Abstract
Kinesin family member C1 (KIFC1), a minus end-directed motor protein, is reported to play an essential role in cancer. This study aimed to analyze KIFC1 expression and examine KIFC1 involvement in cisplatin resistance in bladder cancer (BC). Immunohistochemistry showed that 37 of 78 (47.4%) BC cases were positive for KIFC1. KIFC1-positive cases were associated with high T stage and lymph node metastasis. Kaplan-Meier analysis showed that KIFC1-positive cases were associated with poor prognosis, consistent with the results from public databases. Molecular classification in several public databases indicated that KIFC1 expression was increased in basal type BC. Immunohistochemistry showed that KIFC1-positive cases were associated with basal markers 34βE12, CK5 and CD44. KIFC1 expression was increased in altered TP53 compared to that in wild-type TP53. Immunohistochemistry showed that KIFC1-positive cases were associated with p53-positive cases. P53 knockout by CRISPR-Cas9 induced KIFC1 expression in BC cell lines. Knockdown of KIFC1 by siRNA increased the sensitivity to cisplatin in BC cells. Kaplan-Meier analysis indicated that prognosis was poor among KIFC1-positive BC patients treated with cisplatin-based chemotherapy. Immunohistochemistry showed that KIFC1-positive cases were associated with PD-L1-positive cases. High KIFC1 expression was associated with a favorable prognosis in patients treated with atezolizumab from the IMvigor 210 study. These results suggest that KIFC1 might be a promising biomarker and therapeutic target in BC.
1. Introduction
Bladder cancer (BC) is the 11th most commonly diagnosed cancer worldwide, with approximately 573,000 new cases and 213,000 deaths in 2020 [1]. BC can be classified into two types: non-muscle-invasive BC and muscle-invasive BC (MIBC). In non-MIBC, T1 tumors are an aggressive subtype with 40% recurrence and 15% progression to MIBC at 5 years [2]. MIBC will eventually develop distant metastasis resulting in a 5 year survival rate of <50% [3]. Although standard care for MIBC is neoadjuvant chemotherapy followed by radical cystectomy, about 40% of patients experience relapse [4]. Cisplatin-based chemotherapy is the standard first-line treatment for patients with relapse after radical cystectomy [3]. However, most patients receive few benefits due to cisplatin resistance. Therefore, clarifying the molecular biology of cancer progression and cisplatin resistance is urgently needed in BC.
The presence of more than two centrosomes (centrosome amplification: CA) affects the chromosome segregation machinery and leads to chromosomal instability [5]. Several reports have shown that CA correlates with aggressive features and poor prognosis in BC [6,7]. Although CA causes multipolar spindles and leads to apoptosis, cancer cells overcome these lethal effects through centrosome clustering. Centrosome clustering, defined as the reshaping of transient multipolar spindles into pseudo-bipolar structures, is a well-studied mechanism that allows cancer cells to avoid apoptosis [8]. Kinesin family member C1 encoded by the KIFC1 gene (also called HSET) belongs to the kinesin family member of motor proteins and is implicated in centrosome clustering, microtubule transport and spindle formations during mitosis [9]. A recent study showed that KIFC1 promoted cell growth and epithelial-mesenchymal transition in BC [10]. However, the biological role of KIFC1 in BC has not been fully elucidated.
In this study, we performed immunohistochemistry to analyze the prognostic value of KIFC1 and examined the association between KIFC1 and CD44, CK5, 34βE12, p53 and PD-L1 in BC. We also investigated the association between KIFC1 and molecular classification, analyzed the role of KIFC1 in cisplatin resistance, and performed in silico analysis of the role of KIFC1 in immunotherapy.
2. Materials and Methods
2.1. Tissue Samples
In total, 174 tumors were used in this retrospective study, of which 78 tumors were collected from patients diagnosed as having BC who underwent cystectomy at Hiroshima University Hospital (Hiroshima, Japan) (Supplementary Table S1) and 50 tumors were collected from patients diagnosed as having BC who underwent cystectomy at Kure Medical Center and Chugoku Cancer Center (Kure, Japan) (Supplementary Table S2). In addition, 46 tumors were collected from patients diagnosed as having BC treated with cisplatin-based chemotherapy at Hiroshima University Hospital (Hiroshima, Japan). The Institutional Review Boards of both institutions approved this study (Hiroshima University, IRB# E912; Kure Medical Center/Chugoku Cancer Center: 2019-08).
2.2. Immunohistochemistry
Immunohistochemistry was performed as described previously [11]. We used archival formalin-fixed, paraffin-embedded tissues from the 174 patients with BC for immunohistochemical analysis. Tumor staging was performed according to the TNM (tumor-node-metastasis) classification system [12]. Sections were incubated with anti-KIFC1 antibody (1:100, H00003833-M01, Abnova, Taipei, Taiwan), CD44 (1:200, M7082, Dako, Glastrup, Denmark, USA), CK5 (1:200, M7237, Dako, Glastrup, Denmark), 34βE12 (1:200, GA051, Dako, Glastrup, Denmark), Ki-67 (1:100, M7240, Dako, Glastrup, Denmark), p53 (1:200, M7001, Dako, Glastrup, Denmark) and PD-L1 (1:300, ab205921, Abcam, MA, USA) for 1 h at room temperature. KIFC1 expression in BC was scored in all tumors as positive or negative. When more than 10% of tumor cells were stained, the specimen was considered positive for KIFC1 (according to the median cut-off values rounded off to the nearest 10%). The expressions of CD44, CK5, 34βE12, Ki-67, p53 and PD-L1 were also scored in all tumors as positive or negative. When more than 10% of tumor cells showed staining, the immunostaining of CD44, CK5, 34βE12 was considered positive. When more than 20% of tumor cells showed staining, the immunostaining of Ki-67 was considered positive. p53 staining was evaluated based on the study [13]. Immunostaining of PD-L1 was considered positive according to median cutoff values rounded off to the nearest 5%. Using these definitions, two observers (K.S. and N.O.) without knowledge of the patients’ clinical and pathologic parameters or outcomes independently reviewed immunoreactivity in each specimen.
2.3. In Silico Analysis
The GEPIA web tool was used to determine KIFC1 expression in The Cancer Genome Atlas (TCGA) (BLCA) dataset [14]. The expression array data were downloaded from GEO and Array Express under accession numbers GSE120736 [15], GSE13507 [16], GSE32548 [17], GSE48277 [18], GSE124305 [19], GSE154261 [20], E-MTAB-1803 [21] and E-MTAB-4321 [22]. The data from the study by Sanchez et al. [23] and that from the study by Taber et al. [24] were downloaded. Clinicopathologic characteristics of bladder cancer patients from GSE13507, GSE32548 and GSE48277 (Supplementary Table S3). The data from the IMvigor 210 study was also downloaded from Roche, MA, USA., Data signature analysis was performed with the UCSC web tool [25]. The proliferation signature was referred to the study by Tuan et al. [26] (Supplementary Table S4).
2.4. Cell Lines
Four cell lines derived from human BC (RT4, RT112, 5637 and UMUC3) were provided by the Vancouver Prostate Centre (Vancouver, BC, Canada). The cells were maintained in RPMI 1640 (Nissui Pharmaceutical Co., Ltd., Osaka, Japan) containing 10% fetal bovine serum (BioWhittaker, Walkersville, MD, USA) in a humidified atmosphere with 5% CO2 at 37 °C.
2.5. Western Blotting
Western blotting was performed as described previously [27]. Lysates were solubilized in Laemmli sample buffer by boiling and subjected to 10% SDS-polyacrylamide gel electrophoresis followed by electro-transfer onto a nitrocellulose filter. The membrane was incubated with a primary antibody for KIFC1 (1:500, H00003833-M01, Abnova, Taipei, Taiwan), CD44 (1:1000), and p53 (DO-1) (1:1000, Cell Signaling Technology, Inc., Danvers, MA, USA). Peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG was used in the secondary reaction. Immunocomplexes were visualized with an ECL Western Blot Detection System (Amersham Biosciences, Piscataway, NJ, USA). β-Actin (Sigma-Aldrich, St. Louis, MO, USA) was also stained as a loading control.
2.6. Generation of p53 Knockout Cells
To knock out p53 in RT4 and RT112 cells, we used CRISPR-Cas9 technology, which was performed as described previously [28]. p53 single-guide RNAs (sgRNAs; CRISPR-P53 vector) and scrambled sgRNAs (empty vector) were purchased from ABM Inc. (Richmond, BC, Canada). The sgRNA sequence of the CRISPR-P53 vector was GACGGAAACCGTAGCTGCCC. Lentiviral particles were generated by co-transfection of HEK 293T cells with Cas9-sgRNA constructs and packaging plasmids (GAG, VSVG and REV). After 48 h, the conditioned media containing lentiviral particles were harvested and used to infect cells using Polybrene as the transfection agent. Stable p53 knockout cells were selected by passaging in media containing 4 µg/mL puromycin.
