Isolated Growth Hormone Deficiency and Idiopathic Short Stature: Comparative Efficiency after Growth Hormone Treatment up to Adult Height
Abstract
:1. Introduction and Background
2. Materials and Methods
2.1. Inclusion Criteria for Growth Hormone Deficiency
- Male children that were younger than 14 years old who:
- ◦
- Had heights of more than 2 SD below the mean for age and sex;
- ◦
- Had growth velocities of less than −1 SD for chronological age in the last year, or of less than −1.5 SD over the past 2 years, according to the low-growth-rate definition;
- ◦
- Had received two GH stimulation tests (exercise and clonidine) with GH < 7 ng/mL and delayed bone maturation of greater than or equal to 1 year;
- ◦
- Showed an absence of associated pathology;
- ◦
- Remained prepubertal at least during the 1st year of GH therapy.
2.2. Inclusion Criteria for Idiopathic Short Stature:
- Male children that were younger than 14 years old who:
- ◦
- Had heights of more than 2 SD below the mean for age and sex;
- ◦
- Remained prepubertal at least during the 1st year of GH therapy;
- ◦
- Had growth velocities of less than −1 SD for chronological age in the last year, or of less than −1.5 SD over the past 2 years, according to the low-growth-rate definition;
- ◦
- Had a GH > 7 ng/mL after stimulation test;
- ◦
- Had a birth weight > 2500 g.
2.3. Exclusion Criteria
- Children with short stature due to other causes (hypothyroidism, hypercortisolism, chronic systemic diseases, dysmorphic syndromes, skeletal disorders, etc.);
- Patients with other associated hormone deficiencies, significant anatomical abnormalities, tumor, hypothalamic-pituitary disease, or genetic alterations;
2.4. Ethical Issues
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, D.B.; Macgillivray, M.H.; Alter, C.; Saenger, P.; Anhalt, H.; Hintz, R. Growth Hormone Use in Pediatric Growth Hormone Deficiency and Other Pediatric Growth Disorders. Am. J. Manag. Care 2000, 6, S805–S816. [Google Scholar]
- Bouhours-Nouet, N.; Gatelais, F.; Coutant, R. Lénfant petit avec déficit en hormone de croissance. [Children with growth hormone deficiency]. Med. Ther. Pediatr. 2006, 9, 220–230. [Google Scholar]
- Cianfarani, S.; Tondinelli, T.; Spadoni, G.L.; Scirè, G.; Boemi, S.; Boscherini, B. Height velocity and IGF-I assessment in the diagnosis of childhood onset GH insufficiency: Do we still need a second GH stimulation test? Clin. Endocrinol. 2002, 57, 161–167. [Google Scholar] [CrossRef]
- Gonçalves, J.; Correia, F.; Cardoso, H.; Borges, T.; Oliveira, M. O Papel dos Testes de Estimulação Farmacológica no Diagnóstico da Deficiência de Hormona do Crescimento em Crianças e Adolescentes. [Use of non pharmacological stimulation test in diagnosis for growth hormone deficiency in children and adolescents]. Acta Med. Port. 2014, 27, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.B.; Levy, R.A.; Gut, R.; Germak, J. Trends in Growth Hormone Stimulation Testing and Growth Hormone Dosing In Adult Growth Hormone Deficiency Patients: Results From The Answer Program. Endocr. Pract. 2016, 22, 396–405. [Google Scholar] [CrossRef]
