Machine Learning Prediction Models for Mortality in Intensive Care Unit Patients with Lactic Acidosis
Abstract
1. Introduction
2. Methods
2.1. Patient Population
2.2. Data Collection
2.3. Model Development
2.4. Model Evaluation and Calibration
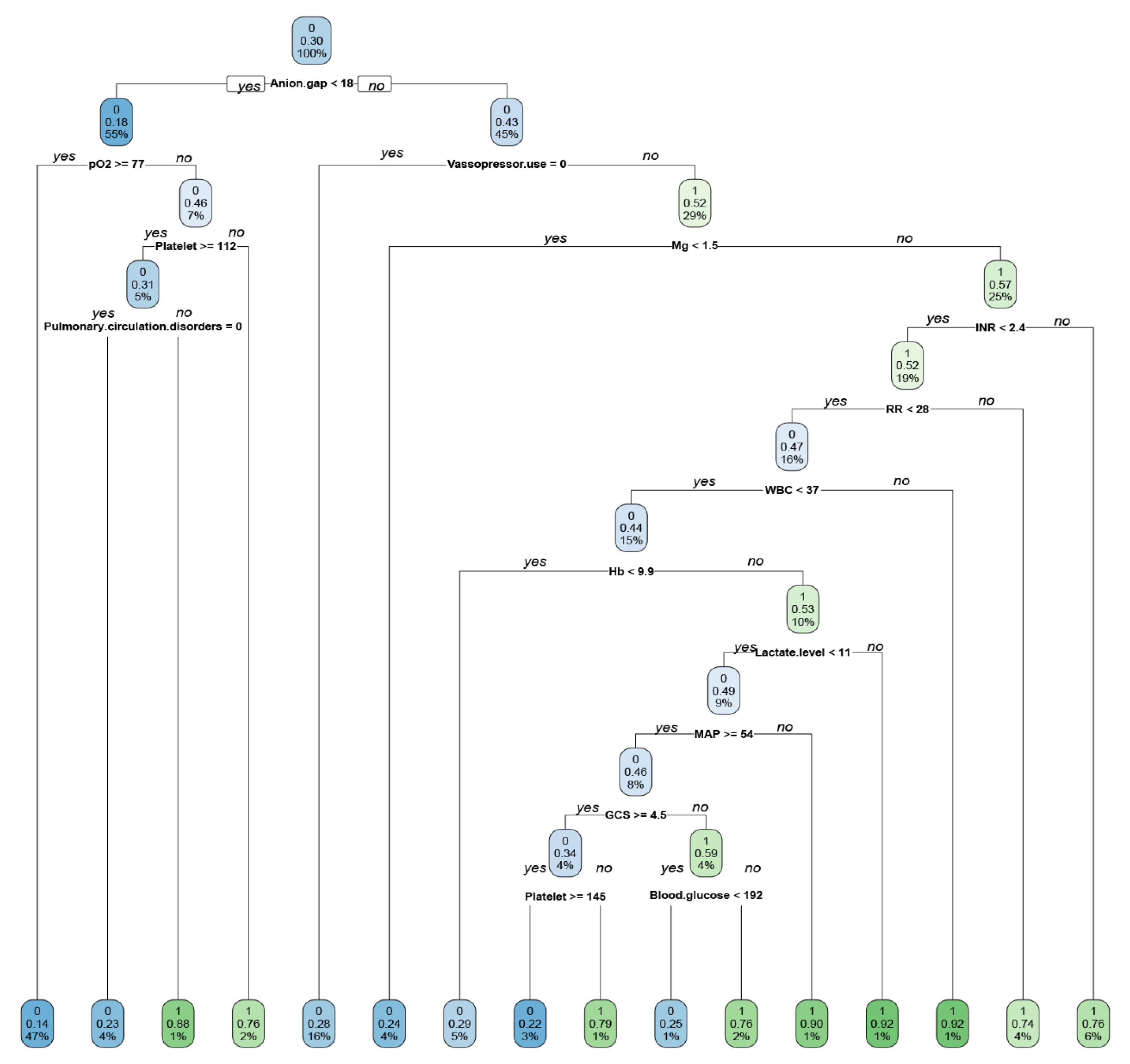
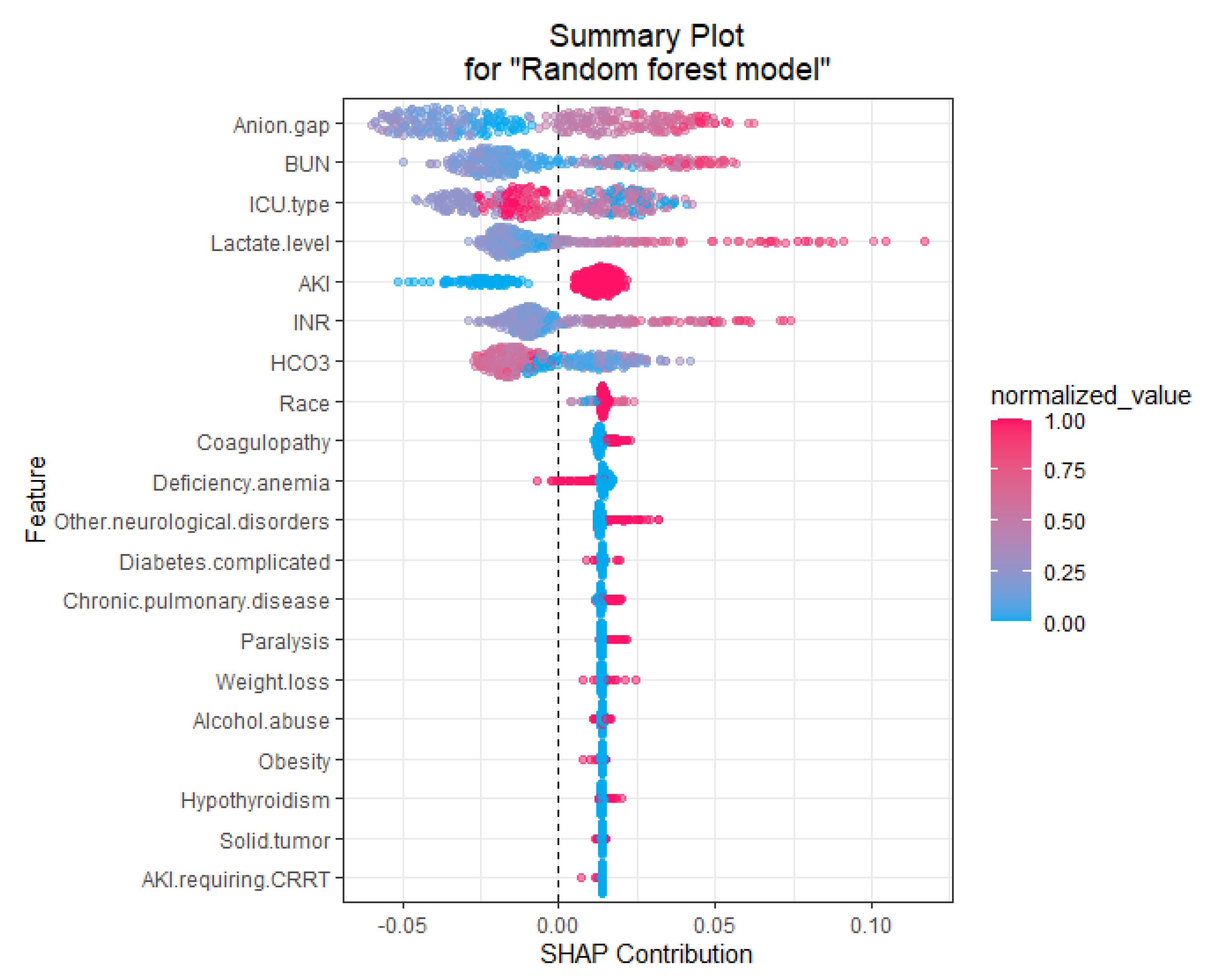
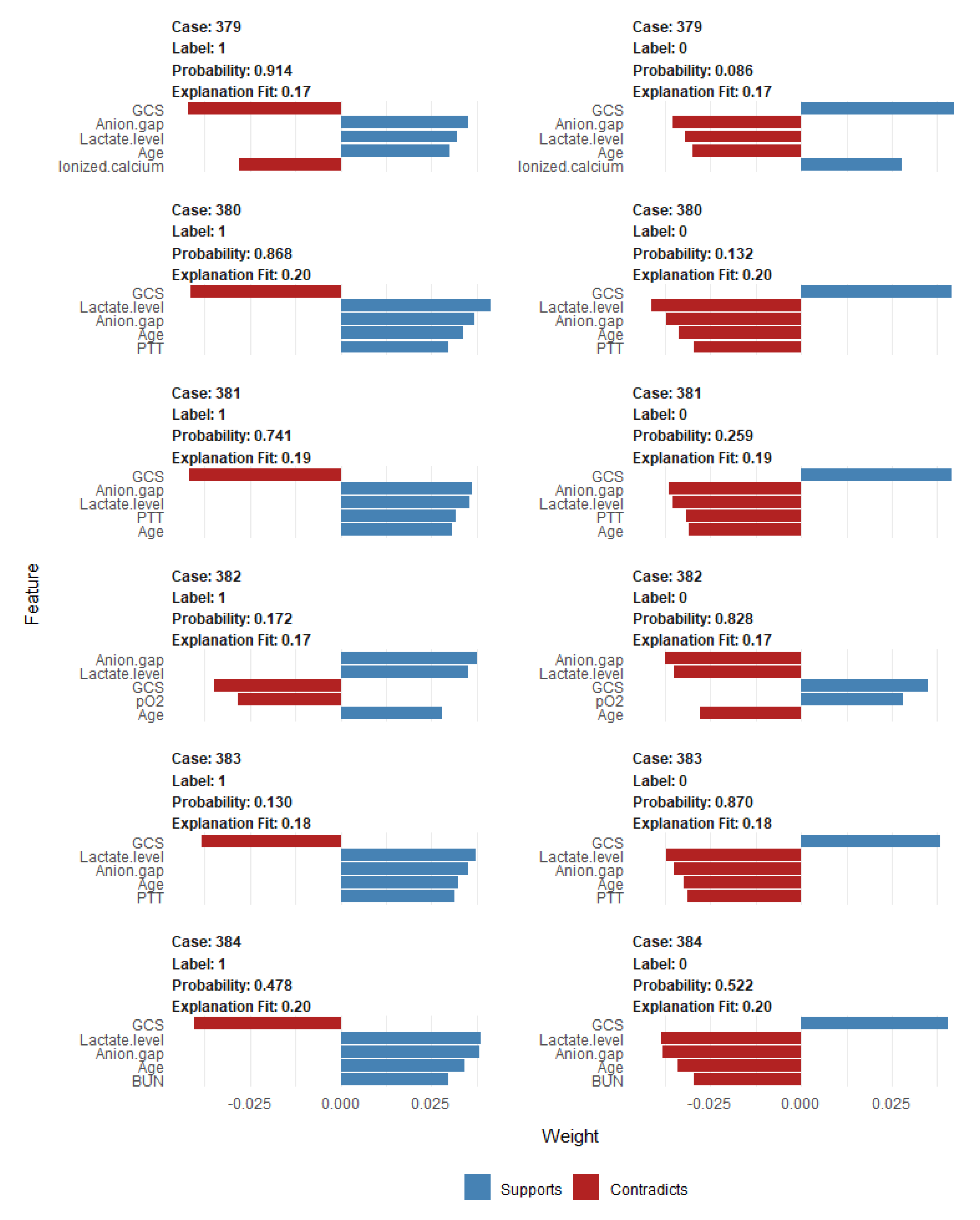
2.5. Explanations of the Features in the ML-Based Prediction Model That Drive Patient-Specific Predictions of Mortality
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vernon, C.; LeTourneau, J.L. Lactic Acidosis: Recognition, Kinetics, and Associated Prognosis. Crit. Care Clin. 2010, 26, 255–283. [Google Scholar] [CrossRef] [PubMed]
- Levy, B. Lactate and shock state: The metabolic view. Curr. Opin. Crit. Care 2006, 12, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, S.; Dellinger, R.P.; Chansky, M.E.; Arnold, R.C.; Schorr, C.; Milcarek, B.; Hollenberg, S.M.; Parrillo, J.E. Serum lactate as a predictor of mortality in patients with infection. Intensiv. Care Med. 2007, 33, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.C.; van Bommel, J.; Woodward, R.; Mulder, P.G.H.; Bakker, J. Association between blood lactate levels, Sequential Organ Failure Assessment subscores, and 28-day mortality during early and late intensive care unit stay: A retrospective observational study. Crit. Care Med. 2009, 37, 2369–2374. [Google Scholar] [CrossRef]
- Levraut, J.; Ciebiera, J.-P.; Chave, S.; Rabary, O.; Jambou, P.; Carles, M.; Grimaud, D. Mild Hyperlactatemia in Stable Septic Patients Is Due to Impaired Lactate Clearance Rather Than Overproduction. Am. J. Respir. Crit. Care Med. 1998, 157, 1021–1026. [Google Scholar] [CrossRef]
- Filho, R.R.; Rocha, L.L.; Corrêa, T.D.; Pessoa, C.M.S.; Colombo, G.; Assuncao, M.S.C. Blood Lactate Levels Cutoff and Mortality Prediction in Sepsis—Time for a Reappraisal? A Retrospective Cohort Study. Shock 2016, 46, 480–485. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Zand, L.; Dillon, J.J.; Qian, Q.; Leung, N. Lactate clearance and metabolic aspects of continuous high-volume hemofiltration. Clin Kidney J 2015, 8, 374–377. [Google Scholar] [CrossRef]
- Kreisberg, R.A. Lactate Homeostasis and Lactic Acidosis. Ann. Intern. Med. 1980, 92, 227–237. [Google Scholar] [CrossRef]
- Bakker, J.; Gris, P.; Coffernils, M.; Kahn, R.J.; Vincent, J.-L. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am. J. Surg. 1996, 171, 221–226. [Google Scholar] [CrossRef]
- Sammour, T.; Kahokehr, A.; Caldwell, S.; Hill, A.G. Venous glucose and arterial lactate as biochemical predictors of mortality in clinically severely injured trauma patients—A comparison with ISS and TRISS. Injury 2009, 40, 104–108. [Google Scholar] [CrossRef]
- Lavery, R.F.; Livingston, D.H.; Tortella, B.J.; Sambol, J.T.; Slomovitz, B.M.; Siegel, J.H. The utility of venous lactate to triage injured patients in the trauma center. J. Am. Coll. Surg. 2000, 190, 656–664. [Google Scholar] [CrossRef]
- O’Connor, E.; Fraser, J.F. The Interpretation of Perioperative Lactate Abnormalities in Patients Undergoing Cardiac Surgery. Anaesth. Intensiv. Care 2012, 40, 598–603. [Google Scholar] [CrossRef]