2.7. Cisplatin Treatment
Cisplatin treatment was performed as described previously [29]. Cisplatin (Nippon Kayaku Co., Ltd., Tokyo, Japan) was obtained and handled according to the manufacturer’s recommendations. Cell lines treated with vehicle (0.5% ethanol) or escalating doses of cisplatin were assessed for cell viability. A WST-1 assay was performed at 48 h after cisplatin chemotherapy [15]. Drug sensitivity curves and IC50 values were calculated using GraphPad Prism 4.0 software (GraphPad Software Inc., San Diego, CA, USA).
2.8. Statistical Analysis
All experiments were repeated at least three times with each sample in triplicate. The results are expressed as the mean ± SD of the triplicate measurements. Sample sizes for relevant experiments were determined by power analysis. Statistical differences were evaluated using the two-tailed Student t-test or Mann-Whitney U-test. One-way analysis of variance (ANOVA) was used to determine whether there were any statistically significant differences. A p-value of <0.05 was considered statistically significant. After a Kaplan-Meier analysis was performed, any statistical difference between the survival curves of the cohorts was determined with the log-rank Mantel-Cox test. Statistical analyses were conducted primarily using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA) or JMP14 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Expression of KIFC1 in BC
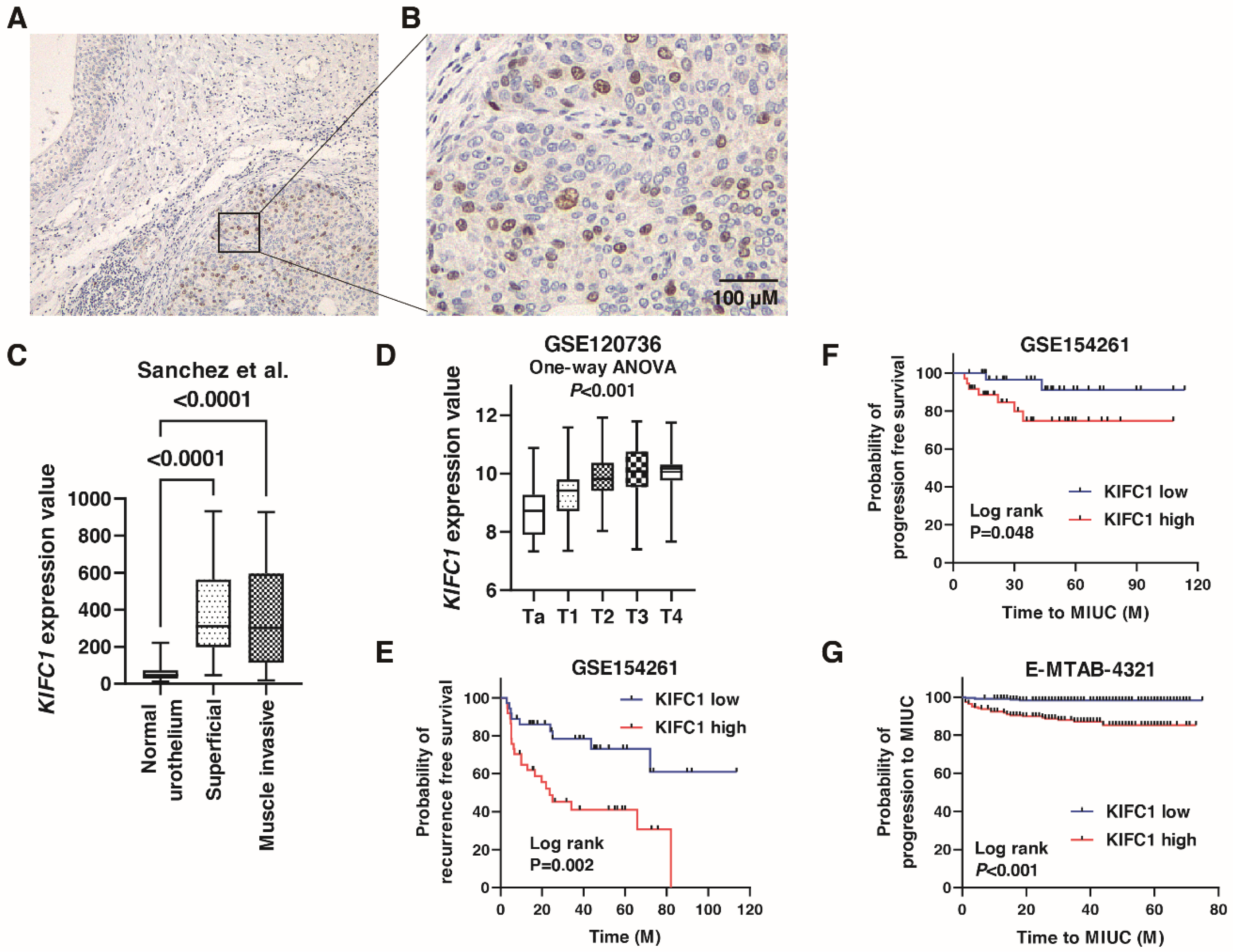
We performed immunohistochemistry to analyze the expression of KIFC1 in 78 BC tissue samples (Hiroshima cohort, Supplementary Table S1). Weak or no staining of KIFC1 was observed in the non-neoplastic urothelium, whereas stronger and more extensive staining was observed in BC tissues (Figure 1A). Staining of KIFC1 was mainly observed in the nucleus in BC (Figure 1B). In total, 37 (47.4%) of the BC cases were considered positive for KIFC1. These positive cases were associated with high T stage and lymph node metastasis (Table 1). KIFC1 expression was increased in superficial BC and MIBC compared to that in normal urothelium in the study by Sanchez et al. [21] (Figure 1C). KIFC1 expression was increased in high T stage cancer in the study GSE120736 (Figure 1D). Of note, high KIFC1 expression was associated with poor recurrence-free survival among the patients with T1 BC in the study GSE154261 (Figure 1E). High KIFC1 expression was also associated with poor progression-free survival among the patients with T1 BC in the studies GSE154261 and E-MTAB-4321 (Figure 1F,G). These results indicate that KIFC1 plays an essential role in progression in BC.
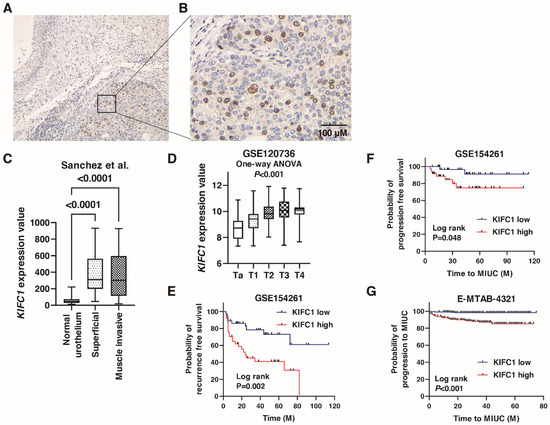
Figure 1.
Expression of KIFC1 in bladder cancer (BC). (A) Immunohistochemical staining of KIFC1 in the non-neoplastic urothelium and BC. Original magnification: 100×. (B) Immunohistochemical staining of KIFC1 in BC. Original magnification: 400×. (C) Box plot of KIFC1 expression in normal urothelium, superficial BC, and muscle-invasive BC from the study by Sanchez et al. [21]. (D) Box plot of KIFC1 expression according to T stage from the study GSE120736. (E) Kaplan-Meier plot of recurrence-free survival of T1 BC patients according to KIFC1 expression from the study (GSE154261). (F,G) Kaplan-Meier plot of progression-free survival of T1 BC patients according to KIFC1 expression after prostatectomy from the studies GSE154261 and E-MTAB-4321.

Table 1.
Relationship between KIFC1 expression and clinicopathologic characteristics in the 78 bladder cancer from Hiroshima cohort.