- Chinoy, A.; Murray, P. Diagnosis of growth hormone deficiency in the paediatric and transitional age. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 737–747. [Google Scholar] [CrossRef]
- Rosenfeld, R.; Cohen, P. Pediatric Endocrinology. Chapter 8: Disorders of Growth Hormone/Insulin-Like Growth Factor Secretion and Action, 3rd ed.; Elsevier: Philadelphia, PA, USA, 2008; pp. 254–334. [Google Scholar]
- Murray, P.; Dattani, M.T.; Clayton, P. Controversies in the diagnosis and management of growth hormone deficiency in childhood and adolescence. Arch. Dis. Child. 2015, 101, 96–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villafuerte, B.; Barrio, R.; Martín-Frías, M.; Alonso, M.; Roldán, B. Auxological characteristics of pediatric patients with permanent or transient isolated growth hormone deficiency. Response to treatment and final height. Endocrinol. Diabetes Nutr. 2019, 66, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Darendeliler, F.; Lindberg, A.; Wilton, P. Response to Growth Hormone Treatment in Isolated Growth Hormone Deficiency versus Multiple Pituitary Hormone Deficiency. Horm. Res. Paediatr. 2011, 76, 42–46. [Google Scholar] [CrossRef] [PubMed]
- López Siguero, J.P.; Martínez-Aedo, M.J.; Paz Cerezo, M.; Martinez Valverde, A. Spontaneous development of idiopathic short stature. Analysis of a group of 42 children followed to their final body height. An. Españoles Pediatría 1998, 48, 261–266. [Google Scholar]
- López-Siguero, J.; García-García, E.; Carralero, I.; Martlnez-Aedo, M. Adult Height in Children with Idiopathic Short Stature Treated with Growth Hormone. J. Pediatr. Endocrinol. Metab. 2000, 13, 1595–1602. [Google Scholar] [CrossRef]
- Secco, A.; Di Iorgi, N.; Napoli, F.; Calandra, E.; Calcagno, A.; Ghezzi, M.; Frassinetti, C.; Fratangeli, N.; Parodi, S.; Benassai, M.; et al. Reassessment of the Growth Hormone Status in Young Adults with Childhood-Onset Growth Hormone Deficiency: Reappraisal of Insulin Tolerance Testing. J. Clin. Endocrinol. Metab. 2009, 94, 4195–4204. [Google Scholar] [CrossRef] [Green Version]
- Sánchez González, E.; Carrascosa Lezcano, A.; Fernández García, J.M.; Ferrández Longás, A.; López De Lara, D.; López-Siguero, J.P. Estudios Españoles de Crecimiento: Situación Actual, Utilidad y Recomendaciones de Uso. [Spanish Studies of Growth: Current Situation, Usefulness and Recommendations of Use]. An. Pediatría 2011, 74, 193-e1. [Google Scholar]