- Ranucci, M.; De Toffol, B.; Isgrò, G.; Romitti, F.; Conti, D.; Vicentini, M. Hyperlactatemia during cardiopulmonary bypass: Determinants and impact on postoperative outcome. Crit. Care 2006, 10, R167. [Google Scholar] [CrossRef]
- Toraman, F.; Evrenkaya, S.; Yuce, M.; Aksoy, N.; Karabulut, H.; Bozkulak, Y.; Alhan, C. Lactic Acidosis after Cardiac Surgery Is Associated with Adverse Outcome. Hear. Surg. Forum 2004, 7, E155–E159. [Google Scholar] [CrossRef]
- Renew, J.R.; Barbara, D.W.; Hyder, J.A.; Dearani, J.A.; Rivera, M.; Pulido, J.N. Frequency and outcomes of severe hyperlactatemia after elective cardiac surgery. J. Thorac. Cardiovasc. Surg. 2016, 151, 825–830. [Google Scholar] [CrossRef]
- Maillet, J.-M.; Le Besnerais, P.; Cantoni, M.; Nataf, P.; Ruffenach, A.; Lessana, A.; Brodaty, D. Frequency, Risk Factors, and Outcome of Hyperlactatemia After Cardiac Surgery. Chest 2003, 123, 1361–1366. [Google Scholar] [CrossRef]
- Demers, P.; Elkouri, S.; Martineau, R.; Couturier, A.; Cartier, R. Outcome with high blood lactate levels during cardiopulmonary bypass in adult cardiac operation. Ann. Thorac. Surg. 2000, 70, 2082–2086. [Google Scholar] [CrossRef]
- Ranucci, M.; Ballotta, A.; Castelvecchio, S.; Baryshnikova, E.; Brozzi, S.; Boncilli, A. Intensive Care Unit Admission Parameters Improve the Accuracy of Operative Mortality Predictive Models in Cardiac Surgery. PLoS ONE 2010, 5, e13551. [Google Scholar] [CrossRef][Green Version]
- Peters, N.; Jay, N.; Barraud, D.; Cravoisy, A.; Nace, L.; Bollaert, P.-E.; Gibot, S. Metformin-associated lactic acidosis in an intensive care unit. Crit. Care 2008, 12, R149. [Google Scholar] [CrossRef]
- Biradar, V.; Moran, J.L.; Peake, S.L.; Peter, J.V. Metformin-associated lactic acidosis (MALA): Clinical profile and outcomes in patients admitted to the intensive care unit. Crit. Care Resusc. 2010, 12, 191–195. [Google Scholar]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008, 36, 296–327. [Google Scholar] [CrossRef]
- Weil, M.H.; Afifi, A.A. Experimental and Clinical Studies on Lactate and Pyruvate as Indicators of the Severity of Acute Circulatory Failure (Shock). Circulation 1970, 41, 989–1001. [Google Scholar] [CrossRef]
- Baysan, M.; Baroni, G.D.; van Boekel, A.M.; Steyerberg, E.W.; Arbous, M.S.; van der Bom, J.G. The Added Value of Lactate and Lactate Clearance in Prediction of In-Hospital Mortality in Critically Ill Patients With Sepsis. Crit. Care Explor. 2020, 2, e0087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, K.; Ni, H.; Fan, H. Predictive value of lactate in unselected critically ill patients: An analysis using fractional polynomials. J. Thorac. Dis. 2014, 6, 995–1003. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chan, K.-S.; Tseng, K.-L.; Weng, S.-F. Prognosis of alcohol-associated lactic acidosis in critically ill patients: An 8-year study. Sci. Rep. 2016, 6, 35368. [Google Scholar] [CrossRef]
- Drolz, A.; Horvatits, T.; Rutter, K.; Landahl, F.; Roedl, K.; Meersseman, P.; Wilmer, A.; Kluwe, J.; Lohse, A.W.; Kluge, S.; et al. Lactate Improves Prediction of Short-Term Mortality in Critically Ill Patients With Cirrhosis: A Multinational Study. Hepatology 2019, 69, 258–269. [Google Scholar] [CrossRef]
