Effect of Adding Midazolam to Dual Prophylaxis for Preventing Postoperative Nausea and Vomiting
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
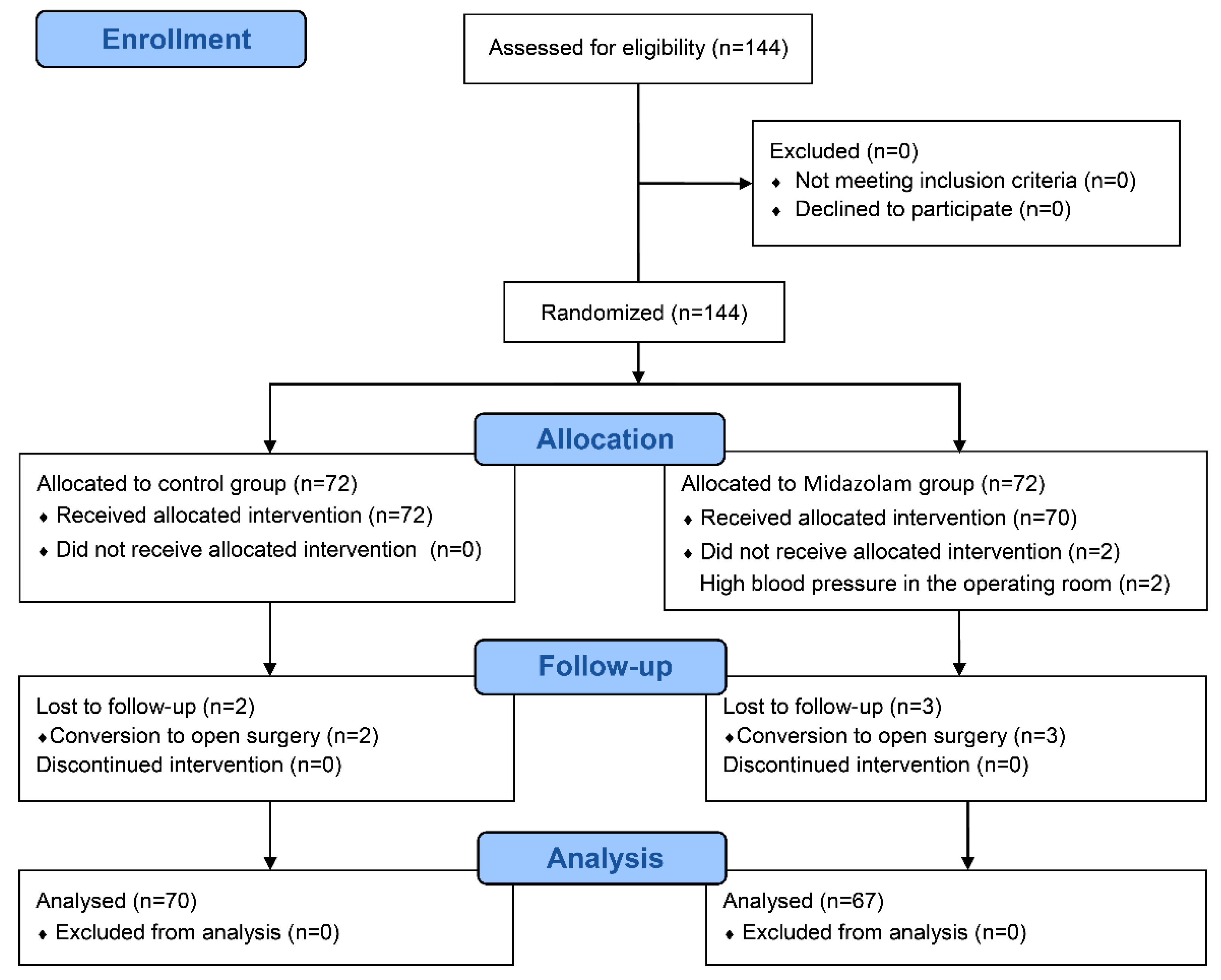
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT3 | 5-hydroxytryptamine |
| ASA | American Society of Anesthesiologists |
| IQR | Interquartile range |
| IV | Intravenous |
| PACU | Post-anesthesia care unit |
| PCA | Patient-controlled analgesia |
| PON | Postoperative nausea |
| PONV | Postoperative nausea and vomiting |
| VNRS | Verbal numerical rating scale |
References
- Cohen, M.M.; Duncan, P.G.; DeBoer, D.P.; Tweed, W.A. The postoperative interview: Assessing risk factors for nausea and vomiting. Anesth. Analg. 1994, 78, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Laara, E.; Koivuranta, M.; Greim, C.A.; Roewer, N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology 1999, 91, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Kranke, P.; Katz, M.H.; Goepfert, C.; Papenfuss, T.; Rauch, S.; Heineck, R.; Greim, C.A.; Roewer, N. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: A randomized controlled trial of factorial design. Br. J. Anaesth. 2002, 88, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef]
- Habib, A.S.; Gan, T.J. The effectiveness of rescue antiemetics after failure of prophylaxis with ondansetron or droperidol: A preliminary report. J. Clin. Anesth. 2005, 17, 62–65. [Google Scholar] [CrossRef]
- Som, A.; Bhattacharjee, S.; Maitra, S.; Arora, M.K.; Baidya, D.K. Combination of 5-HT3 antagonist and dexamethasone is superior to 5-HT3 antagonist alone for PONV prophylaxis after laparoscopic surgeries: A meta-analysis. Anesth. Analg. 2016, 123, 1418–1426. [Google Scholar] [CrossRef]
- Habib, A.S.; El-Moalem, H.E.; Gan, T.J. The efficacy of the 5-HT3 receptor antagonists combined with droperidol for PONV prophylaxis is similar to their combination with dexamethasone. A meta-analysis of randomized controlled trials. Can. J. Anaesth. 2004, 51, 311–319. [Google Scholar] [CrossRef]
- Henzi, I.; Walder, B.; Tramèr, M.R. Dexamethasone for the prevention of postoperative nausea and vomiting: A quantitative systematic review. Anesth. Analg. 2000, 90, 186–194. [Google Scholar] [CrossRef]
- Majumdar, J.R.; Vertosick, E.; Long, M.; Cansino, C.; Assel, M.; Twersky, R. Effects of midazolam on postoperative nausea and vomiting and discharge times in outpatients undergoing cancer-related surgery. AANA J. 2019, 87, 179–183. [Google Scholar]
- Grant, M.C.; Kim, J.; Page, A.J.; Hobson, D.; Wick, E.; Wu, C.L. The effect of intravenous midazolam on postoperative nausea and vomiting: A meta-analysis. Anesth. Analg. 2016, 122, 656–663. [Google Scholar] [CrossRef]