3.2. Prognostic Value of KIFC1 after Cystectomy in BC
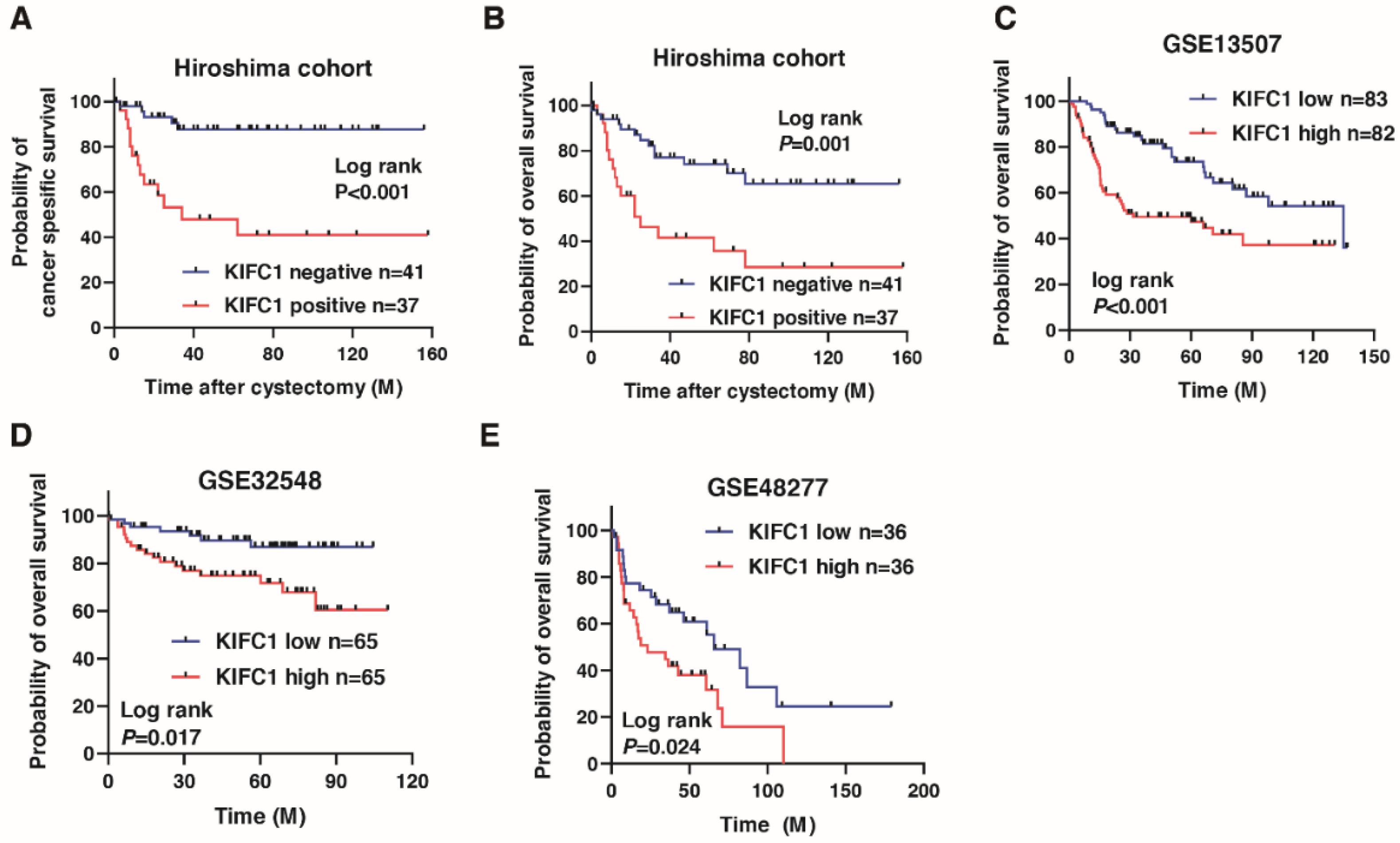
We next analyzed the prognostic value of KIFC1 after cystectomy in BC. A Kaplan-Meier analysis showed that the KIFC1-positive cases were significantly associated with poor cancer-specific survival (hazard ratio 6.443, p < 0.001) and overall survival in BC (hazard ratio 3.159, p < 0.001) in the Hiroshima cohort (Figure 2A,B). To verify our findings, we analyzed the prognostic value of KIFC1 in BC using the public databases. A Kaplan-Meier analysis showed that high KIFC1 expression was significantly associated with poor prognosis in GSE13507, GSE32548 and GSE48277 (Figure 2C–E). We performed univariate and multivariate Cox proportional hazard analyses to evaluate the potential use of KIFC1 expression as a prognostic marker. In the multivariate model, positive KIFC1 expression was independently associated with poor overall survival (hazard ratio 3.121, p = 0.009; Table 2).
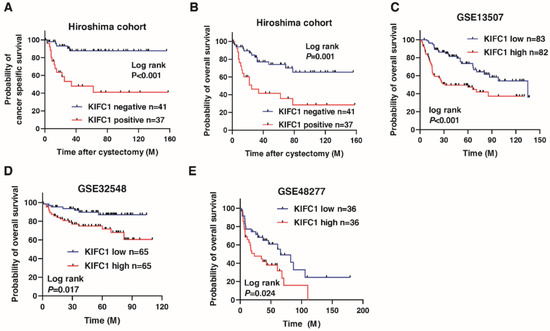
Figure 2.
Prognostic value of KIFC1 after cystectomy in bladder cancer (BC). (A–E) Kaplan-Meier plots of survival of BC patients after cystectomy according to KIFC1 expression in the Hiroshima cohort and public databases (GSE13507, GSE32548, and GSE48277).

Table 2.
Univariate and multivariate Cox regression analysis of overall survival in 78 bladder cancer.
3.3. KIFC1 Is Increased in Basal Type BC
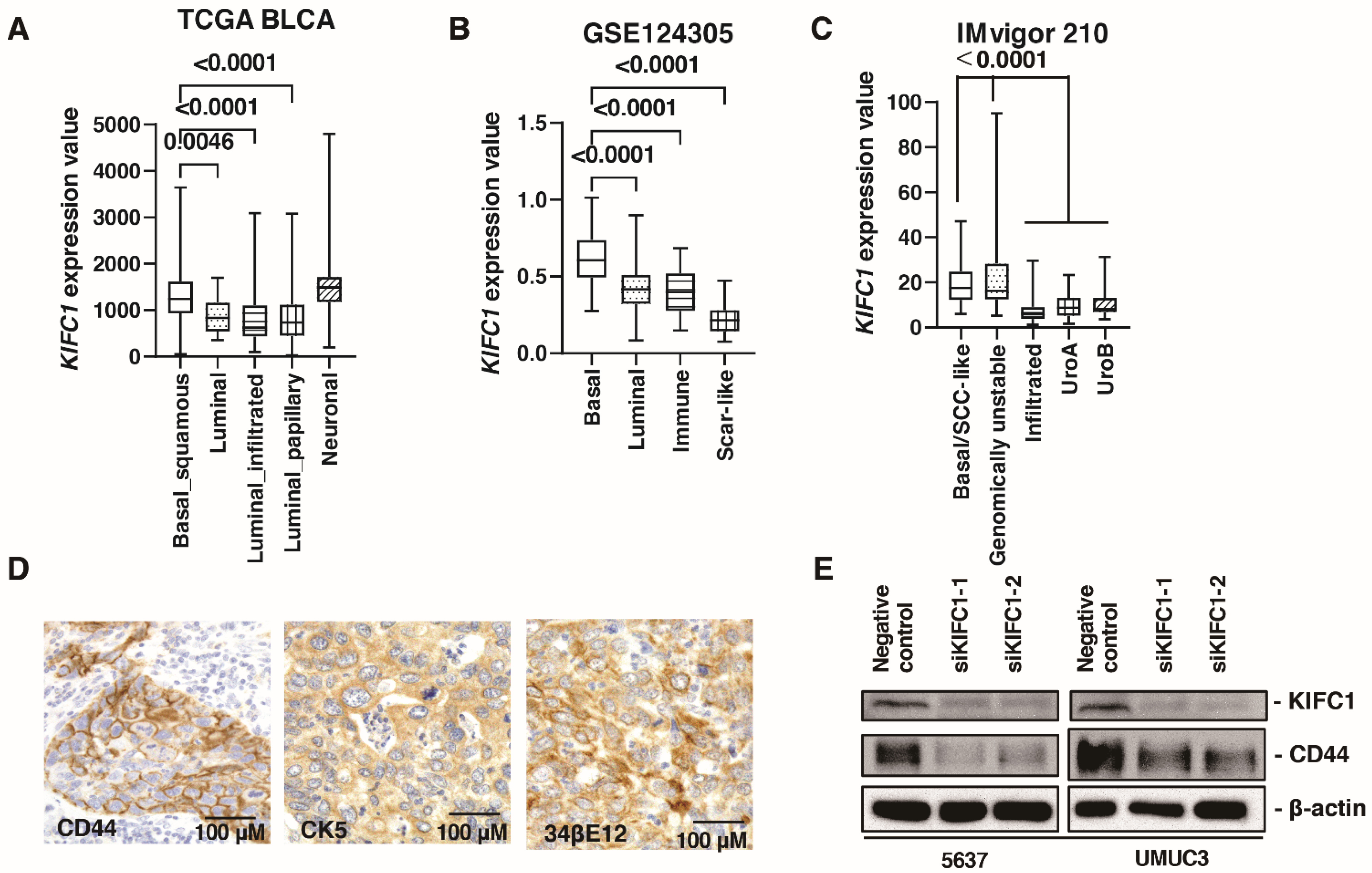
Several recent studies have reported the clinical significance of molecular classifications in BC [30]. Therefore, we analyzed the association between KIFC1 expression and molecular classifications. In TCGA-BLCA, KIFC1 expression was higher in basal/squamous and neuronal type BC than that in other BC types (Figure 3A). In the study GSE124305, KIFC1 expression was higher in basal type BC than that in other BC types (Figure 3B). In the IMvigor 210 study, KIFC1 expression was higher in basal/squamous and genomically unstable type BC than that in other BC types (Figure 3C). These findings indicate that KIFC1 expression was increased in basal type BC. Therefore, we performed immunohistochemistry of basal markers (34βE12, CK5, and CD44) in 50 patients with BC from the Kure cohort. Immunohistochemistry showed that positive KIFC1 cases were associated with positive 34βE12, CK5 and CD44 cases in this cohort (Figure 3D) (Table 3). Of note, western blotting showed that KIFC1 knockdown suppressed CD44 expression in 5637 and UMUC3 cells (Figure 3E), indicating that KIFC1 is involved in basal differentiation.