- Ariza Jiménez, A.B.; Martínez-Aedo Ollero, M.J.; López-Siguero, J.P. Eficacia y seguridad del tratamiento sustitutivo en el déficit aislado de hormona del crecimiento [Efficacy and safety of replacement treatment in isolated growth hormone deficiency]. An. Pediatr. 2019, 90, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Im, M.; Kim, Y.-D.; Han, H.-S. Effect of growth hormone treatment on children with idiopathic short stature and idiopathic growth hormone deficiency. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.A.; Choe, Y.R.; Yang, E.M.; Kim, C.J. Comparison of Growth Hormone Treatment in Patients with Idiopathic Short Stature and Idiopathic Growth Hormone Deficiency. Chonnam. Med. J. 2014, 50, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Bakker, B.; Frane, J.; Anhalt, H.; Lippe, B.; Rosenfeld, R.G. Height Velocity Targets from the National Cooperative Growth Study for First-Year Growth Hormone Responses in Short Children. J. Clin. Endocrinol. Metab. 2008, 93, 352–357. [Google Scholar] [CrossRef] [Green Version]
- Carrascosa, A.; Audí, L.; Fernández-Cancio, M.; Yeste, D.; Gussinye, M.; Campos, A.; Albisu, M.A.; Clemente, M.; Bel, J.; Nosas, R.; et al. Height gain at adult-height age in 184 short patients treated with growth hormone from prepubertal age to near adult-height age is not related to GH secretory status at GH therapy onset. Horm. Res. Paediatr. 2013, 79, 145–156. [Google Scholar] [CrossRef]
- Jeong, H.R.; Kwon, E.B.; Shim, Y.S.; Lee, H.S.; Hwang, J.S. Comparative Study of Growth Hormone Treatment in Children with Idi-opathic Short Stature and Growth Hormone Deficiency. Curr. Drug Metab. 2015, 16, 940–946. [Google Scholar] [CrossRef]
- Schena, L.; Meazza, C.; Pagani, S.; Paganelli, V.; Bozzola, E.; Tinelli, C.; Buzi, F.; Bozzola, M. Efficacy of long-term growth hormone therapy in short non-growth hormone-deficient children. J. Pediatr. Endocrinol. Metab. 2017, 30, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Kaplowitz, P.; Shulman, D.; Frane, J.; Jacobs, J.; Lippe, B. Characteristics of children with the best and poorest first- and second-year growth during rhGH therapy: Data from 25 years of the Genentech national cooperative growth study (NCGS). Int. J. Pediatr. Endocrinol. 2013, 2013, 9. [Google Scholar] [CrossRef] [Green Version]
- Bryant, J.; Baxter, L.; Cave, C.B.; Milne, R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst. Rev. 2007, CD004440. [Google Scholar] [CrossRef] [Green Version]
- Rekers-Mombarg, L.T.; Wit, J.M.; Massa, G.G.; Ranke, M.B.; Buckler, J.M.; Butenandt, O.; Chaussain, J.L.; Frisch, H.; Leiberman, E. Spontaneous growth in idiopathic short stature. European Study Group. Arch. Dis. Child. 1996, 75, 175–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotos, J.F.; Tokar, N.J. Growth hormone significantly increases the adult height of children with idiopathic short stature: Comparison of subgroups and benefit. Int. J. Pediatr. Endocrinol. 2014, 2014, 15. [Google Scholar] [CrossRef] [Green Version]