- Ahn, E.J.; Kang, H.; Choi, G.J.; Baek, C.W.; Jung, Y.H.; Woo, Y.C. The effectiveness of midazolam for preventing postoperative nausea and vomiting: A systematic review and meta-analysis. Anesth. Analg. 2016, 122, 664–676. [Google Scholar] [CrossRef]
- Honarmand, A.; Safavi, M.; Chegeni, M.; Hirmanpour, A.; Nazem, M.; Sarizdi, S.H. Prophylactic antiemetic effects of Midazolam, Ondansetron, and their combination after middle ear surgery. J. Res. Pharm. Pract. 2016, 5, 16–21. [Google Scholar]
- Makhdoom, N.K.; Farid, M.F. Prophylactic antiemetic effects of midazolam, dexamethasone, and its combination after middle ear surgery. Saudi Med. J. 2009, 30, 504–508. [Google Scholar]
- Aldrete, J.A. The post-anesthesia recovery score revisited. J. Clin. Anesth. 1995, 7, 89–91. [Google Scholar] [CrossRef]
- Ryu, J.H.; Jeon, Y.T.; Min, B.; Hwang, J.Y.; Sohn, H.M. Effects of palonosetron for prophylaxis of postoperative nausea and vomiting in high-risk patients undergoing total knee arthroplasty: A prospective, randomized, double-blind, placebo-controlled study. PLoS ONE 2018, 13, e0196388. [Google Scholar] [CrossRef]
- Ham, S.Y.; Shim, Y.H.; Kim, E.H.; Son, M.J.; Park, W.S.; Lee, J.S. Aprepitant for antiemesis after laparoscopic gynaecological surgery: A randomised controlled trial. Eur. J. Anaesthesiol. 2016, 33, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Chandrakantan, A.; Glass, P.S. Multimodal therapies for postoperative nausea and vomiting, and pain. Br. J. Anaesth. 2011, 107 (Suppl. 1), i27–i40. [Google Scholar] [CrossRef] [PubMed]
- Denholm, L.; Gallagher, G. Physiology and pharmacology of nausea and vomiting. Anaesth. Intensive Care Med. 2018, 19, 513–516. [Google Scholar] [CrossRef]
- Apfel, C.C.; Korttila, K.; Abdalla, M.; Kerger, H.; Turan, A.; Vedder, I.; Zernak, C.; Danner, K.; Jokela, R.; Pocock, S.J.; et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N. Engl. J. Med. 2004, 350, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Takano, Y.; Kamiya, H.O. Roles of substance P and NK(1) receptor in the brainstem in the development of emesis. J. Pharmacol. Sci. 2003, 91, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Dando, T.M.; Perry, C.M. Aprepitant: A review of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs 2004, 64, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Hsing, C.H.; Shieh, J.P.; Chien, C.C.; Ho, C.M.; Wang, J.J. The cellular mechanisms of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur. J. Pharmacol. 2014, 722, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Bunce, K.T.; Tyers, M.B. The role of 5-HT in postoperative nausea and vomiting. Br. J. Anaesth. 1992, 69, 60S–62S. [Google Scholar] [CrossRef]