- Hayashi, Y.; Endoh, H.; Kamimura, N.; Tamakawa, T.; Nitta, M. Lactate indices as predictors of in-hospital mortality or 90-day survival after admission to an intensive care unit in unselected critically ill patients. PLoS ONE 2020, 15, e0229135. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, H.; Balling, R.; Beerenwinkel, N.; Kohlbacher, O.; Kumar, S.; Lengauer, T.; Maathuis, M.H.; Moreau, Y.; Murphy, S.A.; Przytycka, T.M.; et al. From hype to reality: Data science enabling personalized medicine. BMC Med. 2018, 16, 1–15. [Google Scholar] [CrossRef]
- Sohail, A.; Arif, F. Supervised and unsupervised algorithms for bioinformatics and data science. Prog. Biophys. Mol. Biol. 2020, 151, 14–22. [Google Scholar] [CrossRef]
- Lin, K.; Hu, Y.; Kong, G. Predicting in-hospital mortality of patients with acute kidney injury in the ICU using random forest model. Int. J. Med. Inform. 2019, 125, 55–61. [Google Scholar] [CrossRef]
- Raita, Y.; Goto, T.; Faridi, M.K.; Brown, D.F.M.; Camargo, C.A., Jr.; Hasegawa, K. Emergency department triage prediction of clinical outcomes using machine learning models. Crit. Care 2019, 23, 64. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.E.W.; Ghassemi, M.M.; Nemati, S.; Niehaus, K.E.; Clifton, D.A.; Clifford, G. Machine Learning and Decision Support in Critical Care. Proc. IEEE 2016, 104, 444–466. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.B.; Thorsen-Meyer, H.-C.; Belling, K.; Nielsen, A.P.; Thomas, C.E.; Chmura, P.; Lademann, M.; Moseley, P.L.; Heimann, M.; Dybdahl, L.; et al. Survival prediction in intensive-care units based on aggregation of long-term disease history and acute physiology: A retrospective study of the Danish National Patient Registry and electronic patient records. Lancet Digit. Health 2019, 1, e78–e89. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Chen, C.-M.; Hsieh, C.-C.; Chao, C.-M.; Lai, C.-C. Comparison of machine learning models for the prediction of mortality of patients with unplanned extubation in intensive care units. Sci. Rep. 2018, 8, 17116. [Google Scholar] [CrossRef]
- Manz, C.R.; Chen, J.; Liu, M.; Chivers, C.; Regli, S.H.; Braun, J.; Draugelis, M.; Hanson, C.W.; Shulman, L.N.; Schuchter, L.M.; et al. Validation of a Machine Learning Algorithm to Predict 180-Day Mortality for Outpatients With Cancer. JAMA Oncol. 2020, 6, 1723. [Google Scholar] [CrossRef]
- Johnson, A.E.W.; Pollard, T.J.; Shen, L.; Lehman, L.-W.H.; Feng, M.; Ghassemi, M.; Moody, B.; Szolovits, P.; Anthony Celi, L.; Mark, R.G. MIMIC-III, a freely accessible critical care database. Sci. Data 2016, 3, 160035. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. J. Br. Surg. 2015, 102, 148–158. [Google Scholar] [CrossRef]
- Singh, D.; Singh, B. Investigating the impact of data normalization on classification performance. Appl. Soft Comput. 2020, 97, 105524. [Google Scholar] [CrossRef]
- Harvey, H.B.; Sotardi, S.T. The Pareto Principle. J. Am. Coll. Radiol. 2018, 15, 931. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Huang, J.-C.; Tsai, Y.-C.; Wu, P.-Y.; Lien, Y.-H.; Chien, C.-Y.; Kuo, C.-F.; Hung, J.-F.; Chen, S.-C.; Kuo, C.-H. Predictive modeling of blood pressure during hemodialysis: A comparison of linear model, random forest, support vector regression, XGBoost, LASSO regression and ensemble method. Comput. Methods Programs Biomed. 2020, 195, 105536. [Google Scholar] [CrossRef] [PubMed]
