 nmol/h/mg protein (IQ: 0.21–0.55) | 24 |
| Age at diagnosis (Median, IQ) | 8.1 y. (1.8–42.3) | 31 |
| Age at last follow-up (Median, IQ) | 48.7 y. (30.3–55.1) | 31 |
| Body mass index (Median, IQ) | 24.2 kg/m2 (21.5–27.6) | 31 |
| Consanguinity | 51.6% | 31 |
| Adrenal gland abnormality | 72.2% | 18 |
| Spleen size (Median, IQ) | 18 cm (17–22) | 23 |
| DLCO (Median, IQ) | 51.5% (IQ: 42–69) | 24 |
| Haemoglobin (Median, IQ) | 14.3 g/dL (13.7–14.9) | 26 |
| Platelets (Median, IQ) | 130 g/L (98–140) | 31 |
| HDL cholesterol (Median, IQ) | 0.25 g/L (0.18–0.30) | 21 |
| Tobacco exposure | 53.6% | 28 |
| Alcohol exposure | 42.3% | 26 |
| Cancer | 5 | 31 |
| Death | 3 | 31 |
| Cancer | No Cancer | p-Value | |
|---|---|---|---|
| n | 5 | 26 | |
| Gender male/female | 4/1 | 15/11 | 0.6 |
| Consanguinity (n, %) | 3 (60%) | 13 (50%) | 1 |
| Median age at last visit (IQ) (years) | 53.8 (51.4–57.7) | 46.9 (30.0–51.7) | 0.2 |
| BMI (kg/m2) | 25.7 (25.2–29.4) | 24.0 (21.4–26.9) | 0.09 |
| Spleen diameter (cm) | 25 (22.5–25) | 18 (17–20) | 0.04 |
| n available data | 3 | 19 | |
| Platelets (g/L) | 197 (91–274) | 131 (103–146) | 0.5 |
| DLCO (%) | 29.5 (17.8–43.0) | 58.5 (49.8–69.5) | 0.01 |
| n available data | 4 | 20 | |
| HDL cholesterol (g/L) | 0.27 (0.18–0.29) | 0.27 (0.20–0.32) | 0.7 |
| n available data | 5 | 16 | |
| Tobacco use n (%) | 5/5 (100) | 10/23 (43.5) | 0.004 |
| Alcohol use n (%) | 2/5 (40) | 9/20 (45) | 1 |
| Death n (%) | 3 (60) | 0 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauhin, W.; Levade, T.; Vanier, M.T.; Froissart, R.; Lidove, O. Prevalence of Cancer in Acid Sphingomyelinase Deficiency. J. Clin. Med. 2021, 10, 5029. https://doi.org/10.3390/jcm10215029
Mauhin W, Levade T, Vanier MT, Froissart R, Lidove O. Prevalence of Cancer in Acid Sphingomyelinase Deficiency. Journal of Clinical Medicine. 2021; 10(21):5029. https://doi.org/10.3390/jcm10215029
Chicago/Turabian StyleMauhin, Wladimir, Thierry Levade, Marie T. Vanier, Roseline Froissart, and Olivier Lidove. 2021. "Prevalence of Cancer in Acid Sphingomyelinase Deficiency" Journal of Clinical Medicine 10, no. 21: 5029. https://doi.org/10.3390/jcm10215029
APA StyleMauhin, W., Levade, T., Vanier, M. T., Froissart, R., & Lidove, O. (2021). Prevalence of Cancer in Acid Sphingomyelinase Deficiency. Journal of Clinical Medicine, 10(21), 5029. https://doi.org/10.3390/jcm10215029