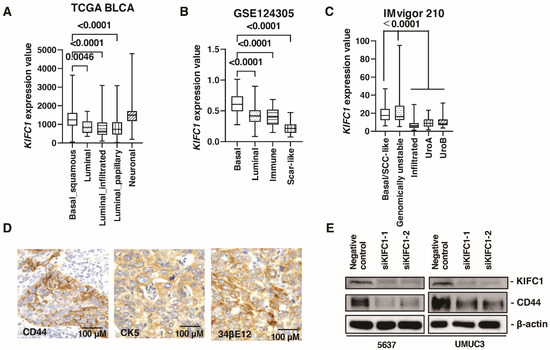
Figure 3.
KIFC1 is increased in basal type bladder cancer (BC). (A–C) Box plot of KIFC1 expression by molecular classification in these studies: TCGA BLCA, GSE124305, IMvigor 210. (D) Representative immunohistochemical images of CD44, CK5 and 34βE12 expression in BC. Original magnification: 400×. (E) Western blotting of KIFC1 and CD44 in 5637 and UMUC3 cells transfected with KIFC1 or negative control siRNAs. β-Actin was used as a loading control.

Table 3.
Relationship between KIFC1 expression and 34βE12, CK5, CD44 and PD-L1 in the 50 bladder cancer from Kure cohort.
3.4. KIFC1 Is Involved in Cell Proliferation in BC
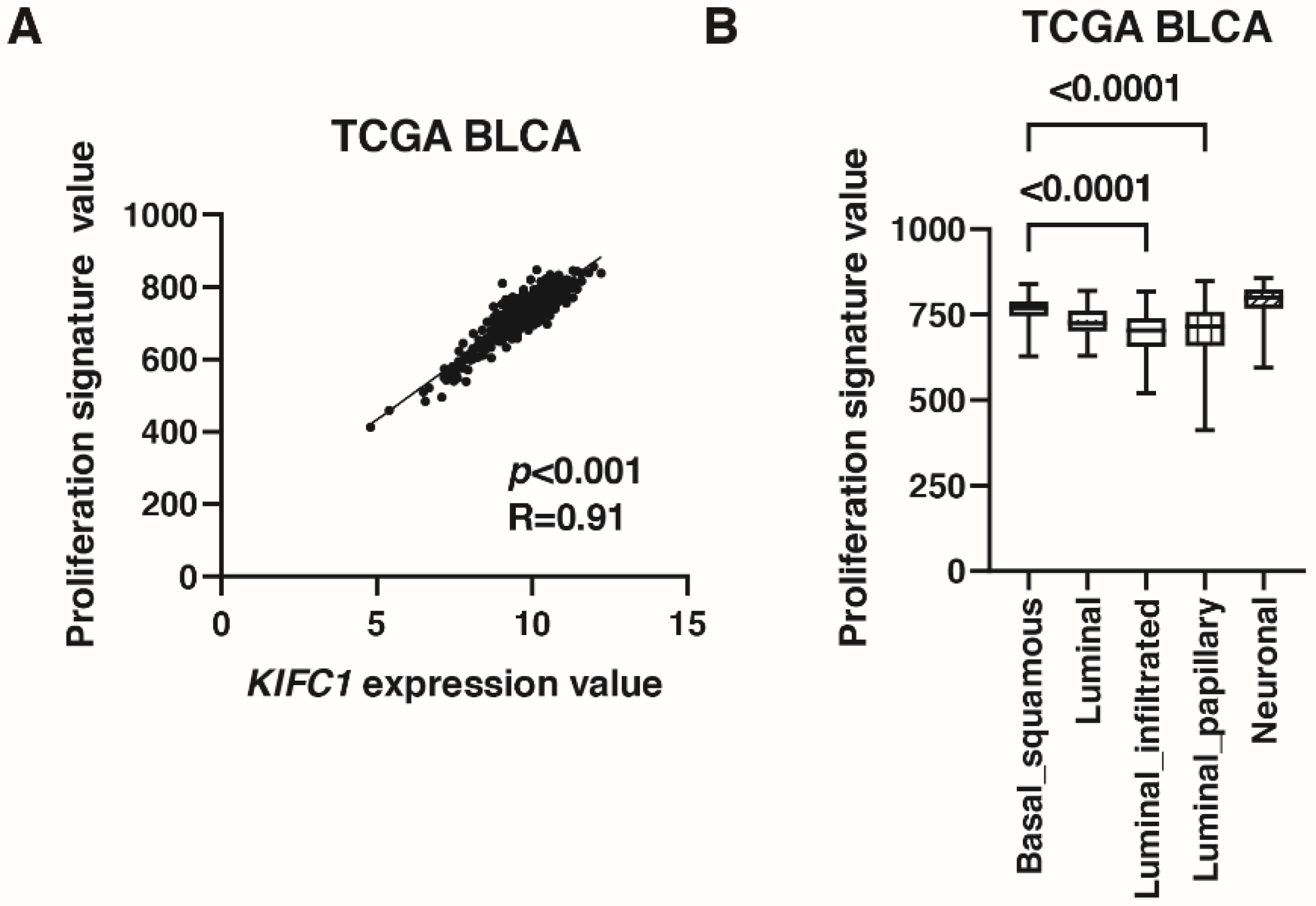
As mentioned in the introduction, KIFC1 promotes bladder cancer cell proliferation in vitro [10]. Therefore, we validated this finding by immunohistochemistry and signature analysis. Immunohistochemistry showed that positive KIFC1 cases were associated with positive Ki-67 cases in Hiroshima cohort (Table 4). What is more, KIFC1 expression was positively correlated with proliferation signature value [26] in TCGA cohort (Figure 4A). Proliferation signature value was increased in basal/squamous type than that in luminal infiltrated and luminal papillary types (Figure 4B).

Table 4.
Relationship between KIFC1 expression and Ki-67 in the 58 bladder cancer from Hiroshima cohort.
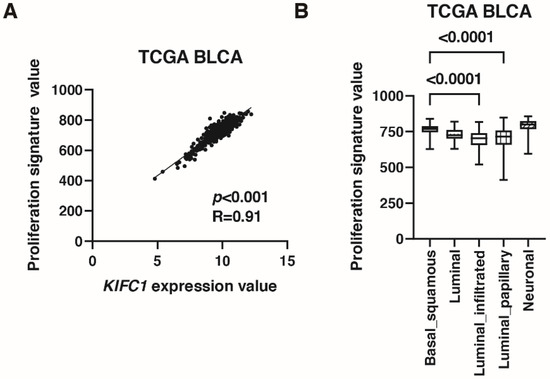
Figure 4.
KIFC1 is associated with proliferation in bladder cancer (BC). (A) The correlation between KIFC1 expression and proliferation signature value in TCGA BLCA. Spearman’s correlation coefficients and p-values are indicated. (B) Box plot of proliferation signature value by molecular classification in TCGA BLCA.