- Muchlinski, D.; Siroky, D.; He, J.; Kocher, M. Comparing Random Forest with Logistic Regression for Predicting Class-Imbalanced Civil War Onset Data. Political Anal. 2016, 24, 87–103. [Google Scholar] [CrossRef]
- Bergstra, J.; Bardenet, R.; Bengio, Y.; Kégl, B. Algorithms for hyper-parameter optimization. Adv. Neural Inf. Process. Syst. 2011, 24, 2546–2554. [Google Scholar]
- McGee, S. Simplifying likelihood ratios. J. Gen. Intern. Med. 2002, 17, 647–650. [Google Scholar] [CrossRef]
- Zou, K.H.; O’Malley, A.J.; Mauri, L. Receiver-Operating Characteristic Analysis for Evaluating Diagnostic Tests and Predictive Models. Circulation 2007, 115, 654–657. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Macheret, F.; Gabriel, R.A.; Ohno-Machado, L. A tutorial on calibration measurements and calibration models for clinical prediction models. J. Am. Med. Inform. Assoc. 2020, 27, 621–633. [Google Scholar] [CrossRef]
- Stekhoven, D.J.; Bühlmann, P. MissForest—Non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef]
- Moreno, R.P.; Metnitz, P.G.H.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.A.; Iapichino, G.; Edbrooke, D.; Capuzzo, M.; Le Gall, J.-R. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005, 31, 1345–1355. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Ma, C.; Wang, X.Q.; Seelye, S.; Zhu, J.; Waljee, A.K. Variation in model performance by data cleanliness and classification methods in the prediction of 30-day ICU mortality, a US nationwide retrospective cohort and simulation study. BMJ Open 2020, 10, e041421. [Google Scholar] [CrossRef]
- Fika, S.; Nanas, S.; Baltopoulos, G.; Charitidou, E.; Myrianthefs, P. A novel mortality prediction model for the current population in an adult intensive care unit. Heart Lung 2018, 47, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Loreto, M.; Lisboa, T.; Moreira, V.P. Early prediction of ICU readmissions using classification algorithms. Comput. Biol. Med. 2020, 118, 103636. [Google Scholar] [CrossRef] [PubMed]
- Handelman, G.S.; Kok, H.K.; Chandra, R.V.; Razavi, A.H.; Lee, M.J.; Asadi, H. eDoctor: Machine learning and the future of medicine. J. Intern. Med. 2018, 284, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Chik, C.; Hayes, G.M.; Menard, J. Development of a veterinary trauma score (VetCOT) in canine trauma patients with per-formance evaluation and comparison to the animal trauma triage score: A VetCOT registry study. J. Vet. Emerg. Crit. Care 2021. [Google Scholar] [CrossRef]
- Kasapoğlu, U.S.; Kaçmaz, O.; Gök, A.; Yildiz Eglen, M.; Şayan, H.; Çolak, F. Prognostic factors for 30-days mortality in eighty years aged and older critically ill patients: A single center retrospec-tive cohort study. Turk. J. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Lim, J.-H.; Jeon, Y.; Ahn, J.-S.; Kim, S.; Kim, D.K.; Lee, J.P.; Ryu, D.-R.; Seong, E.Y.; Ahn, S.Y.; Baek, S.H.; et al. GDF-15 Predicts In-Hospital Mortality of Critically Ill Patients with Acute Kidney Injury Requiring Continuous Renal Replacement Therapy: A Multicenter Prospective Study. J. Clin. Med. 2021, 10, 3660. [Google Scholar] [CrossRef]