- Paltoglou, G.; Dimitropoulos, I.; Kourlaba, G.; Charmandari, E. The effect of treatment with recombinant human growth hormone (rhGH) on linear growth and adult height in children with idiopathic short stature (ISS): A systematic review and metaanalysis. J. Pediatr. Endocrinol. Metab. 2020, 33, 1577–1588. [Google Scholar] [CrossRef]
- Wit, J.M.; Rekers-Mombarg, L.T.M. Final Height Gain by GH Therapy in Children with Idiopathic Short Stature Is Dose Dependent. J. Clin. Endocrinol. Metab. 2002, 87, 604–611. [Google Scholar] [CrossRef]
- Deodati, A.; Cianfarani, S. Impact of growth hormone therapy on adult height of children with idiopathic short stature: Systematic review. BMJ 2011, 342, c7157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkelstein, B.; Imperiale, T.; Speroff, T.; Marrero, U.; Radcliffe, D.; Cuttler, L. Effect of growth hormone therapy on height in children with idiopathic short stature: A meta-analysis. Arch. Pediatr. Adolesc. Med. 2002, 156, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Smuel, K.; Kauli, R.; Lilos, P.; Laron, Z. Growth, development, puberty and adult height before and during treatment in children with congenital isolated growth hormone deficiency. Growth Horm. IGF Res. 2015, 25, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Reiter, E.O.; Price, D.A.; Wilton, P.; Albertsson-Wikland, K.; Ranke, M.B. Effect of Growth Hormone (GH) Treatment on the Near-Final Height of 1258 Patients with Idiopathic GH Deficiency: Analysis of a Large International Database. J. Clin. Endocrinol. Metab. 2006, 91, 2047–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P.; Germak, J.; Rogol, A.; Weng, W.; Kappelgaard, A.; Rosenfeld, R.; American Norditropin Study Group. Variable degree of growth hormone (GH) and insulin-like growth factor (IGF) sensitivity in children with idiopathic short stature compared with GH-deficient patients: Evidence from an IGF-based dosing study of short children. J. Clin. Endocrinol. Metab. 2010, 95, 2089–2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmati, S.; Pourattar, N.; Azami, M.; Depisheh, A.; Najafi, R.; Sayehmiri, K. The Effect of Growth Hormone Treatment on Adult Height of Children with Idiopathic Short Stature: A Systematic Review and Meta-Analyses. J. Endocrinol. Metab. 2017, 7, 45–54. [Google Scholar] [CrossRef] [Green Version]
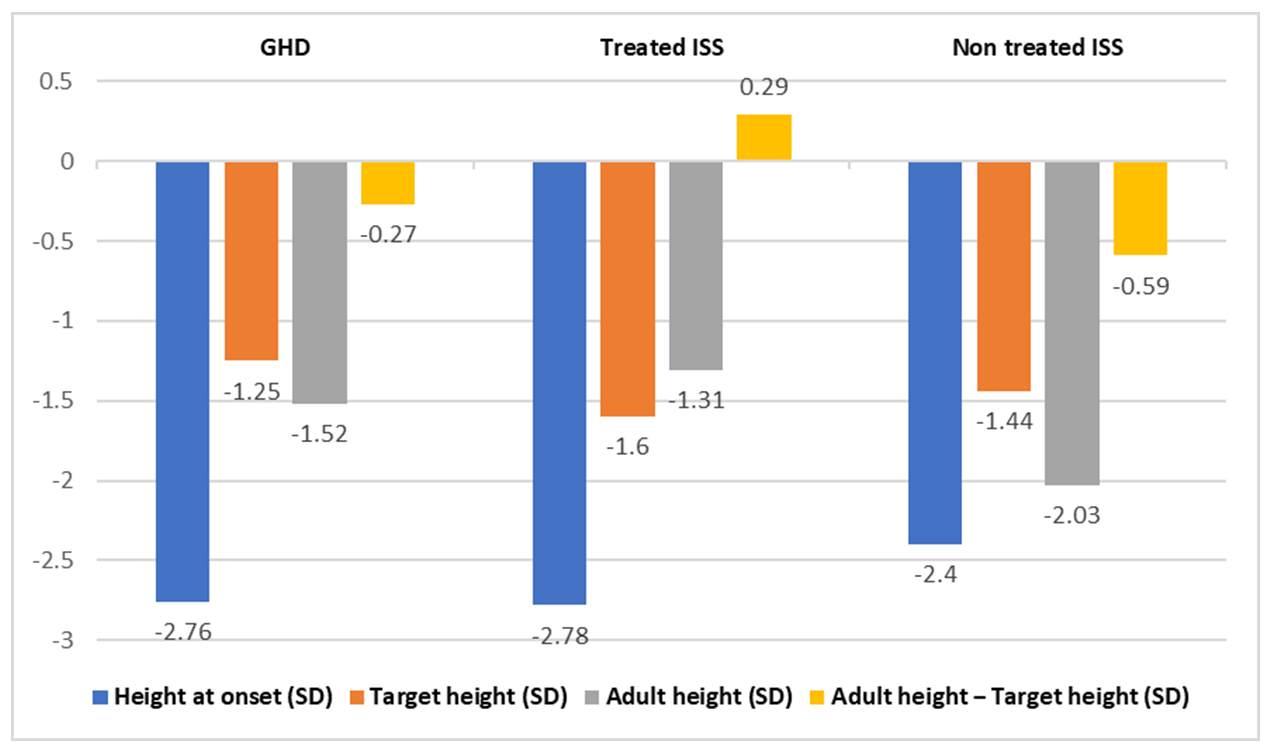
| Group A Means (n 67) | Group B [12] Means (n 30) | Group C [11] Means (n 42) | p Value | |
|---|---|---|---|---|
| Age at start of GH treatment (years) | 9.99 | 11.1 | 10.8 | 0.010 * |
| Age at onset of puberty (years) | 12.5 | 13.4 | 13.2 | 0.000 * |
| Initial Bone Age/Initial Age | 0.71 | 0.85 | 0.87 | 0.217 |
| Height at start of GH treatment (SD) | −2.76 | −2.78 | −2.4 | 0.020 * |
