Old and New Calcineurin Inhibitors in Lupus Nephritis
Abstract
:1. Introduction
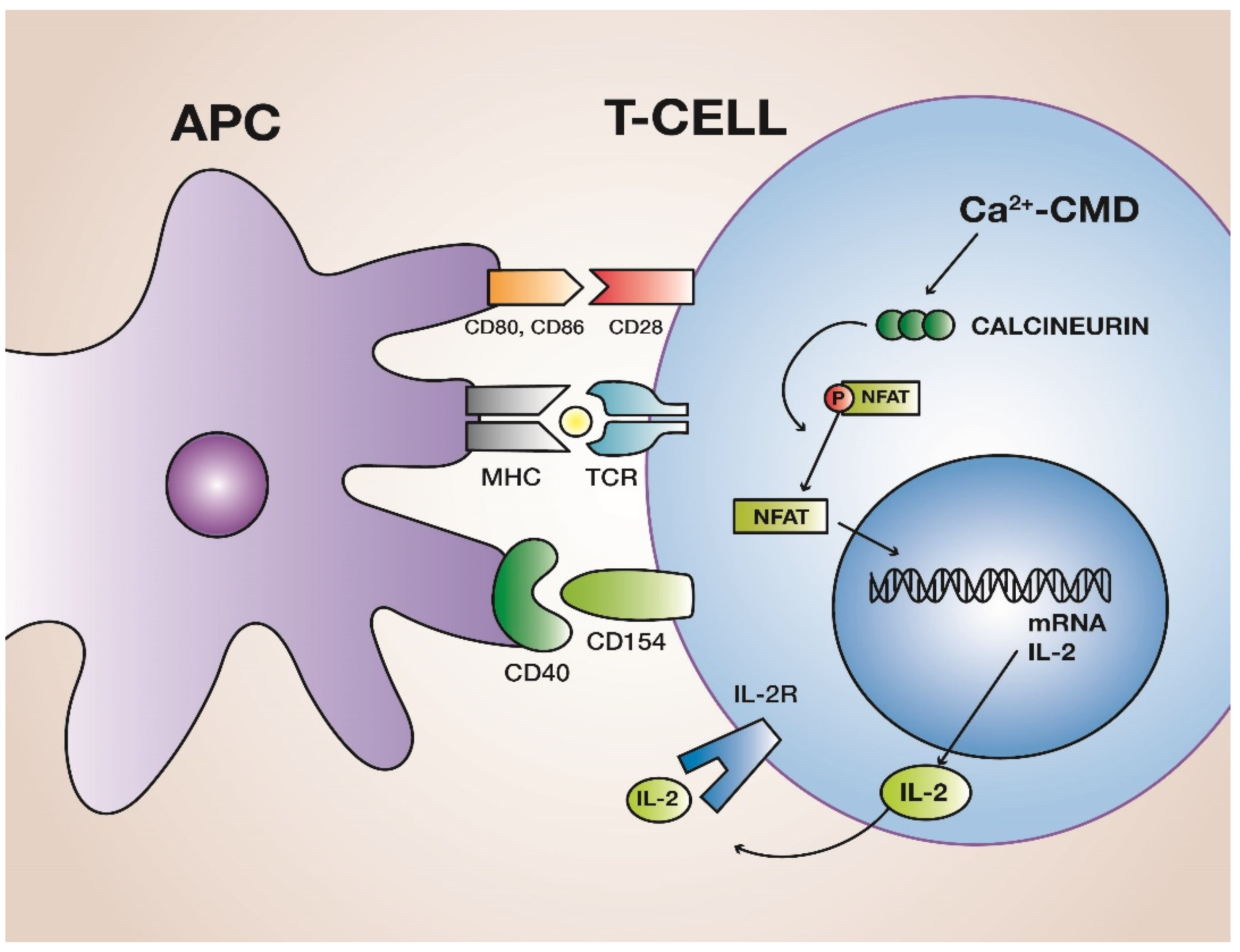
2. Calcineurin
3. Calcineurin Inhibitors
4. Old CNIs in Lupus Nephritis
5. New CNI in Lupus Nephritis
6. CNI and Pregnancy
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The Classification of Glomerulonephritis in Systemic Lupus Erythematosus Revisited. J. Am. Soc. Nephrol. 2004, 15, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C.; Kwok, R.C.L.; Yip, P.S.F. Effect of Renal Disease on the Standardized Mortality Ratio and Life Expectancy of Patients with Systemic Lupus Erythematosus. Arthritis Rheum. 2013, 65, 2154–2160. [Google Scholar] [CrossRef]
- Yap, D.Y.H.; Tang, C.S.O.; Ma, M.K.M.; Lam, M.F.; Chan, T.M. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol. Dial. Transplant. 2012, 27, 3248–3254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusnak, F.; Mertz, P. Calcineurin: Form and Function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [CrossRef] [PubMed]
- Parra, V.; Rothermel, B.A. Calcineurin signaling in the heart: The importance of time and place. J. Mol. Cell. Cardiol. 2017, 103, 121–136. [Google Scholar] [CrossRef] [Green Version]
- Penny, C.J.; Gold, M.G. Mechanisms for localising calcineurin and CaMKII in dendritic spines. Cell. Signal. 2018, 49, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Roy, J.; Cyert, M.S. Identifying New Substrates and Functions for an Old Enzyme: Calcineurin. Cold Spring Harb. Perspect. Biol. 2020, 12, a035436. [Google Scholar] [CrossRef]
- Yamada, A.; Salama, A.D.; Sayegh, M.H. The Role of Novel T Cell Costimulatory Pathways in Autoimmunity and Transplantation. J. Am. Soc. Nephrol. 2002, 13, 559–575. [Google Scholar] [CrossRef]
- Hogan, P.G.; Chen, L.; Nardone, J.; Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003, 17, 2205–2232. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.K.; Eleonora, T.; Dimitre, R.S.; Alexander, M.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, eaat1482. [Google Scholar] [CrossRef] [Green Version]
- Sijpkens, Y.W.J.; Doxiadis, I.I.N.; Mallat, M.J.K.; de Fijter, J.W.; Bruijn, J.A.; Claas, F.H.J.; Paul, L.C. Early versus late acute rejection episodes in renal transplantation. Transplantation 2003, 75, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C.; Villa, M.; Cesana, B.; Montagnino, G.; Tarantino, A. Risk factors for late kidney allograft failure. Kidney Int. 2002, 62, 1848–1854. [Google Scholar] [CrossRef] [Green Version]
- Issa, N.; Kukla, A.; Ibrahim, H.N. Calcineurin Inhibitor Nephrotoxicity: A Review and Perspective of the Evidence. Am. J. Nephrol. 2013, 37, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.S.; Collins, K.; Laurence, A.; O’Shea, J.J. The Current STATus of lymphocyte signaling: New roles for old players. Curr. Opin. Immunol. 2009, 21, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.-A. Voclosporin: First Approval. Drugs 2021, 81, 605–610. [Google Scholar] [CrossRef]
- Li, Y.; Palmisano, M.; Sun, D.; Zhou, S. Pharmacokinetic Disposition Difference Between Cyclosporine and Voclosporin Drives Their Distinct Efficacy and Safety Profiles in Clinical Studies. Clin. Pharmacol. 2020, 12, 83–96. [Google Scholar] [CrossRef]