- Kahneman, D.; Lovallo, D.; Sibony, O. Before you make that big decision. Harv. Bus. Rev. 2011, 89, 50. [Google Scholar]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Rashidi, H.H.; Tran, N.K.; Betts, E.V.; Howell, L.P.; Green, R. Artificial Intelligence and Machine Learning in Pathology: The Present Landscape of Supervised Methods. Acad. Pathol. 2019, 6, 2374289519873088. [Google Scholar] [CrossRef]
- Wasserman, L.; Roeder, K. High-dimensional variable selection. Ann. Stat. 2009, 37, 2178–2201. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Nair, B.; Vavilala, M.S.; Horibe, M.; Eisses, M.J.; Adams, T.; Liston, D.E.; Low, D.K.-W.; Newman, S.-F.; Kim, J.; et al. Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat. Biomed. Eng. 2018, 2, 749–760. [Google Scholar] [CrossRef]

| Characteristics | All (n = 1919) | Training Set (n = 1535) | Testing Set (n = 384) | p-Value |
|---|---|---|---|---|
| Age (years) | 61.8 ± 17.1 | 61.5 ± 17.0 | 63.0 ± 17.3 | 0.13 |
| Male sex | 1118 (58) | 889 (58) | 229 (60) | 0.54 |
| Race | 0.85 | |||
| White | 1560 (81) | 1246 (81) | 314 (82) | |
| Black | 152 (8) | 124 (8) | 28 (7) | |
| Hispanic | 79 (4) | 56 (4) | 14 (4) | |
| Other | 128 (7) | 100 (7) | 28 (7) | |
| ICU type | 0.75 | |||
| Cardiac ICU | 206 (11) | 164 (11) | 42 (11) | |
| Cardiac surgery ICU | 467 (24) | 375 (24) | 92 (24) | |
| Medical ICU | 605 (32) | 475 (31) | 130 (34) | |
| Surgical ICU | 295 (15) | 243 (16) | 52 (13) | |
| Trauma/surgical ICU | 346 (18) | 278 (18) | 68 (18) | |
| Elixhauser Comorbidities | ||||
| Congestive heart failure | 456 (24) | 370 (24) | 86 (22) | 0.48 |
| Valvular disease | 352 (18) | 282 (18) | 70 (18) | 0.95 |
| Pulmonary circulation disorders | 133 (7) | 110 (7) | 23 (6) | 0.42 |
| Peripheral vascular disease | 286 (15) | 227 (15) | 59 (15) | 0.78 |
| Hypertension | 884 (46) | 694 (45) | 190 (49) | 0.13 |
| Paralysis | 55 (3) | 36 (2) | 19 (5) | 0.006 |
| Neurologic disorders | 174 (9) | 131 (9) | 43 (11) | 0.10 |
| Chronic pulmonary disease | 266 (14) | 204 (13) | 62 (16) | 0.15 |
| Uncomplicated diabetes | 385 (20) | 307 (20) | 78 (20) | 0.89 |
| Complicated diabetes | 73 (4) | 61 (4) | 12 (3) | 0.44 |
| Hypothyroidism | 134 (7) | 108 (7) | 26 (7) | 0.86 |
| Liver disease | 291 (15) | 240 (16) | 51 (13) | 0.25 |
| Peptic ulcer | 1 (0.05) | 1 (0.05) | 0 (0) | 0.62 |
| AIDS/HIV | 27 (1) | 21 (1) | 6 (2) | 0.77 |
| Lymphoma | 52 (3) | 41 (3) | 11 (3) | 0.83 |
| Metastatic cancer | 136 (7) | 105 (7) | 31 (8) | 0.40 |
| Solid tumor | 128 (7) | 103 (7) | 25 (7) | 0.97 |
| Rheumatoid arthritis | 41 (2) | 36 (2) | 5 (1) | 0.20 |
| Coagulopathy | 500 (26) | 395 (26) | 105 (27) | 0.52 |
| Obesity | 97 (5) | 74 (5) | 23 (6) | 0.35 |
| Weight loss | 68 (4) | 55 (4) | 13 (3) | 0.85 |
| Fluid and electrolyte disorders | 843 (44) | 676 (44) | 167 (43) | 0.85 |
| Blood loss anemia | 36 (2) | 29 (2) | 7 (2) | 0.93 |
| Deficiency anemia | 275 (14) | 232 (15) | 43 (11) | 0.05 |
| Alcohol abuse | 199 (10) | 168 (11) | 31 (8) | 0.10 |
| Drug abuse | 70 (4) | 57 (4) | 13 (3) | 0.76 |
| Psychosis | 71 (4) | 60 (4) | 11 (3) | 0.33 |
| Depression | 104 (5) | 78 (5) | 26 (7) | 0.19 |
| Chronic kidney disease | 25 (1) | 23 (1) | 2(1) | 0.13 |
| Body weight (kg) | 81.7 ± 21.0 | 81.9 ± 20.7 | 81.4 ± 22.1 | 0.72 |
| Vital signs | ||||