3.5. KIFC1 Is Associated with Genomic Instability in BC
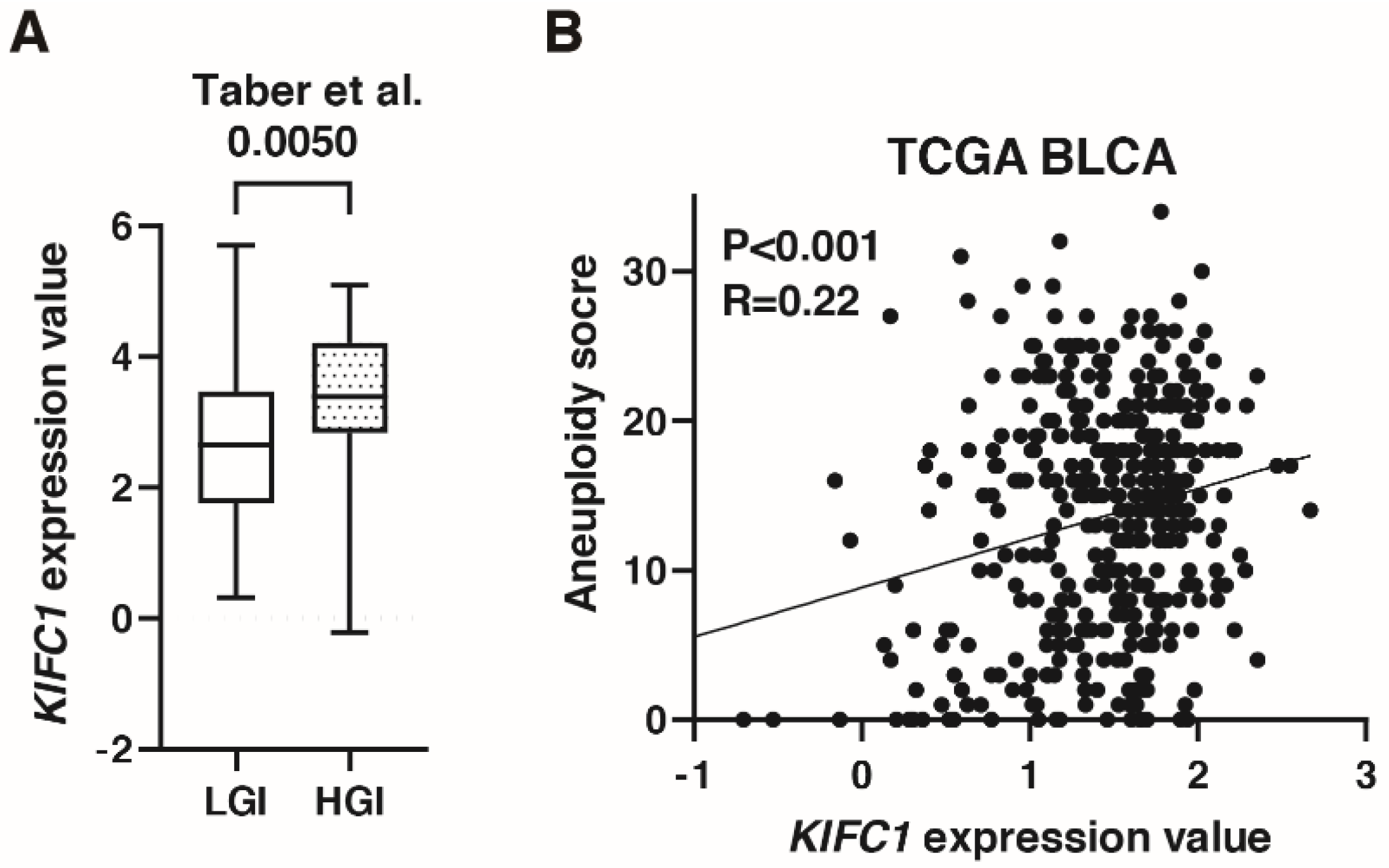
A recent study has shown that KIFC1 phosphorylation induces chromosomal instability in breast cancer [31]. As shown in Figure 3C, KIFC1 expression was increased in genomically unstable type BC in the IMvigor 210 study. In the study by Taber et al. [22], KIFC1 expression was higher in high genomic instability than in low genomic instability groups (Figure 5A). KIFC1 expression was positively correlated with aneuploidy score in TCGA-BLCA (Figure 5B).
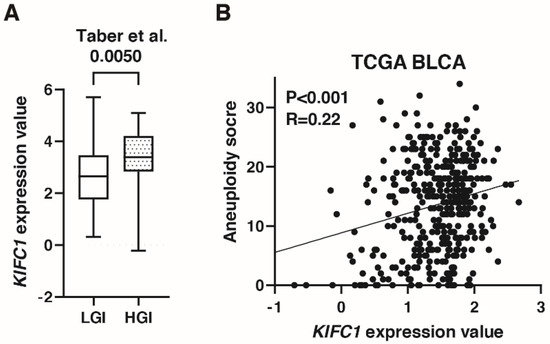
Figure 5.
KIFC1 is associated with genomic instability in bladder cancer (BC). (A) Box plot of KIFC1 expression in low and high genomic instability groups in the study by Taber et al. [22] (B) The correlation between KIFC1 expression and aneuploidy score in TCGA BLCA. Spearman’s correlation coefficients and p-values are indicated.
3.6. KIFC1 Is Regulated by p53 in BC
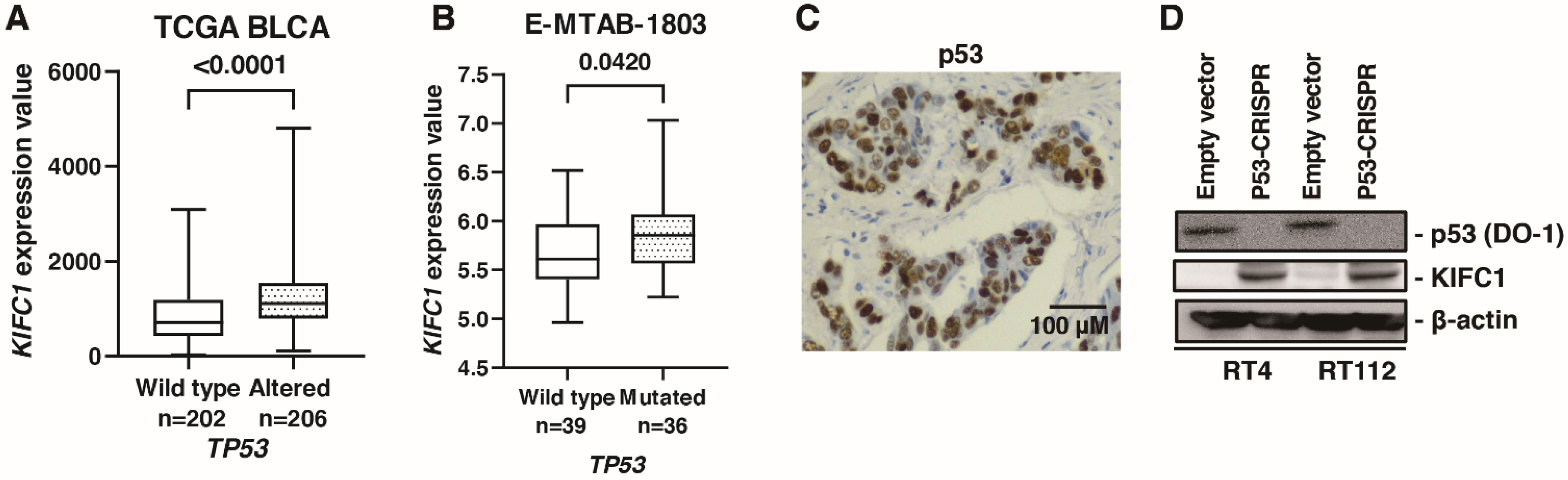
A comprehensive sequencing study found that half of the patients with MIBC had a TP53 mutation [32], indicating p53 pathway plays an essential role in biology of BC. What is more, cancers with a loss of p53 showed increased genomic instability [33]. Therefore, we analyzed the association between KIFC1 and p53. In TCGA-BLCA, gene alteration of KIFC1 was associated with gene alteration of TP53 (Table 5). Furthermore, mRNA KIFC1 expression was increased in the case of TP53 alteration in TCGA-BLCA and E-MTAB-1803 (Figure 6A,B). Then, we performed immunohistochemistry of p53 in 58 bladder cancer patients. We considered p53 overexpression and p53 complete absence of expression as altered-type p53 based on the study [13]. Immunohistochemistry showed that positive KIFC1 cases were associated with altered-type p53 cases in 58 patients with BC from the Hiroshima cohort (Figure 6C) (Table 6). Then, we established p53 knockout cells using CRISPR-Cas9 in RT4 and RT112 cells, which are p53 wild type BC cell lines [34] to analyze the effect of p53 knockout on KIFC1 expression. Western blotting showed that KIFC1 expression was upregulated in the TP53 knockout cells (Figure 6D).

Table 5.
Relationship between KIFC1 and TP53 gene status in gene alterations in the TCGA BLCA.
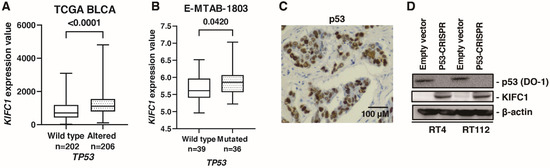
Figure 6.
KIFC1 is regulated by p53 in bladder cancer (BC). (A) Box plot of KIFC1 expression in wild-type and altered TP53 in TCGA BLCA. (B) Box plot of KIFC1 expression in wild-type and mutated TP53 in E-MTAB-1803. (C) Representative immunohistochemical images of p53 overexpression in BC. Original magnification: 400×. (D) Western blotting of p53 (DO-1) and KIFC1 in RT4 and RT112 cells transfected with empty vector or p53-CRISPR. β-Actin was used as a loading control.

Table 6.
Relationship between KIFC1 expression and p53 in the 58 bladder cancer from Hiroshima cohort.