- De Jonge, H.; de Loor, H.; Verbeke, K.; Vanrenterghem, Y.; Kuypers, D.R. In Vivo CYP3A4 Activity, CYP3A5 Genotype, and Hematocrit Predict Tacrolimus Dose Requirements and Clearance in Renal Transplant Patients. Clin. Pharmacol. Ther. 2012, 92, 366–375. [Google Scholar] [CrossRef]
- Callaghan, M.; Whelan, A.; Feighery, C.; Bresnihan, B. IL-2 enhances polyclonal IgM but not IgM-rheumatoid factor synthesis by activated human peripheral blood B cells. Clin. Exp. Immunol. 1993, 93, 212–217. [Google Scholar] [CrossRef]
- Penafuerte, C.; Ng, S.; Bautista-Lopez, N.; Birman, E.; Forner, K.; Galipeau, J. B Effector Cells Activated by a Chimeric Protein Consisting of IL-2 and the Ectodomain of TGF-β Receptor II Induce Potent Antitumor Immunity. Cancer Res. 2012, 72, 1210–1220. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, S.L.; Crabtree, G.R. The mechanism of action of cyclosporin A and FK506. Immunol. Today 1992, 13, 136–142. [Google Scholar] [CrossRef]
- Barbarino, J.M.; Owusu Obeng, A.; Klein, T.E.; Altman, R.B. PharmGKB summary: Voriconazole pathway, pharmacokinetics. Pharmacogenet. Genom. 2017, 27, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bîrsan, T.; Dambrin, C.; Freitag, D.; Yatscoff, R.; Morris, R. The novel calcineurin inhibitor ISA247: A more potent immunosuppressant than cyclosporine in vitro. Transpl. Int. 2005, 17, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Donnelly, M.; Merscher-Gomez, S.; Chang, Y.H.; Franz, S.; Delfgaauw, J.; Chang, J.-M.; Choi, H.Y.; Campbell, K.N.; Kim, K.; et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 2008, 14, 931–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.-C.; Fornoni, A.; Weins, A.; Hakroush, S.; Maiguel, D.; Sageshima, J.; Chen, L.; Ciancio, G.; Faridi, M.H.; Behr, D.; et al. Abatacept in B7-1-Positive Proteinuric Kidney Disease. N. Engl. J. Med. 2013, 369, 2416–2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Shi, W.; Ma, J.; Sloan, A.; Faul, C.; Wei, C.; Reiser, J.; Yang, Y.; Liu, S.; Wang, W. The calcineurin-NFAT pathway allows for urokinase receptor-mediated beta3 integrin signaling to cause podocyte injury. J. Mol. Med. 2012, 90, 1407–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedigo, C.E.; Ducasa, G.M.; Leclercq, F.; Sloan, A.; Mitrofanova, A.; Hashmi, T.; Molina-David, J.; Ge, M.; Lassenius, M.I.; Forsblom, C.; et al. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J. Clin. Investig. 2016, 126, 3336–3350. [Google Scholar] [CrossRef] [Green Version]
- Zea, A.H.; Stewart, T.; Ascani, J.; Tate, D.J.; Finkel-Jimenez, B.; Wilk, A.; Reiss, K.; Smoyer, W.E.; Aviles, D.H. Activation of the IL-2 Receptor in Podocytes: A Potential Mechanism for Podocyte Injury in Idiopathic Nephrotic Syndrome? PLoS ONE 2016, 11, e0157907. [Google Scholar] [CrossRef]