| Temperature (F) | 97.2 ± 2.2 | 97.2 ± 2.2 | 97.4 ± 2.0 | 0.23 |
| Heart rate (per minutes) | 97 ± 21 | 97 ± 21 | 97 ± 22 | 0.47 |
| Systolic blood pressure (mmHg) | 117 ± 26 | 117 ± 26 | 117 ± 24 | 0.86 |
| Diastolic blood pressure (mmHg) | 62 ± 15 | 62 ± 15 | 62 ± 15 | 0.91 |
| Mean blood pressure (mmHg) | 81 ± 21 | 82 ± 22 | 80 ± 18 | 0.32 |
| Respiratory rate (per minutes) | 17 ± 9 | 17 ± 9 | 17 ± 9 | 0.95 |
| Oxygen saturation (%) | 97 ± 5 | 97 ± 5 | 97 ± 5 | 0.18 |
| Glasgow coma score | 7.9 ± 4.9 | 8.3 ± 4.9 | 7.8 ± 4.9 | 0.05 |
| Vasopressor use | 1230 (64) | 984 (64) | 246 (64) | 0.99 |
| Ventilator use | 1608 (84) | 1285 (84) | 323 (84) | 0.85 |
| Any renal replacement therapies | 54 (3) | 44 (3) | 10 (3) | 0.78 |
| Hemodialysis | 35 (2) | 29 (2) | 6 (2) | 0.67 |
| CRRT | 22 (1) | 18 (1) | 4 (1) | 0.83 |
| Acute kidney injury | 1401 (73) | 1117 (73) | 284 (74) | 0.64 |
| Laboratory data | ||||
| BUN (mg/dL) | 27 ± 21 | 27 ± 20 | 28 ± 23 | 0.28 |
| eGFR (mL/min/1.73 m2) | 68 ± 31 | 68 ± 31 | 67 ± 29 | 0.65 |
| Sodium (mEq/L) | 138 ± 5 | 138 ± 6 | 139 ± 5 | 0.38 |
| Potassium (mEq/L) | 4.4 ± 0.9 | 4.3 ± 0.9 | 4.4 ± 0.9 | 0.45 |
| Chloride (mEq/L) | 106 ± 7 | 107 ± 6 | 106 ± 7 | 0.79 |
| Bicarbonate (mEq/L) | 20 ± 5 | 20 ± 5 | 20 ± 5 | 0.81 |
| Anion gap (mEq/L) | 18 ± 6 | 18 ± 5 | 18 ± 6 | 0.57 |
| Total calcium (mg/dL) | 8.2 ± 1.2 | 8.2 ± 1.2 | 8.2 ± 1.1 | 0.91 |
| Ionized calcium (mmol/L) | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.1 | 0.60 |
| Phosphate (mg/dL) | 4.1 ± 1.8 | 4.1 ± 1.7 | 4.2 ± 1.9 | 0.29 |
| Magnesium (mg/dL) | 1.9 ± 0.5 | 1.9 ± 0.5 | 2.0 ± 0.5 | 0.60 |
| Lactate (mmol/L) | 6.2 ± 2.6 | 6.2 ± 2.6 | 6.1 ± 2.5 | 0.45 |
| Glucose (mg/dL) | 179 ± 89 | 179 ± 88 | 180 ± 91 | 0.89 |
| Hemoglobin (g/dL) | 10.6 ± 2.3 | 10.6 ± 2.4 | 10.6 ± 2.3 | 0.98 |
| WBC (109 cells/L) | 14.1 ± 8.3 | 14.0 ± 8.6 | 14.2 ± 7.2 | 0.73 |
| Platelet (109 cells/L) | 170 ± 103 | 178 ± 102 | 187 ± 105 | 0.13 |
| pH | 7.31 ± 0.12 | 7.31 ± 0.12 | 7.31 ± 0.12 | 0.94 |
| pCO2 (mmHg) | 39 ± 11 | 39 ± 11 | 39 ± 11 | 0.96 |
| pO2 (mmHg) | 209 ± 133 | 209 ± 133 | 210 ± 134 | 0.80 |
| INR | 1.8 ± 1.0 | 1.8 ± 1.1 | 1.8 ± 1.0 | 0.98 |
| PTT (second) | 49 ± 30 | 49 ± 30 | 48 ± 31 | 0.82 |
| Culture data | ||||
| Positive blood culture | 197 (10) | 158 (10) | 39 (10) | 0.94 |
| Positive urine culture | 205 (11) | 171 (11) | 34 (9) | 0.19 |
| Positive sputum culture | 284 (15) | 220 (14) | 64 (17) | 0.25 |
| Hospital death | 571 (30) | 457 (30) | 114 (30) | 0.97 |
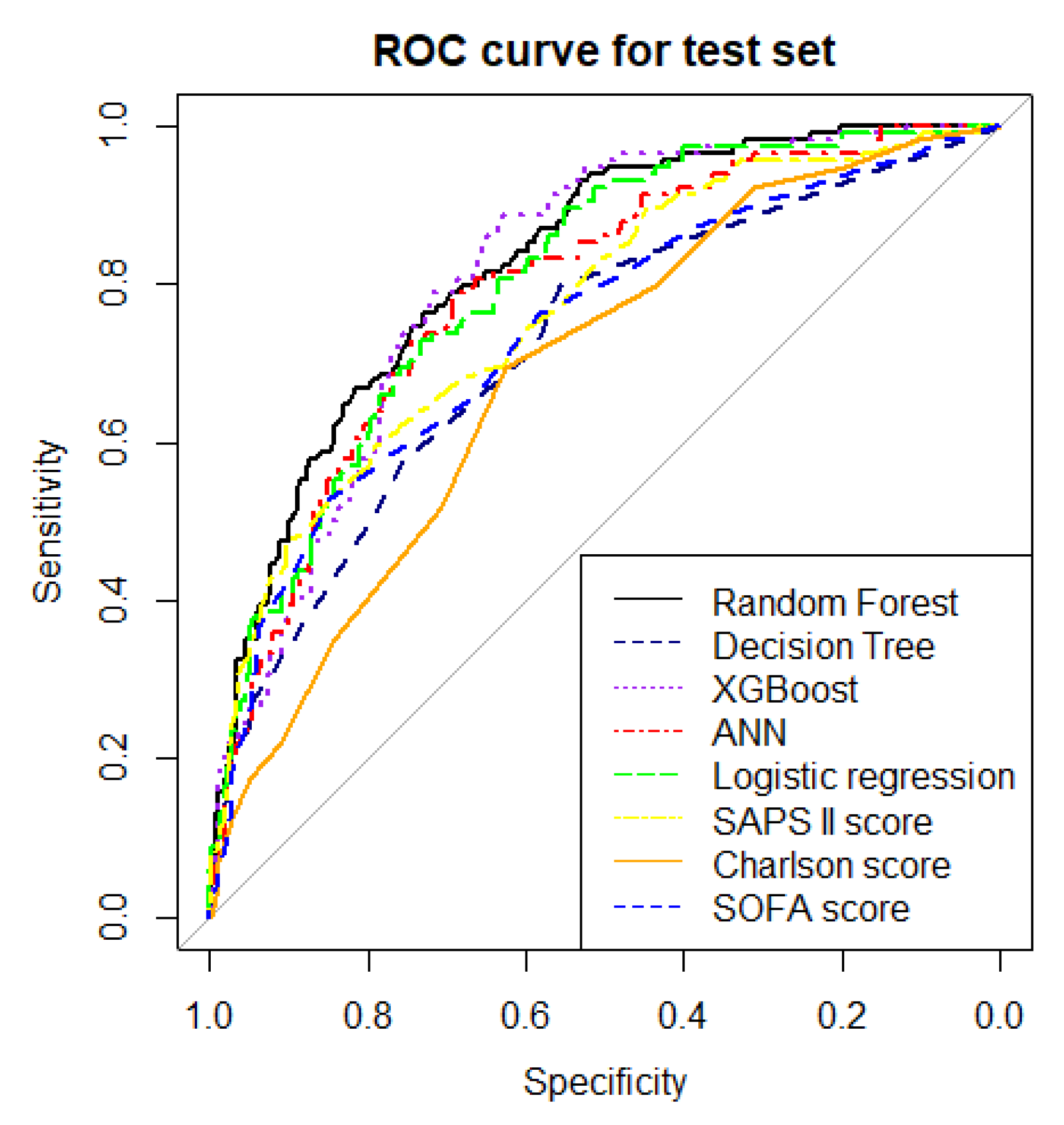
| Model | Error Rate of Test Data Set | Accuracy | Precision | MCC | F1 Score | AUROC in the Test Set | Brier Score |
|---|---|---|---|---|---|---|---|
| Random forest model | 21.4% | 0.79 | 0.72 | 0.45 | 0.56 | 0.83 (0.79–0.87) | 0.15 |
| Decision tree | 26.7% | 0.73 | 0.59 | 0.30 | 0.44 | 0.71 (0.66–0.77) | 0.19 |
| XGBoost | 25.0% | 0.75 | 0.60 | 0.36 | 0.52 | 0.81 (0.76–0.85) | 0.18 |
| ANN | 25.0% | 0.75 | 0.67 | 0.33 | 0.42 | 0.79 (0.74–0.84) | 0.19 |
| Multivariable logistic regression | 22.9% | 0.77 | 0.67 | 0.41 | 0.54 | 0.81 (0.79–0.83) | 0.16 |
| SOFA score | 25.5% | 0.74 | 0.67 | 0.30 | 0.39 | 0.74 (0.68–0.80) | 0.17 |
| SAPS II score | 23.2% | 0.77 | 0.71 | 0.39 | 0.49 | 0.77 (0.71–0.82) | 0.17 |