- Takada, K.; Murai, T.; Kanayama, T.; Koshikawa, N. Effects of midazolam and flunitrazepam on the release of dopamine from rat striatum measured by in vivo microdialysis. Br. J. Anaesth. 1993, 70, 181–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Florio, T. The use of midazolam for persistent postoperative nausea and vomiting. Anaesth. Intensive Care 1992, 20, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.P.; Dom, P.M.; Ramirez, A.M.; O’Flaherty, J.E. Preoperative intravenous midazolam: Benefits beyond anxiolysis. J. Clin. Anesth. 2004, 16, 177–183. [Google Scholar] [CrossRef]
- Lee, Y.; Wang, J.J.; Yang, Y.L.; Chen, A.; Lai, H.Y. Midazolam vs ondansetron for preventing postoperative nausea and vomiting: A randomised controlled trial. Anaesthesia 2007, 62, 18–22. [Google Scholar] [CrossRef]
- Prakash, K.; Meshram, T.; Jain, P. Midazolam versus dexamethasone-ondansetron in preventing post-operative nausea-vomiting in patients undergoing laparoscopic surgeries. Acta Anaesthesiol. Scand. 2021, 65, 870–876. [Google Scholar] [CrossRef]
- Fredman, B.; Lahav, M.; Zohar, E.; Golod, M.; Paruta, I.; Jedeikin, R. The effect of midazolam premedication on mental and psychomotor recovery in geriatric patients undergoing brief surgical procedures. Anesth. Analg. 1999, 89, 1161–1166. [Google Scholar] [CrossRef]
- Weingarten, T.N.; Bergan, T.S.; Narr, B.J.; Schroeder, D.R.; Sprung, J. Effects of changes in intraoperative management on recovery from anesthesia: A review of practice improvement initiative. BMC Anesthesiol. 2015, 15, 54. [Google Scholar] [CrossRef]
- Brosius, K.K.; Bannister, C.F. Oral midazolam premedication in preadolescents and adolescents. Anesth. Analg. 2002, 94, 31–36. [Google Scholar] [CrossRef]
- Cho, E.J.; Yoon, S.Z.; Cho, J.E.; Lee, H.W. Comparison of the effects of 0.03 and 0.05 mg/kg midazolam with placebo on prevention of emergence agitation in children having strabismus surgery. Anesthesiology 2014, 120, 1354–1361. [Google Scholar] [CrossRef]
- Yeo, J.; Jung, J.; Ryu, T.; Jeon, Y.H.; Kim, S.; Baek, W. Antiemetic efficacy of dexamethasone combined with midazolam after middle ear surgery. Otolaryngol.-Head Neck Surg. 2009, 141, 684–688. [Google Scholar] [CrossRef]
- Abdelhamid, S.A.; Kamel, M.S. A prospective controlled study to assess the antiemetic effect of midazolam following intragastric balloon insertion. J. Anaesthesiol. Clin. Pharmacol. 2014, 30, 383–386. [Google Scholar] [CrossRef]