- Liao, R.; Liu, Q.; Zheng, Z.; Fan, J.; Peng, W.; Kong, Q.; He, H.; Yang, S.; Chen, W.; Tang, X.; et al. Tacrolimus Protects Podocytes from Injury in Lupus Nephritis Partly by Stabilizing the Cytoskeleton and Inhibiting Podocyte Apoptosis. PLoS ONE 2015, 10, e0132724. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Jiang, H.; Ying, M.; Xie, Z.; Li, X.; Wang, H.; Zhao, J.; Lin, C.; Wang, Y.; Feng, S.; et al. Calcineurin inhibitors cyclosporin A and tacrolimus protect against podocyte injury induced by puromycin aminonucleoside in rodent models. Sci. Rep. 2016, 6, 32087. [Google Scholar] [CrossRef]
- Bennett, W.M.; DeMattos, A.; Meyer, M.M.; Andoh, T.; Barry, J.M. Chronic cyclosporine nephropathy: The Achilles’ heel of immunosuppressive therapy. Kidney Int. 1996, 50, 1089–1100. [Google Scholar] [CrossRef] [Green Version]
- Naesens, M.; Kuypers, D.R.J.; Sarwal, M. Calcineurin inhibitor nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481–508. [Google Scholar] [CrossRef] [Green Version]
- Curtis, J.J. Hypertensinogenic mechanism of the calcineurin inhibitors. Curr. Hypertens. Rep. 2002, 4, 377–380. [Google Scholar] [CrossRef]
- Blankenstein, K.I.; Borschewski, A.; Labes, R.; Paliege, A.; Boldt, C.; McCormick, J.A.; Ellison, D.H.; Bader, M.; Bachmann, S.; Mutig, K. Calcineurin inhibitor cyclosporine A activates renal Na-K-Cl cotransporters via local and systemic mechanisms. Am. J. Physiol. Physiol. 2017, 312, F489–F501. [Google Scholar] [CrossRef] [PubMed]
- Øzbay, L.A.; Smidt, K.; Mortensen, D.M.; Carstens, J.; Jørgensen, K.A.; Rungby, J. Cyclosporin and tacrolimus impair insulin secretion and transcriptional regulation in INS-1E beta-cells. Br. J. Pharmacol. 2011, 162, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakkera, H.A.; Kudva, Y.; Kaplan, B. Calcineurin Inhibitors: Pharmacologic Mechanisms Impacting Both Insulin Resistance and Insulin Secretion Leading to Glucose Dysregulation and Diabetes Mellitus. Clin. Pharmacol. Ther. 2017, 101, 114–120. [Google Scholar] [CrossRef]
- Li, Z.; Sun, F.; Zhang, Y.; Chen, H.; He, N.; Chen, H.; Song, P.; Wang, Y.; Yan, S.; Zheng, S. Tacrolimus Induces Insulin Resistance and Increases the Glucose Absorption in the Jejunum: A Potential Mechanism of the Diabetogenic Effects. PLoS ONE 2015, 10, e0143405. [Google Scholar] [CrossRef]
- Triñanes, J.; Rodriguez-Rodriguez, A.E.; Brito-Casillas, Y.; Wagner, A.; de Vries, A.P.J.; Cuesto, G.; Acebes, A.; Salido, E.; Torres, A.; Porrini, E. Deciphering Tacrolimus-Induced Toxicity in Pancreatic β Cells. Am. J. Transplant. 2017, 17, 2829–2840. [Google Scholar] [CrossRef]
- Kolic, J.; Beet, L.; Overby, P.; Cen, H.H.; Panzhinskiy, E.; Ure, D.R.; Cross, J.L.; Huizinga, R.B.; Johnson, J.D. Differential Effects of Voclosporin and Tacrolimus on Insulin Secretion From Human Islets. Endocrinology 2020, 161, bqaa162. [Google Scholar] [CrossRef]
- Tavori, H.; Rashid, S.; Fazio, S. On the function and homeostasis of PCSK9: Reciprocal interaction with LDLR and additional lipid effects. Atherosclerosis 2015, 238, 264–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponticelli, C.; Arnaboldi, L.; Moroni, G.; Corsini, A. Treatment of dyslipidemia in kidney transplantation. Expert Opin. Drug Saf. 2020, 19, 257–267. [Google Scholar] [CrossRef]