| Target height (SD) | −1.25 | −1.6 | −1.44 | 0.050 * |
| Predicted adult height (SD) | −1.22 | −2.09 | −2.03 | 0.240 |
| Height at onset of puberty (SD) | −1.96 | −2.38 | −2.74 | 0.090 |
| Pubertal gain (cm) | 24.96 | 26.3 | 23.75 | 0.017 * |
| Adult height (SD) | −1.52 | −1.31 | −2.03 | 0.537 |
| Adult height—Target height (SD) | −0.27 | +0.29 | −0.59 | 0.001 * |
| Adult height—Predicted adult height (SD) | −0.3 | 0.78 | 0.05 | 0.596 |
| Adult height—Height at onset of GH treatment (SD) IGF1 (SD) at start of treatment IGF1 (SD) at end of treatment GH posology (mg/kg/day) | 1.24 −1.27 0.023 0.028 | 1.47 0.24 1.63 0.062 | 0.39 0.22 0.34 - | 0.080 0.028 * 0.026 * 0.063 |
| p Value Group B [12] vs. Group C [11] | p Value Group A vs. Group B [12] | p Value Group A vs. Group C [11] | |
|---|---|---|---|
| Chronological age | 0.958 (NS) | 0.033 * | 0.025 * |
| Age at onset of puberty (years) | 0.816 (NS) | 0.022 * | 0.017 * |
| Height at start of GH treatment (H) SD | <0.050 * | 0.002 * | 0.044 * |
| Target height (TH) SD | 0.903 (NS) | 1.797 (NS) | 0.045 * |
| Predicted adult height (PAH) SD | 0.324 (NS) | 0.290 (NS) | 0.274 (NS) |
| Height at onset of puberty (PH) SD | 1.972 (NS) | 0.047 * | 0.461 (NS) |
| Pubertal gain (cm) | <0.050 * | 0.017 * | 0.016 * |
| Adult height (AH) SD | <0.050 * | 0.047 * | 0.217 (NS) |
| AH-H (SD) | <0.050 * | 0.181 (NS) | 0.415 (NS) |
| AH-TH (SD) | <0.050 * | 0.062 (NS) | 1.691 (NS) |
| AH-PAH (SD) | <0.050 * | 0.430 (NS) | 0.285 (NS) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariza-Jimenez, A.-B.; Leiva Gea, I.; Martinez-Aedo Ollero, M.J.; Lopez-Siguero, J.P. Isolated Growth Hormone Deficiency and Idiopathic Short Stature: Comparative Efficiency after Growth Hormone Treatment up to Adult Height. J. Clin. Med. 2021, 10, 4988. https://doi.org/10.3390/jcm10214988
Ariza-Jimenez A-B, Leiva Gea I, Martinez-Aedo Ollero MJ, Lopez-Siguero JP. Isolated Growth Hormone Deficiency and Idiopathic Short Stature: Comparative Efficiency after Growth Hormone Treatment up to Adult Height. Journal of Clinical Medicine. 2021; 10(21):4988. https://doi.org/10.3390/jcm10214988
Chicago/Turabian StyleAriza-Jimenez, Ana-Belen, Isabel Leiva Gea, Maria Jose Martinez-Aedo Ollero, and Juan Pedro Lopez-Siguero. 2021. "Isolated Growth Hormone Deficiency and Idiopathic Short Stature: Comparative Efficiency after Growth Hormone Treatment up to Adult Height" Journal of Clinical Medicine 10, no. 21: 4988. https://doi.org/10.3390/jcm10214988
APA StyleAriza-Jimenez, A.-B., Leiva Gea, I., Martinez-Aedo Ollero, M. J., & Lopez-Siguero, J. P. (2021). Isolated Growth Hormone Deficiency and Idiopathic Short Stature: Comparative Efficiency after Growth Hormone Treatment up to Adult Height. Journal of Clinical Medicine, 10(21), 4988. https://doi.org/10.3390/jcm10214988