3.7. KIFC1 Is Involved in Cisplatin Resistance in BC
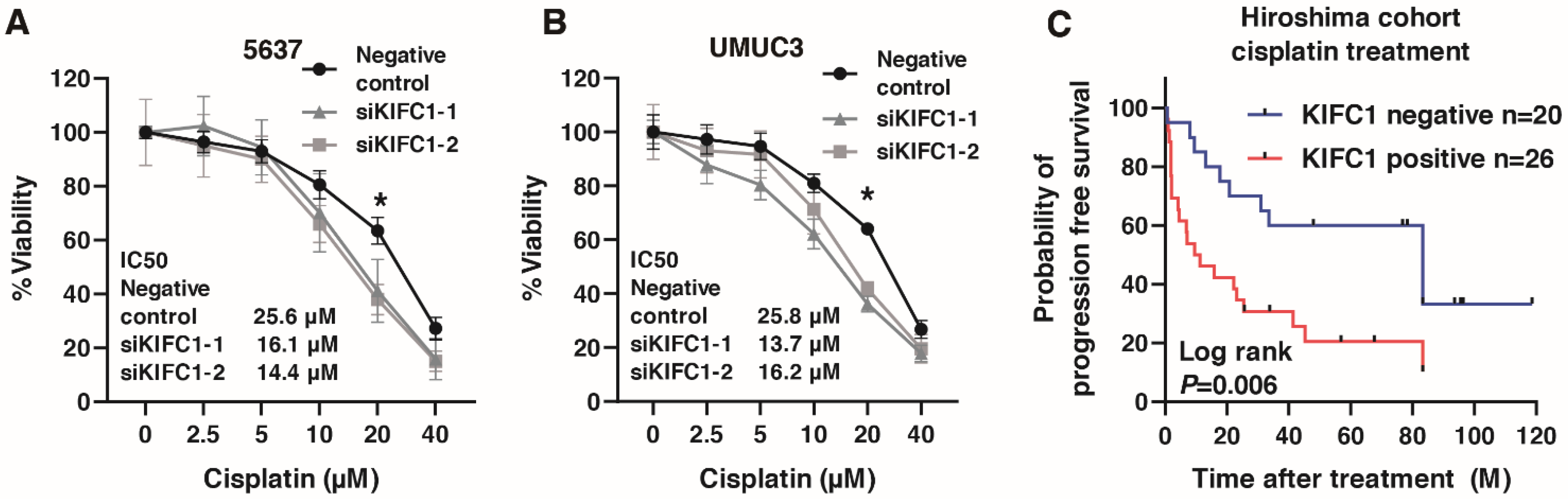
Recent studies have shown that KIFC1 is involved in cisplatin resistance in breast cancer [31,35]. Therefore, we analyzed the involvement of KIFC in cisplatin resistance in BC. We performed WST-1 assays to measure cell viability under various concentrations of cisplatin in 5637 and UMUC3 cells transfected with negative control small interfering RNA (siRNA) and KIFC1 siRNA. KIFC1 knockdown increased the sensitivity to cisplatin in the 5637 and UMUC3 cells (Figure 7A,B). Then, to analyze the prognostic value of KIFC for cisplatin treatment, we performed immunohistochemistry of KIFC in 46 patients with advanced BC treated with cisplatin-based chemotherapy (Table 7). KIFC1 positive cases was not associated with the response to cisplatin-based chemotherapy (Table 8). Kaplan-Meier analysis showed that KIFC1-positive cases were associated with poor prognosis after cisplatin-based chemotherapy (Figure 7C). These results suggest that KIFC1 may be a prognostic marker for cisplatin-based chemotherapy.
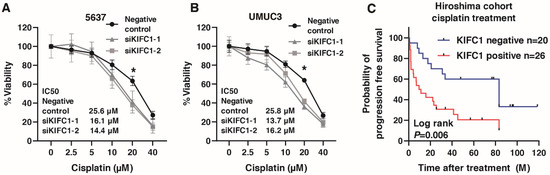
Figure 7.
KIFC1 is involved in cisplatin resistance in bladder cancer (BC). (A,B) The dose-dependent effects of cisplatin on the viability of 5637 and UMUC3 cells transfected with negative control siRNAs and KIFC1 siRNAs. * p < 0.05. IC50 values are indicated. (C) Kaplan-Meier plot of survival of BC patients treated with cisplatin-based chemotherapy according to KIFC1 expression in the Hiroshima cohort.

Table 7.
Clinicopathologic characteristics of 46 bladder cancer patients who were treated with cisplatin based chemotherapy.

Table 8.
Relationship between KIFC1 expression and the response to cisplatin based chemotherapy in Hiroshima cohort.
3.8. KIFC1 Is Associated with PD-L1 and Favorable Prognosis after PD-L1 Inhibition in BC
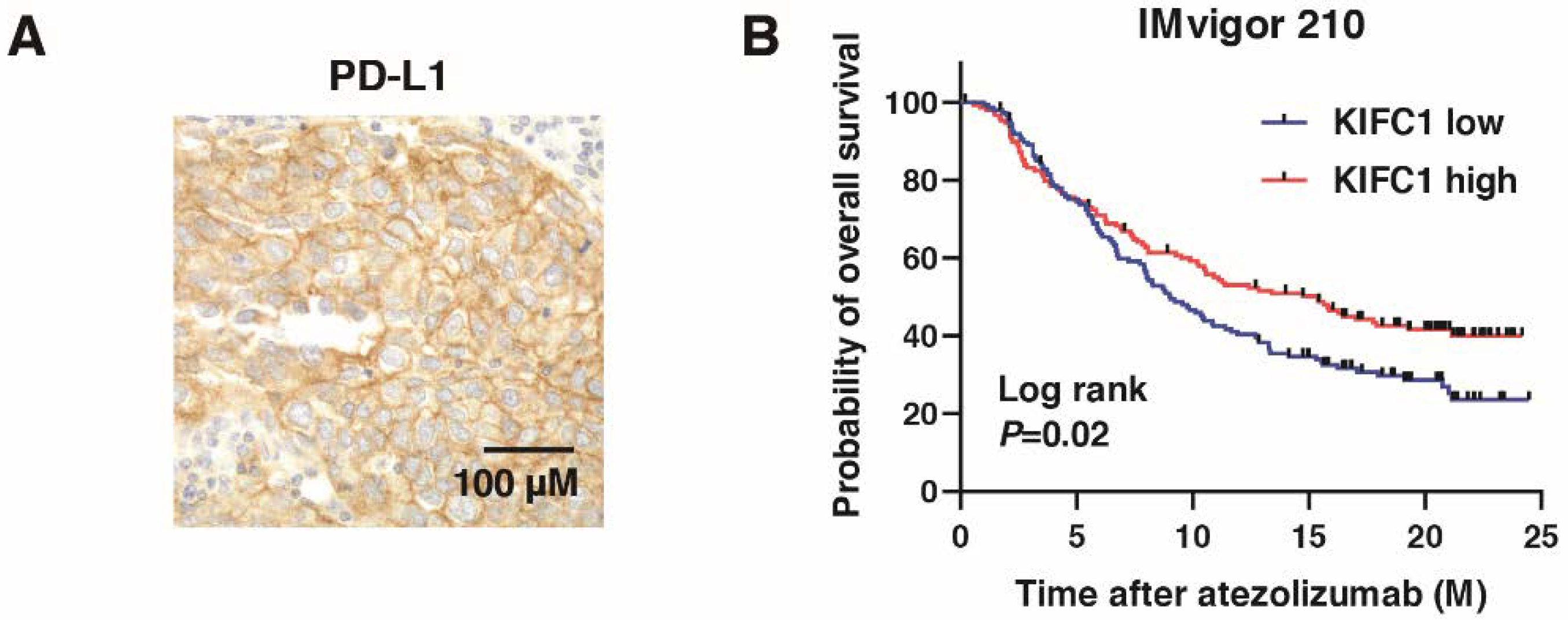
We performed immunohistochemistry of PD-L1 in BC (Figure 8A), which showed that KIFC1-positive cases were significantly associated with PD-L1-positive cases (Table 3). PD-L1 is used as a biomarker for immune checkpoint inhibitors (ICI) [36]. Therefore, we analyzed the role of KIFC1 for ICI. High KIFC1 expression was significantly associated with the favorable outcome (complete response/partial response) (Table 9). Kaplan-Meier analysis showed that high KIFC1 expression was significantly associated with favorable overall survival in BC treated with atezolizumab (Figure 8B). These results suggest that KIFC1 may be a useful marker for atezolizumab treatment.
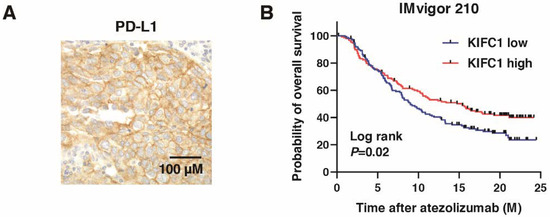
Figure 8.
KIFC1 expression is associated with PD-L1 expression and favorable prognosis after PD-L1 inhibition in bladder cancer (BC). (A) Representative immunohistochemical images of KIFC1 and PD-L1 expression in BC. Original magnification: 400×. (B) Kaplan-Meier plot of survival of BC patients treated with atezolizumab according to KIFC1 expression in the IMvigor 210 study.

Table 9.
Relationship between KIFC1 expression and the response to atezolizumab in IMvigor210 cohort.
4. Discussion
Molecular mechanisms of cisplatin resistance are believed to be caused by multiple factors including drug uptake and efflux, detoxification, DNA repair and apoptosis [37]. However, strategies to overcome cisplatin resistance are not well established. A recent study showed that KIFC1 phosphorylation by ATM and ATR kinase is involved in drug resistance in breast cancer [31]. Another study reported that KIFC1 knockdown increased the sensitivity to cisplatin in breast cancer [35]. In the present study, we showed that knockdown of KIFC1 increased the sensitivity to cisplatin, which is the first report to analyze the involvement of KIFC1 in cisplatin resistance in BC. These results suggest that KIFC1 may play an essential role in cisplatin resistance. In this study, western blotting showed that KIFC1 knockdown suppressed CD44 expression in BC cell lines. Previously, we showed that KIFC1 is associated with CD44 in prostate cancer and gastric cancer [38,39]. Several studies have reported that CD44 is involved in cisplatin resistance in BC [40,41], which may help to explain why KIFC1 increased the sensitivity to cisplatin. Collectively, these results suggest that knockdown of KIFC1 increased the sensitivity to cisplatin partly through CD44 in BC.