- Fuhrmann, A.; Lopes, P.C.; Sereno, J.; Pedro, J.; Espinoza, D.O.; Pereira, M.J.; Reis, F.; Eriksson, J.W.; Carvalho, E. Molecular mechanisms underlying the effects of cyclosporin A and sirolimus on glucose and lipid metabolism in liver, skeletal muscle and adipose tissue in an in vivo rat model. Biochem. Pharmacol. 2014, 88, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Brocks, D.; Chaudhary, H.; Ben-Eltriki, M.; Elsherbiny, M.; El-Kadi, A. Effects of serum lipoproteins on cyclosporine A cellular uptake and renal toxicity in vitro. Can. J. Physiol. Pharmacol. 2014, 92, 140–148. [Google Scholar] [CrossRef]
- Ponticelli, C.; Podestà, M.A.; Moroni, G. Hyperuricemia as a trigger of immune response in hypertension and chronic kidney disease. Kidney Int. 2020, 98, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Faravelli, I.; Velardo, D.; Podestà, M.A.; Ponticelli, C. Immunosuppression-related neurological disorders in kidney transplantation. J. Nephrol. 2021, 34, 539–555. [Google Scholar] [CrossRef]
- Clarke, H.; Ryan, M.P. Cyclosporine A-induced alterations in magnesium homeostasis in the rat. Life Sci. 1999, 64, 1295–1306. [Google Scholar] [CrossRef]
- Lea, J.P.; Sands, J.M.; McMahon, S.J.; Tumlin, J.A. Evidence that the inhibition of Na+/K+-ATPase activity by FK506 involves calcineurin. Kidney Int. 1994, 46, 647–652. [Google Scholar] [CrossRef] [Green Version]
- Peleg, Y.; Bomback, A.S.; Radhakrishnan, J. The evolving role of calcineurin inhibitors in treating lupus nephritis. Clin. J. Am. Soc. Nephrol. 2020, 15, 1066–1072. [Google Scholar] [CrossRef]
- Favre, H.; Miescher, P.A.; Huang, Y.P.; Chatelanat, F.; Mihatsch, M.J. Ciclosporin in the Treatment of Lupus Nephritis. Am. J. Nephrol. 1989, 9, 57–60. [Google Scholar] [CrossRef]
- Hussein, M.M.; Mooij, J.M.; Roujouleh, H. Cyclosporine in the treatment of lupus nephritis including two patients treated during pregnancy. Clin. Nephrol. 1993, 40, 160–163. [Google Scholar] [PubMed]
- Manger, K.; Kalden, J.R.; Manger, B. Cyclosporin a in the treatment of systemic lupus erythematosus: Results of an open clinical study. Rheumatology 1996, 35, 669–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dostál, C.; Tesař, V.; Rychlík, I.; Zabka, J.; Vencovskŷ, J.; Bartûňková, J.; Stejskalová, A.; Tegzova, D. Effect of 1 year cyclosporine a treatment on the activity and renal involvement of systemic lupus erythematosus: A pilot study. Lupus 1998, 7, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.W.; Yang, L.Y.; Chen, W.P.; Lin, C.Y. Clinical efficacy of cyclosporin a neoral in the treatment of paediatric lupus nephritis with heavy proteinuria. Br. J. Rheumatol. 1998, 37, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Austin, H.A.; Illei, G.G.; Braun, M.J.; Balow, J.E. Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J. Am. Soc. Nephrol. 2009, 20, 901–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavada, J.; Pešickova, S.S.; Ryšava, R.; Olejarova, M.; Horák, P.; Hrnčíř, Z.; Rychlík, I.; Havrda, M.; Vítova, J.; Lukáč, J. Cyclosporine A or intravenous cyclophosphamide for lupus nephritis: The Cyclofa-Lune study. Lupus 2010, 19, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Moroni, G.; Doria, A.; Mosca, M.; Alberighi, O.D.C.; Ferraccioli, G.; Todesco, S.; Manno, C.; Altieri, P.; Ferrara, R.; Greco, S. A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin. J. Am. Soc. Nephrol. 2006, 1, 925–932. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Hajialilo, M.; Rasi Hashemi, S.S.; Malek Mahdavi, A.; Gojazadeh, M.; Khabbazi, A. Low dose cyclosporine A in the treatment of resistant proliferative lupus nephritis. Mod. Rheumatol. 2018, 28, 523–529. [Google Scholar] [CrossRef]