- Oh, S.K.; Lee, I.O.; Lim, B.G.; Jeong, H.; Kim, Y.S.; Ji, S.G.; Park, J.S. Comparison of the Analgesic Effect of Sufentanil versus Fentanyl in Intravenous Patient-Controlled Analgesia after Total Laparoscopic Hysterectomy: A Randomized, Double-blind, Prospective Study. Int. J. Med. Sci. 2019, 16, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.H.; Choi, G.J.; Kang, H.; Baek, C.W.; Jung, Y.H.; Woo, Y.C.; Oh, J.; Park, Y.H. Effects of sugammadex vs. pyridostigmine-glycopyrrolate on post-operative nausea and vomiting: Propensity score matching. Acta Anaesthesiol. Scand. 2017, 61, 39–45. [Google Scholar] [CrossRef]
- Kang, H.Y.; Park, S.W.; Lee, S.; Jeon, J.M.; Oh, I.D.; Choi, J.H. Effect of prophylactic palonosetron and sugammadex on postoperative nausea and vomiting in patients undergoing microvascular decompression under propofol-maintained anesthesia: A retrospective observational study. Medicine 2018, 97, e13237. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Kang, K.S.; Yoo, S.H.; Chung, J.H.; Chung, J.W.; Seo, Y.; Chung, H.S.; Jeon, H.R.; Gong, H.Y.; Lee, H.Y.; et al. A comparison of oxycodone and fentanyl in intravenous patient-controlled analgesia after laparoscopic hysterectomy. Korean J. Anesthesiol. 2015, 68, 261–266. [Google Scholar] [CrossRef] [PubMed]
- DREAMS Trial Collaborators; West Midlands Research Collaborative. Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: Randomised controlled trial (DREAMS Trial). BMJ (Clin. Res. Ed.) 2017, 357, j1455. [Google Scholar]
- Apfel, C.C.; Heidrich, F.M.; Jukar-Rao, S.; Jalota, L.; Hornuss, C.; Whelan, R.P.; Zhang, K.; Cakmakkaya, O.S. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br. J. Anaesth. 2012, 109, 742–753. [Google Scholar] [CrossRef]
| Control Group (n = 70) | Midazolam Group (n = 67) | p-Value | |
|---|---|---|---|
| Age (years) | 39.0 [31.0–44.0] | 37.0 [30.0–44.0] | 0.93 |
| ASA physical status (I/II) | 58/12 | 51/16 | 0.44 |
| BMI (kg/m2) | 22.6 [20.8–25.6] | 23.2 [20.9–25.8] | 0.52 |
| Non-smokers | 57 (81%) | 60 (90%) | 0.27 |
| Previous PONV or motion sickness history | 31 (44%) | 21 (31%) | 0.17 |
| Apfel’s risk score 1 for PONV | 0.76 | ||
| 2 | 7 (10%) | 7 (10%) | |
| 3 | 38 (54%) | 40 (60%) | |
| 4 | 25 (36%) | 20 (30%) | |
| Type of surgery | 0.18 | ||
| Hysterectomy | 15 (21%) | 15 (22%) | |
| Myomectomy | 23 (33%) | 13 (19%) | |
| Adnexal surgery 2 | 32 (46%) | 39 (58%) | |
| Duration of anesthesia (min) | 125.0 [100.0–155.0] | 110.0 [92.5–130.0] | 0.06 |
| Duration of surgery (min) | 87.5 [65.0–125.0] | 75.0 [57.5–97.5] | 0.05 |