Immunohistochemistry in the present study showed that KIFC1-positive cases were associated with basal markers CK5, 34βE12 and CD44. KIFC1 expression was increased in basal type BC in some molecular classifications. These results suggest that KIFC1 is involved in basal differentiation. The response of basal type BC to chemotherapy is controversial. A study by Choi et al. showed that p53-like type BC is resistant to neoadjuvant chemotherapy compared to basal and luminal type BC [18]. The study by Seiler et al., found that basal type BC benefitted more from neoadjuvant cisplatin-based chemotherapy than other BC subtypes [42]. The study by Taber et al., reported that basal type BC is associated with a poor response to cisplatin-based chemotherapy [24]. In the present study, Kaplan-Meier analysis showed that KIFC1-positive cases were associated with poor prognosis after cisplatin-based chemotherapy in BC. Although further studies are needed, KIFC1 may be promising as a basal marker in BC.
A recent study found that DNA-damaging treatments induce KIFC1 expression and KIFC1-dependent centrosome clustering [31]. Centrosome clustering contributes to chromosomal instability [43]. Indeed, our in silico analysis showed that KIFC1 expression was increased in high genomic instability and genomically unstable BC subtypes. Loss of p53 promotes genomic instability in cancer cells [33]. In addition, in silico analysis showed that KIFC1 alteration was associated with TP53 alteration, and mRNA KIFC1 expression was increased in patients with altered TP53. Immunohistochemistry showed that KIFC1-positive cases were associated with p53-positive cases. What is more, western blotting revealed that knockout of p53 induced KIFC1 expression in BC cell lines. Taken together, these results suggest that the interaction between p53 and KIFC1 may play an essential role in BC development and progression.
In the present study, immunohistochemistry showed that KIFC1-positive cases were associated with PD-L1 positive cases. Recent studies have shown that PD-L1 expression is increased in the basal/squamous subtype in BC [44,45]. In our study, KIFC1 expression was also increased in the basal/squamous subtype. These results indicate that there may not be direct interaction between KIFC1 and PD-L1. Although PD-L1 is used as a biomarker for ICI, the clinical utility is limited [36]. In our study, in silico analysis showed that high KIFC1 expression was associated with favorable prognosis in BC patients treated with atezolizumab. Immunohistochemistry showed that KIFC1 positive cases were associated with poor prognosis in BC patients treated with cisplatin-based chemotherapy. These results suggest that KIFC1 may serve as a potential biomarker for drug selection.
As we mentioned above, KIFC1 knockdown suppressed CD44 expression in BC cell lines. However, the mechanism is still unclear. A recent review reported that there is an inverse relationship between proliferation and differentiation [46]. Indeed, cisplatin reduces cell survival and induces differentiation of stem cells in breast cancer [47]. In our study, we showed that KIFC1 was associated with cell proliferation signature. A previous study showed that KIFC1 promotes cell proliferation [10]. These findings indicate that KIFC1 knockdown may induce differentiation, which may help to explain why KIFC1 knockdown suppressed CD44.
This study has some limitations. First, although we used siRNA to evaluate the function of KIFC1 in BC, an overexpression model is needed to verify our findings. Second, immunohistochemistry showed that KIFC1-positive cases were associated with poor prognosis after cisplatin-based chemotherapy, but the number of samples was relatively small. Third, because KIFC1-positive cases were associated with favorable prognosis in BC treated with atezolizumab, in the future, we will analyze the prognostic value of KIFC1 in immune therapy using immunohistochemistry. Fourth, although we showed that KIFC1 knockdown promoted the sensitivity to cisplatin, the effect was not very dramatic. KIFC1 was involved in bladder cancer proliferation, indicating that this involvement in proliferation may affect the sensitivity to cisplatin. In the future, we will validate the effect of KIFC1 on cisplatin sensitivity in vivo analysis.
In conclusion, the present study revealed that high expression of KIFC1 was associated with poor prognosis in BC, which was consistent with the findings from the public databases. KIFC1 expression was increased in genomic instability and alteration of TP53. p53 knockout induced KIFC1 expression, and KIFC1 knockdown increased the sensitivity to cisplatin. Furthermore, prognosis was poor in the KIFC1-positive patients treated with cisplatin, whereas in patients treated with atezolizumab, KIFC1 expression was associated with PD-L1 expression and a favorable prognosis. The data presented here highlight the great potential of KIFC1 as a possible biomarker and therapeutic target in BC.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10214837/s1, Table S1: Clinicopathologic characteristics of 78 bladder cancer patients who were treated with cystectomy from Hiroshima cohort, Table S2: Clinicopathologic characteristics of 50 bladder cancer patients who were treated with cystectomy from Kure cohort, Table S3: Clinicopathologic characteristics of bladder cancer patients from GSE13507, GSE32548, and GSE48277, Table S4: Proliferation signature.
Author Contributions
Conceptualization, Y.S., J.T., W.Y. and N.H.; Methodology, K.I.; Software, K.I.; Validation, Y.S.; Formal Analysis, Q.T.P., K.S. and N.O.; Investigation, K.G. and H.N.; Resources, H.K.; Data Curation, K.K.; Writing—Original Draft Preparation, Y.S.; Writing—Review and Editing, T.H.; Visualization, Y.S.; Supervision, J.T.; Project Administration, K.G. and T.H.; Funding Acquisition, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Japan Society for the Promotion of Science [19K18586].
Institutional Review Board Statement
This study was conducted in accordance with the Ethical Guidance for Human Genome/Gene Research of the Japanese Government. This study was approved by the Institutional Review Board of Hiroshima University Hospital (approval no. E-688) and the Institutional Review Board of Kure Medical Center and Chugoku Cancer Center (approval no. 2020-30).
Informed Consent Statement
Regarding the samples for immunohistochemistry, 124 samples were collected from patients at Hiroshima University Hospital, and 50 were collected from patients at Kure Medical Center and Chugoku Cancer Center. Written comprehensive approvals for basic or clinical research were obtained from each patient.
Data Availability Statement
All data generated or analyzed during this study are included either in this article or in the Supplementary Information Files.
Acknowledgments
This work was carried out with the kind cooperation of the Research Center for Molecular Medicine of the Faculty of Medicine of Hiroshima University. We also thank the Analysis Center of Life Science of Hiroshima University for the use of their facilities.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- van den Bosch, S.; Alfred Witjes, J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: A systematic review. Eur. Urol. 2011, 60, 493–500. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Comperat, E.M.; Cowan, N.C.; Gakis, G.; Hernandez, V.; Linares Espinos, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Kaur, J.; Jaczko, M.; Wei, G.; Toss, M.S.; Rakha, E.A.; Janssen, E.A.M.; Soiland, H.; Kucuk, O.; Reid, M.D.; et al. Centrosome amplification: A quantifiable cancer cell trait with prognostic value in solid malignancies. Cancer Metastasis Rev. 2021, 40, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Caraway, N.P.; Sabichi, A.L.; Zhang, H.Z.; Ruitrok, A.; Grossman, H.B.; Gu, J.; Lerner, S.P.; Lippman, S.; Katz, R.L. Centrosomal abnormality is common in and a potential biomarker for bladder cancer. Int. J. Cancer 2003, 106, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Matsuyama, H.; Furuya, T.; Oga, A.; Yoshihiro, S.; Okuda, M.; Kawauchi, S.; Sasaki, K.; Naito, K. Centrosome hyperamplification predicts progression and tumor recurrence in bladder cancer. Clin. Cancer Res. 2004, 10, 6449–6455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milunovic-Jevtic, A.; Mooney, P.; Sulerud, T.; Bisht, J.; Gatlin, J.C. Centrosomal clustering contributes to chromosomal instability and cancer. Curr. Opin. Biotechnol. 2016, 40, 113–118. [Google Scholar] [CrossRef]