- Sumethkul, K.; Kitumnuaypong, T.; Angthararak, S.; Pichaiwong, W. Low-dose cyclosporine for active lupus nephritis: A dose titration approach. Clin. Rheumatol. 2019, 38, 2151–2159. [Google Scholar] [CrossRef]
- Argolini, L.M.; Frontini, G.; Elefante, E.; Saccon, F.; Binda, V.; Tani, C.; Scotti, I.; Carli, L.; Gatto, M.; Esposito, C. Multicentric study comparing cyclosporine, mycophenolate mofetil and azathioprine in the maintenance therapy of lupus nephritis: 8 years follow up. J. Nephrol. 2021, 34, 389–398. [Google Scholar] [CrossRef]
- Moroni, G.; Doria, A.; Ponticelli, C. Cyclosporine (CsA) in lupus nephritis: Assessing the evidence. Nephrol. Dial. Transplant. 2009, 24, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Szeto, C.-C.; Kwan, B.-H.; Lai, F.-M.; Tam, L.-S.; Li, E.-M.; Chow, K.-M.; Gang, W.; Li, P.-T. Tacrolimus for the treatment of systemic lupus erythematosus with pure class V nephritis. Rheumatology 2008, 47, 1678–1681. [Google Scholar] [CrossRef] [Green Version]
- Miyasaka, N.; Kawai, S.; Hashimoto, H. Efficacy and safety of tacrolimus for lupus nephritis: A placebo-controlled double-blind multicenter study. Mod. Rheumatol. 2009, 19, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Lanata, C.M.; Mahmood, T.; Fine, D.M.; Petri, M. Combination therapy of mycophenolate mofetil and tacrolimus in lupus nephritis. Lupus 2010, 19, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Uchino, A.; Tsukamoto, H.; Nakashima, H.; Yoshizawa, S.; Furugo, I.; Mitoma, H.; Oryoji, K.; Shimoda, T.; Niiro, H.; Tada, Y. Tacrolimus is effective for lupus nephritis patients with persistent proteinuria. Clin. Exp. Rheumatol. 2010, 28, 6–12. [Google Scholar] [PubMed]
- Ramachandran, R.; Dheerendra, P.C.; Kumar, V.; Sakhuja, V.; Jha, V.; Kohli, H.S.; Rathi, M. Maintenance therapy with tacrolimus in lupus nephritis. Lupus 2012, 21, 1258. [Google Scholar] [CrossRef]
- Deng, J.; Huo, D.; Wu, Q.; Yang, Z.; Liao, Y. A meta-analysis of randomized controlled trials comparing tacrolimus with intravenous cyclophosphamide in the induction treatment for lupus nephritis. Tohoku J. Exp. Med. 2012, 227, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Song, G.G. Relative efficacy and safety of tacrolimus, mycophenolate mofetil, and cyclophosphamide as induction therapy for lupus nephritis: A Bayesian network meta-analysis of randomized controlled trials. Lupus 2015, 24, 1520–1528. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Liu, Z.; Xing, C.; Fu, P.; Ni, Z.; Chen, J.; Lin, H.; Liu, F.; He, Y. Multitarget therapy for induction treatment of lupus nephritis: A randomized trial. Ann. Intern. Med. 2015, 162, 18–26. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Zhou, M.; Liu, Z.; Chen, J.; Xing, C.; Lin, H.; Ni, Z.; Fu, P.; Liu, F. Multitarget therapy for maintenance treatment of lupus nephritis. J. Am. Soc. Nephrol. 2017, 28, 3671–3678. [Google Scholar] [CrossRef]