| Charlson score | 28.4% | 0.72 | 0.73 | 0.16 | 0.13 | 0.69 (0.63–0.74) | 0.19 |
| KERRYPNX | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Characteristics | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Age per 10 years | 1.10 (1.03–1.17) | 0.005 | 1.16 (1.07–1.26) | 0.001 |
| Male sex | 0.93 (0.75–1.16) | 0.52 | ||
| Race | ||||
| White | 1 (reference) | 1 (reference) | ||
| Black | 1.05 (0.70–1.56) | 0.83 | ||
| Hispanic | 0.37 (0.18–0.75) | 0.006 | ||
| Other | 0.93 (0.59–1.46) | 0.75 | ||
| ICU type | ||||
| Cardiac ICU | 1.21 (0.85–1.73) | 0.30 | 0.73 (0.48–1.12) | 0.15 |
| Cardiac surgery ICU | 0.21 (0.15–0.30) | <0.001 | 0.26 (0.16–0.42) | <0.001 |
| Medical ICU | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Surgical ICU | 0.56 (0.40–0.77) | 0.001 | 0.67 (0.45–1.00) | 0.05 |
| Trauma/surgical ICU | 0.43 (0.31–0.60) | <0.001 | 0.82 (0.54–1.25) | 0.35 |
| Elixhauser Comorbidities | 1.20 (0.93–1.54) | 0.16 | ||
| Congestive heart failure | 0.51 (0.37–0.71) | <0.001 | ||
| Valvular disease | 1.21 (0.80–1.83) | 0.36 | ||
| Pulmonary circulation disorders | 0.80 (0.58–1.11) | 0.18 | ||
| Peripheral vascular disease | 0.75 (0.60–0.94) | 0.01 | ||
| Hypertension | 0.91 (0.43–1.89) | 0.79 | ||
| Paralysis | 1.17 (0.80–1.71) | 0.43 | ||
| Neurologic disorders | 0.98 (0.71–1.35) | 0.90 | ||
| Chronic pulmonary disease | 0.95 (0.73–1.26) | 0.74 | ||
| Uncomplicated diabetes | 0.69 (0.38–1.27) | 0.24 | ||
| Complicated diabetes | 0.81 (0.52–1.27) | 0.37 | ||
| Hypothyroidism | 1.80 (1.35–2.39) | <0.001 | ||
| Liver disease | 2.17 (0.92–5.15) | 0.08 | ||
| AIDS/HIV | 3.12 (1.67–5.84) | <0.001 | ||
| Lymphoma | 2.29 (1.53–3.41) | <0.001 | ||
| Metastatic cancer | 0.74 (0.47–1.18) | 0.21 | ||
| Solid tumor | 0.91 (0.43–1.89) | 0.79 | ||
| Rheumatoid arthritis | 1.77 (1.39–2.25) | <0.001 | 1.96 (1.22–3.15) | 0.005 |
| Coagulopathy | 0.49 (0.27–0.90) | 0.02 | ||
| Obesity | 0.97 (0.53–1.75) | 0.91 | ||
| Weight loss | 1.92 (1.54–2.40) | <0.001 | ||
| Fluid and electrolyte disorders | 0.90 (0.39–2.04) | 0.80 | 0.49 (0.25–0.99) | 0.04 |
| Blood loss anemia | 0.61 (0.43–0.85) | 0.003 | ||
| Deficiency anemia | 0.94 (0.66–1.34) | 0.72 | ||
| Alcohol abuse | 1.00 (0.56–1.79) | 0.99 | ||
| Drug abuse | 0.50 (0.34–0.74) | <0.001 | ||
| Psychosis | 0.64 (0.34–1.20) | 0.16 | ||
| Depression | 0.92 (0.56–1.53) | 0.76 | ||
| Chronic kidney disease | 0.49 (0.17–1.46) | 0.20 | ||
| Body weight per 5 kg | 0.98 (0.96–1.01) | 0.25 | ||
| Vital signs | ||||
| Temperature per 1 F | 0.96 (0.91–1.01) | 0.08 | ||
| Heart rate per 10 times/minute | 1.11 (1.05–1.16) | <0.001 | ||
| Systolic per 10 mmHg | 0.92 (0.88–0.96) | <0.001 | ||
| Diastolic BP per 5 mmHg | 0.97 (0.93–1.01) | 0.09 | 1.05 (1.01–1.10) | 0.03 |
| Mean BP per 5 mmHg | 0.97 (0.94–0.99) | 0.02 | ||
| Respiratory rate per 1 time/minute | 1.05 (1.04–1.06) | <0.001 | 1.02 (1.00–1.03) | 0.04 |
| Oxygen saturation per 1 percent | 0.93 (0.91–0.95) | <0.001 | ||
| Glasgow coma score per 1 unit | 1.00 (0.98–1.02) | 0.91 | ||
| Vasopressor use | 2.19 (1.71–2.79) | <0.001 | 2.11 (1.54–2.89) | <0.001 |
| Ventilator use | 1.31 (0.96–1.79) | 0.09 | 1.81 (1.21–2.70) | 0.004 |
| Any renal replacement therapies | 1.36 (0.73–2.54) | 0.33 | ||
| Hemodialysis | 1.68 (0.80–3.55) | 0.17 | ||