- Rath, O.; Kozielski, F. Kinesins and cancer. Nat. Rev. Cancer 2012, 12, 527–539. [Google Scholar] [CrossRef]
- Xiao, K.H.; Teng, K.; Ye, Y.L.; Tan, L.; Chen, M.K.; Liang, H.T.; Feng, Z.H.; Duan, J.L.; Deng, M.H.; Wei, W.S.; et al. Kinesin family member C1 accelerates bladder cancer cell proliferation and induces epithelial-mesenchymal transition via Akt/GSK3beta signaling. Cancer Sci. 2019, 110, 2822–2833. [Google Scholar] [CrossRef]
- Sekino, Y.; Oue, N.; Mukai, S.; Shigematsu, Y.; Goto, K.; Sakamoto, N.; Sentani, K.; Hayashi, T.; Teishima, J.; Matsubara, A.; et al. Protocadherin B9 promotes resistance to bicalutamide and is associated with the survival of prostate cancer patients. Prostate 2019, 79, 234–242. [Google Scholar] [CrossRef]
- Paner, G.P.; Stadler, W.M.; Hansel, D.E.; Montironi, R.; Lin, D.W.; Amin, M.B. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur. Urol. 2018, 73, 560–569. [Google Scholar] [CrossRef]
- Kobel, M.; Piskorz, A.M.; Lee, S.; Lui, S.; LePage, C.; Marass, F.; Rosenfeld, N.; Mes Masson, A.M.; Brenton, J.D. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J. Pathol. Clin. Res. 2016, 2, 247–258. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Song, B.N.; Kim, S.K.; Mun, J.Y.; Choi, Y.D.; Leem, S.H.; Chu, I.S. Identification of an immunotherapy-responsive molecular subtype of bladder cancer. EBioMedicine 2019, 50, 238–245. [Google Scholar] [CrossRef]
- Kim, W.J.; Kim, E.J.; Kim, S.K.; Kim, Y.J.; Ha, Y.S.; Jeong, P.; Kim, M.J.; Yun, S.J.; Lee, K.M.; Moon, S.K.; et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol. Cancer 2010, 9, 3. [Google Scholar] [CrossRef]
- Lindgren, D.; Sjodahl, G.; Lauss, M.; Staaf, J.; Chebil, G.; Lovgren, K.; Gudjonsson, S.; Liedberg, F.; Patschan, O.; Mansson, W.; et al. Integrated genomic and gene expression profiling identifies two major genomic circuits in urothelial carcinoma. PLoS ONE 2012, 7, e38863. [Google Scholar] [CrossRef]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Seiler, R.; Gibb, E.A.; Wang, N.Q.; Oo, H.Z.; Lam, H.M.; van Kessel, K.E.; Voskuilen, C.S.; Winters, B.; Erho, N.; Takhar, M.M.; et al. Divergent Biological Response to Neoadjuvant Chemotherapy in Muscle-invasive Bladder Cancer. Clin. Cancer. Res. 2019, 25, 5082–5093. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Groeneveld, C.S.; Jordan, B.; Lin, X.; McLaughlin, K.A.; Das, A.; Fall, L.A.; Fantini, D.; Taxter, T.J.; Mogil, L.S.; et al. Identification of Differential Tumor Subtypes of T1 Bladder Cancer. Eur. Urol. 2020, 78, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Rebouissou, S.; Bernard-Pierrot, I.; de Reynies, A.; Lepage, M.L.; Krucker, C.; Chapeaublanc, E.; Herault, A.; Kamoun, A.; Caillault, A.; Letouze, E.; et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci. Transl. Med. 2014, 6, 244–291. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, J.; Lamy, P.; Nordentoft, I.; Algaba, F.; Hoyer, S.; Ulhoi, B.P.; Vang, S.; Reinert, T.; Hermann, G.G.; Mogensen, K.; et al. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell 2016, 30, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carbayo, M.; Socci, N.D.; Lozano, J.; Saint, F.; Cordon-Cardo, C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J. Clin. Oncol. 2006, 24, 778–789. [Google Scholar] [CrossRef]
- Taber, A.; Christensen, E.; Lamy, P.; Nordentoft, I.; Prip, F.; Lindskrog, S.V.; Birkenkamp-Demtroder, K.; Okholm, T.L.H.; Knudsen, M.; Pedersen, J.S.; et al. Molecular correlates of cisplatin-based chemotherapy response in muscle invasive bladder cancer by integrated multi-omics analysis. Nat. Commun. 2020, 11, 4858. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repecka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Tan, T.Z.; Rouanne, M.; Tan, K.T.; Huang, R.Y.; Thiery, J.P. Molecular Subtypes of Urothelial Bladder Cancer: Results from a Meta-cohort Analysis of 2411 Tumors. Eur. Urol. 2019, 75, 423–432. [Google Scholar] [CrossRef]
- Sekino, Y.; Han, X.; Babasaki, T.; Miyamoto, S.; Kobatake, K.; Kitano, H.; Ikeda, K.; Goto, K.; Inoue, S.; Hayashi, T.; et al. TUBB3 is associated with PTEN, neuroendocrine differentiation, and castration resistance in prostate cancer. Urol. Oncol. 2021, 39, 368.e1–368.e9. [Google Scholar] [CrossRef]
- Sekino, Y.; Han, X.; Babasaki, T.; Miyamoto, S.; Kitano, H.; Kobayashi, G.; Goto, K.; Inoue, S.; Hayashi, T.; Teishima, J.; et al. TUBB3 Is Associated with High-Grade Histology, Poor Prognosis, p53 Expression, and Cancer Stem Cell Markers in Clear Cell Renal Cell Carcinoma. Oncology 2020, 98, 689–698. [Google Scholar] [CrossRef]
- Sekino, Y.; Sakamoto, N.; Ishikawa, A.; Honma, R.; Shigematsu, Y.; Hayashi, T.; Sentani, K.; Oue, N.; Teishima, J.; Matsubara, A.; et al. Transcribed ultraconserved region Uc.63+ promotes resistance to cisplatin through regulation of androgen receptor signaling in bladder cancer. Oncol. Rep. 2019, 41, 3111–3118. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reynies, A.; Allory, Y.; Sjodahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Sun, L.; Meng, L.; Hu, C.; Wang, X.; Shi, Z.; Hu, C.; Han, Y.; Yang, Q.; Cao, L.; et al. The ATM and ATR kinases regulate centrosome clustering and tumor recurrence by targeting KIFC1 phosphorylation. Nat. Commun. 2021, 12, 20. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.E25. [Google Scholar] [CrossRef]
- Muller, P.A.; Vousden, K.H. p53 mutations in cancer. Nat. Cell Biol. 2013, 15, 2–8. [Google Scholar] [CrossRef]
- Warrick, J.I.; Walter, V.; Yamashita, H.; Chung, E.; Shuman, L.; Amponsa, V.O.; Zheng, Z.; Chan, W.; Whitcomb, T.L.; Yue, F.; et al. FOXA1, GATA3 and PPAR Cooperate to Drive Luminal Subtype in Bladder Cancer: A Molecular Analysis of Established Human Cell Lines. Sci. Rep. 2016, 6, 38531. [Google Scholar] [CrossRef]
- Patel, N.; Weekes, D.; Drosopoulos, K.; Gazinska, P.; Noel, E.; Rashid, M.; Mirza, H.; Quist, J.; Braso-Maristany, F.; Mathew, S.; et al. Integrated genomics and functional validation identifies malignant cell specific dependencies in triple negative breast cancer. Nat. Commun. 2018, 9, 1044. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother Cancer 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in Our Understanding of the Molecular Mechanisms of Action of Cisplatin in Cancer Therapy. J. Exp. Pharmacol. 2021, 13, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Sekino, Y.; Oue, N.; Shigematsu, Y.; Ishikawa, A.; Sakamoto, N.; Sentani, K.; Teishima, J.; Matsubara, A.; Yasui, W. KIFC1 induces resistance to docetaxel and is associated with survival of patients with prostate cancer. Urol. Oncol. 2017, 35, e13–e31. [Google Scholar] [CrossRef]
- Oue, N.; Mukai, S.; Imai, T.; Pham, T.T.; Oshima, T.; Sentani, K.; Sakamoto, N.; Yoshida, K.; Yasui, W. Induction of KIFC1 expression in gastric cancer spheroids. Oncol. Rep. 2016, 36, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, M.; Kikuchi, E.; Tanaka, N.; Kosaka, T.; Mikami, S.; Saya, H.; Oya, M. Variant isoforms of CD44 involves acquisition of chemoresistance to cisplatin and has potential as a novel indicator for identifying a cisplatin-resistant population in urothelial cancer. BMC Cancer 2018, 18, 113. [Google Scholar] [CrossRef]
- Ogihara, K.; Kikuchi, E.; Okazaki, S.; Hagiwara, M.; Takeda, T.; Matsumoto, K.; Kosaka, T.; Mikami, S.; Saya, H.; Oya, M. Sulfasalazine could modulate the CD44v9-xCT system and enhance cisplatin-induced cytotoxic effects in metastatic bladder cancer. Cancer Sci. 2019, 110, 1431–1441. [Google Scholar] [CrossRef]
- Seiler, R.; Ashab, H.A.D.; Erho, N.; van Rhijn, B.W.G.; Winters, B.; Douglas, J.; Van Kessel, K.E.; Fransen van de Putte, E.E.; Sommerlad, M.; Wang, N.Q.; et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur. Urol. 2017, 72, 544–554. [Google Scholar] [CrossRef]
- Ganem, N.J.; Godinho, S.A.; Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 2009, 460, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, C.; Kim, Y.A.; Moon, K.C. PD-L1 Expression in Muscle-Invasive Urinary Bladder Urothelial Carcinoma According to Basal/Squamous-Like Phenotype. Front. Oncol. 2020, 10, 527385. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.; Liu, S.K.; Vesprini, D.; Xu, B.; Downes, M.R. Basal-subtype bladder tumours show a ‘hot’ immunophenotype. Histopathology 2018, 73, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, P.; Hassiotou, F.; Blancafort, P.; Filgueira, L. Cisplatin induces differentiation of breast cancer cells. Front. Oncol. 2013, 3, 134. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).