| Duration of emergence (min) | 7.0 [5.0–9.0] | 8.0 [6.0–10.0] | 0.24 |
| Duration of PACU stay (min) | 55.0 [45.0–60.0] | 55.0 [45.0–63.0] | 0.43 |
| Control Group (n = 70) | Midazolam Group (n = 67) | p-Value | |
|---|---|---|---|
| Nausea | |||
| PACU | 14 (20%) | 5 (7%) | 0.06 |
| PACU discharge to 6 h after surgery | 21 (30%) | 12 (18%) | 0.15 |
| 6 to 24 h after surgery | 22 (31%) | 13 (19%) | 0.16 |
| Severity of nausea (mild/moderate/severe) | |||
| PACU | 9/3/2 | 2/1/2 | 0.46 |
| PACU discharge to 6 h after surgery | 17/2/2 | 9/0/3 | 0.30 |
| 6 to 24 h after surgery | 18/0/4 | 12/0/1 | 0.72 |
| Retching or vomiting | |||
| PACU | 2 (3%) | 2 (3%) | 1.00 |
| PACU discharge to 6 h after surgery | 2 (3%) | 2 (3%) | 1.00 |
| 6 to 24 h after surgery | 4 (6%) | 1 (2%) | 0.37 |
| Rescue antiemetics | |||
| PACU | 5 (7%) | 3 (5%) | 0.72 |
| PACU discharge to 6 h after surgery | 4 (6%) | 3 (5%) | 1.00 |
| 6 to 24 h after surgery | 4 (6%) | 1 (2%) | 0.37 |
| PCA discontinuation | 4 (6%) | 1 (2%) | 0.37 |
| Complete response 1 | 41 (59%) | 48 (72%) | 0.11 |
| Control Group (n = 70) | Midazolam Group (n = 67) | p-Value | |
|---|---|---|---|
| Pain VNRS | |||
| PACU | 3.0 [2.0–4.0] | 4.0 [2.0–4.0] | 0.41 |
| PACU discharge to 6 h after surgery | 2.0 [2.0–3.0] | 2.0 [2.0–3.0] | 0.30 |
| 6 to 24 h after surgery | 2.0 [1.0–2.0] | 1.0 [1.0–2.0] | 0.18 |
| Rescue analgesics | |||
| PACU | 35 (50%) | 33 (50%) | 1.00 |
| PACU discharge to 6 h after surgery | 3 (4%) | 3 (5%) | 1.00 |
| 6 to 24 h after surgery | 4 (6%) | 7 (10%) | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Yi, I.K.; Han, J.Y.; Na, E.D.; Park, C.; Kim, J.Y. Effect of Adding Midazolam to Dual Prophylaxis for Preventing Postoperative Nausea and Vomiting. J. Clin. Med. 2021, 10, 4857. https://doi.org/10.3390/jcm10214857
Lee J, Yi IK, Han JY, Na ED, Park C, Kim JY. Effect of Adding Midazolam to Dual Prophylaxis for Preventing Postoperative Nausea and Vomiting. Journal of Clinical Medicine. 2021; 10(21):4857. https://doi.org/10.3390/jcm10214857
Chicago/Turabian StyleLee, Jiyoung, In Kyong Yi, Jung Youn Han, Eun Duc Na, Chunghyun Park, and Jong Yeop Kim. 2021. "Effect of Adding Midazolam to Dual Prophylaxis for Preventing Postoperative Nausea and Vomiting" Journal of Clinical Medicine 10, no. 21: 4857. https://doi.org/10.3390/jcm10214857
APA StyleLee, J., Yi, I. K., Han, J. Y., Na, E. D., Park, C., & Kim, J. Y. (2021). Effect of Adding Midazolam to Dual Prophylaxis for Preventing Postoperative Nausea and Vomiting. Journal of Clinical Medicine, 10(21), 4857. https://doi.org/10.3390/jcm10214857