- Mok, C.C.; Ho, L.Y.; Ying, S.K.Y.; Leung, M.C.; To, C.H.; Ng, W.L. Long-term outcome of a randomised controlled trial comparing tacrolimus with mycophenolate mofetil as induction therapy for active lupus nephritis. Ann. Rheum. Dis. 2020, 79, 1070–1076. [Google Scholar] [CrossRef]
- Cattran, D.C.; Feehally, J.; Cook, H.T.; Liu, Z.H.; Fervenza, F.C.; Mezzano, S.A.; Floege, J.; Nachman, P.H.; Gipson, D.S.; Praga, M. Kidney disease: Improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. Suppl. 2012, 2, 139–274. [Google Scholar]
- Fanouriakis, A.; Kostopoulou, M.; Cheema, K.; Anders, H.-J.; Aringer, M.; Bajema, I.; Boletis, J.; Frangou, E.; Houssiau, F.A.; Hollis, J. 2019 update of the joint European league against rheumatism and European renal association—European Dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann. Rheum. Dis. 2020, 79, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Mayo, P.R.; Huizinga, R.B.; Ling, S.Y.; Freitag, D.G.; Aspeslet, L.J.; Foster, R.T. Voclosporin food effect and single oral ascending dose pharmacokinetic and pharmacodynamic studies in healthy human subjects. J. Clin. Pharmacol. 2013, 53, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Solomons, N.; Pendergraft, W.F., III; Dooley, M.A.; Tumlin, J.; Romero-Diaz, J.; Lysenko, L.; Navarra, S.V.; Huizinga, R.B.; Adzerikho, I. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 2019, 95, 219–231. [Google Scholar] [CrossRef]
- Rovin, B.H.; Teng, Y.K.O.; Ginzler, E.M.; Arriens, C.; Caster, D.J.; Romero-Diaz, J.; Gibson, K.; Kaplan, J.; Lisk, L.; Navarra, S. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2070–2080. [Google Scholar] [CrossRef]
- Chambers, C.D.; Tutuncu, Z.N.; Johnson, D.; Jones, K.L. Human pregnancy safety for agents used to treat rheumatoid arthritis: Adequacy of available information and strategies for developing post-marketing data. Arthritis Res. Ther. 2006, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Moroni, G.; Ponticelli, C. Pregnancy in women with systemic lupus erythematosus (SLE). Eur. J. Intern. Med. 2016, 32, 7–12. [Google Scholar] [CrossRef]
- Bramham, K.; Chusney, G.; Lee, J.; Lightstone, L.; Nelson-Piercy, C. Breastfeeding and tacrolimus: Serial monitoring in breast-fed and bottle-fed infants. Clin. J. Am. Soc. Nephrol. 2013, 8, 563–567. [Google Scholar] [CrossRef]
- Parikh, S.V.; Almaani, S.; Brodsky, S.; Rovin, B.H. Update on Lupus Nephritis: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 76, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGraw, J.; Waller, D. Cytochrome P450 variations in different ethnic populations. Expert Opin. Drug Metab. Toxicol. 2012, 8, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Moroni, G.; Ponticelli, C. AURORA 1 reports efficacy of voclosporin in lupus nephritis. Nat. Rev. Nephrol. 2021, 17, 637–638. [Google Scholar] [CrossRef] [PubMed]
| CYP450 Inhibitors | CYP450 Inducers |
|---|---|
| CNIs Bioavailability | |
| Increased | Decreased |
| Older age | Childhood |
| Obesity | Malnutrition |
| Smoking | Biliary diversion |