| CRRT | 0.91 (0.32–2.56) | 0.85 | ||
| Acute kidney injury | 3.45 (2.55–4.67) | <0.001 | 2.10 (1.49–2.96) | <0.001 |
| Laboratory data | ||||
| BUN per 1 mg/dL | 1.03 (1.02–1.03) | <0.001 | ||
| eGFR per 10 mL/min/1.73 m2 | 0.90 (0.87–0.94) | <0.001 | ||
| Sodium per 1 mEq/L | 1.00 (0.98–1.02) | 0.72 | ||
| Potassium per 1 mEq/L | 1.03 (0.92–1.16) | 0.60 | ||
| Chloride per 1 mEq/L | 0.95 (0.94–0.97) | <0.001 | 0.97 (0.95–0.99) | 0.01 |
| Bicarbonate per 1 mEq/L | 0.90 (0.87–0.92) | <0.001 | ||
| Anion gap per 1 mEq/L | 1.14 (1.12–1.17) | <0.001 | 1.04 (1.01–1.08) | 0.009 |
| Total calcium per 1 mg/dL | 0.88 (0.80–0.96) | 0.006 | ||
| Ionized calcium per 1 mmol/L | 0.06 (0.03–0.13) | 0.06 | 0.19 (0.08–0.46) | <0.001 |
| Phosphate per 1 mg/dL | 1.29 (1.21–1.37) | <0.001 | ||
| Magnesium per 1 mg/dL | 1.48 (1.21–1.81) | <0.001 | 1.54 (1.18–2.02) | 0.002 |
| Lactate per 1 mmol/L | 1.25 (1.20–1.31) | <0.001 | 1.11 (1.04–1.17) | 0.001 |
| Glucose per 1 mg/dL | 1.00 (1.00–1.00) | 0.14 | ||
| Hemoglobin per 1 g/dL | 1.06 (1.01–1.11) | 0.02 | ||
| WBC per 109 cells/L | 1.01 (1.00–1.02) | 0.13 | ||
| Platelet per 109 cells/L | 1.00 (1.00–1.00) | 0.12 | ||
| pH per 1 unit | 0.04 (0.02–0.10) | <0.001 | ||
| pCO2 per 1 mmHg | 0.99 (0.98–0.99) | 0.04 | ||
| pO2 per 1 mmHg | 1.00 (1.00–1.00) | <0.001 | 0.99 (0.99–1.00) | 0.004 |
| INR per 1 unit | 1.62 (1.43–1.84) | <0.001 | 1.17 (1.03–1.33) | 0.02 |
| PTT per 1 s | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.00–1.01) | 0.003 |
| Culture data | ||||
| Positive blood culture | 2.49 (1.79–3.48) | <0.001 | ||
| Positive urine culture | 2.05 (1.53–2.74) | <0.001 | ||
| Positive sputum culture | 1.90 (1.37–2.63) | <0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattharanitima, P.; Thongprayoon, C.; Kaewput, W.; Qureshi, F.; Qureshi, F.; Petnak, T.; Srivali, N.; Gembillo, G.; O’Corragain, O.A.; Chesdachai, S.; et al. Machine Learning Prediction Models for Mortality in Intensive Care Unit Patients with Lactic Acidosis. J. Clin. Med. 2021, 10, 5021. https://doi.org/10.3390/jcm10215021
Pattharanitima P, Thongprayoon C, Kaewput W, Qureshi F, Qureshi F, Petnak T, Srivali N, Gembillo G, O’Corragain OA, Chesdachai S, et al. Machine Learning Prediction Models for Mortality in Intensive Care Unit Patients with Lactic Acidosis. Journal of Clinical Medicine. 2021; 10(21):5021. https://doi.org/10.3390/jcm10215021
Chicago/Turabian StylePattharanitima, Pattharawin, Charat Thongprayoon, Wisit Kaewput, Fawad Qureshi, Fahad Qureshi, Tananchai Petnak, Narat Srivali, Guido Gembillo, Oisin A. O’Corragain, Supavit Chesdachai, and et al. 2021. "Machine Learning Prediction Models for Mortality in Intensive Care Unit Patients with Lactic Acidosis" Journal of Clinical Medicine 10, no. 21: 5021. https://doi.org/10.3390/jcm10215021
APA StylePattharanitima, P., Thongprayoon, C., Kaewput, W., Qureshi, F., Qureshi, F., Petnak, T., Srivali, N., Gembillo, G., O’Corragain, O. A., Chesdachai, S., Vallabhajosyula, S., Guru, P. K., Mao, M. A., Garovic, V. D., Dillon, J. J., & Cheungpasitporn, W. (2021). Machine Learning Prediction Models for Mortality in Intensive Care Unit Patients with Lactic Acidosis. Journal of Clinical Medicine, 10(21), 5021. https://doi.org/10.3390/jcm10215021