| Liver disease | Diarrhea |
| Grapefruit | Antiepileptic drugs |
| Antifungal azoles | Nafcillin |
| Macrolides | Oxacillin |
| Quinolones | Rifampicin |
| Non-dihydropyridine CCBs | Rifabutin |
| P-Glycoprotein Inhibitors | P-Glycoprotein Inducers |
|---|---|
| Decrease CNIs Plasma Levels and Increase Intracellular Concentration | Increase CNIs Plasma Levels and Decrease Intracellular Concentration |
| Macrolides (erythromycin, clarithromycin) | Antiepileptic Drugs (carbamazepine, phenobarbital, phenytoin) |
| Proton pump inhibitors (omeprazole, lansoprazole) | Glucocorticoids (dexamethasone) |
| Calcium channel blockers (verapamil, diltiazem, felodipine, nifedipine) | Rifampicin |
| Antiarrhythmic drugs (amiodarone, quinidine) | Tenofovir |
| Antidepressant drugs (paroxetine, sertraline) | Antidepressant drugs (nefazodone, trazodone) |
| Antifungal drugs (ketoconazole) | Alpha-blockers (prazosin) |
| Cyclosporine | Doxorubicin |
| Fenofibrates |
| Chronic nephrotoxicity Similar for CsA and TAC. | Dyslipidemia More frequent with CsA. |
| Thrombotic microangiopathy Dose-dependent. Similar for CsA and TAC. | Gum hyperplasia Typical of CsA. Aggravated by simultaneous use of CCB. |
| Neurological complications More frequent with TAC. | Increased hair growth Typical of CsA. TAC may rarely cause alopecia. |
| Diabetes More frequent with TAC. | Hearing loss More frequent with TAC. |
| Gastrointestinal troubles Similar for CsA and TAC. | Electrolyte disorders Hypomagnesemia, hyperkalemia. |
| Hypertension More frequent with CsA. | Hyperuricemia Similar for CsA and TAC. |
| Author, Year | Study Type | Study Population | Drugs | Follow-Up | Endpoint | Results | Notes | |
|---|---|---|---|---|---|---|---|---|
| CsA—Induction | Fu [53] | RCT | 40 children | CsA Neoral vs. prednisolone + CYC | 1 year | Reduction of proteinuria | Reduction of proteinuria with both drugs | Neoral well tolerated, better growth rate. |
| Austin [54] | RCT | 42 patients (LN class V) | Prednisone vs. prednisone + CsA vs. prednisone + IV CYC | 1 year | Remission of proteinuria | 27% with prednisone, 60% with IV CYC, and 83% with CsA | Significantly more relapse of nephrotic syndrome after CsA than after IV CYC | |
| Zavada [55] | RCT | 40 patients (active LN) | Regimens based on CYC vs. CsA | End of induction and maintenance phase | Remission and response to therapy | CYC (24% remission, 52% response); CsA (26% remission, 43% response) | Transient increase in blood pressure and reversible decrease in GFR with CsA | |
| Sheikholesla-mi [57] | OS | 27 patients (resistant LN) | CsA added to steroid + MMF or steroid + CYC | 40.7 ± 24.9 months | Complete and partial renal remission | 66.9% and 25.7% | With CsA stable creatinine, <proteinuria and anti-dsDNA titer, >of C3 and C4 | |
| Sumethkul [58] | OS | 62 patients (active LN) | CsA + MMF and prednisolone, or CsA + prednisolone | 1 year | Complete and partial renal remission | 90.3% and 40.8% | Non-renal activity including arthritis, alopecia, hematologic and cutaneous conditions improved in all patients. | |
| CsA—Maintenance | Moroni [56] | RCT | 75 patients (LN class IV) | CsA vs. AZA (both after steroids and CYC) | 4 years | Lupus flares | 7 lupus flares with CsA, 8 with AZA | No difference in creatinine clearance. In both groups activity index decreased significantly and the chronicity index slightly increased. |
| Argolini [59] | OS | 106 patients | CsA vs. MMF vs. AZA | 8 years | Complete remission | 79.4% of CsA vs. 83.3% of MMF and 77.8% of AZA | Flares-free survival curves and incidence of side-effects were not different. | |
| TAC | Miyasaka [62] | RCT | 63 patients (persistent LN) | TAC vs. placebo | 28 weeks | Change in LNDAI | 31% decrease with TAC, vs. 38% increase with placebo | Treatment-related adverse events occurred in 93% with TAC and 80% with placebo. |
| Deng [66] | MA | 225 Chinese patients—5 RCTs | TAC (oral or IV) vs. IV CYC for induction therapy | \ | Complete remission, response rate, serum albumin, anti-dsDNA, proteinuria, SLEDAI | TAC superior in all endpoints | TAC safer than IV CYC | |
| Lee [67] | MA | 972 Korean patients—9 RCTs | TAC vs. MMF vs. IV CYC for induction therapy | \ | Overall response rate (complete remission plus partial remission) | TAC showed higher overall response rate than CYC than MMF | Better overall response with MMF. Less serious infections with TAC. | |
| Liu [68] | RCT | 368 patients | TAC + MMF vs. IV CYC (both preceded by pulse MMP) | 24 weeks | Complete remission | 46% with TAC plus MMF vs. 26% with IV CYC showed complete remission | Higher overall response incidence with TAC plus MMF. Shorter median time to overall response with TAC. | |
| Zhang [69] | CT | 206 patients | TAC + MMF vs. AZA + prednisone | 2 years | Renal relapse | Similar cumulative renal relapse rates | More adverse events with AZA. | |
| Mok [70] | RCT | 150 patients | MMF vs. TAC, + high-dose prednisolone | 10 years | Complete response | Complete renal response rate similar between MMF (59%) and TAC (62%) | Proteinuric and nephritic renal flares in 34% and 37% of the MMF, and 53% and 30% of the TAC groups. | |
| VCS | Rovin [74] | RCT | 265 patients | VCS vs. placebo, with MMF and rapidly tapered low-dose oral corticosteroids | 24 weeks | Complete response | 33% complete renal response in low-dose VCS group, 27% in high-dose VCS group, 19% in placebo group | More serious adverse events in VCS groups. More deaths in the low-dose group (11%) and high-dose VCS (2%) than placebo (1.1%). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponticelli, C.; Reggiani, F.; Moroni, G. Old and New Calcineurin Inhibitors in Lupus Nephritis. J. Clin. Med. 2021, 10, 4832. https://doi.org/10.3390/jcm10214832
Ponticelli C, Reggiani F, Moroni G. Old and New Calcineurin Inhibitors in Lupus Nephritis. Journal of Clinical Medicine. 2021; 10(21):4832. https://doi.org/10.3390/jcm10214832
Chicago/Turabian StylePonticelli, Claudio, Francesco Reggiani, and Gabriella Moroni. 2021. "Old and New Calcineurin Inhibitors in Lupus Nephritis" Journal of Clinical Medicine 10, no. 21: 4832. https://doi.org/10.3390/jcm10214832
APA StylePonticelli, C., Reggiani, F., & Moroni, G. (2021). Old and New Calcineurin Inhibitors in Lupus Nephritis. Journal of Clinical Medicine, 10(21), 4832. https://doi.org/10.3390/jcm10214832






