Infection of Human Cells by SARS-CoV-2 and Molecular Overview of Gastrointestinal, Neurological, and Hepatic Problems in COVID-19 Patients
Abstract
:1. Introduction
2. Gastrointestinal Pathogenesis of SARS-CoV-2
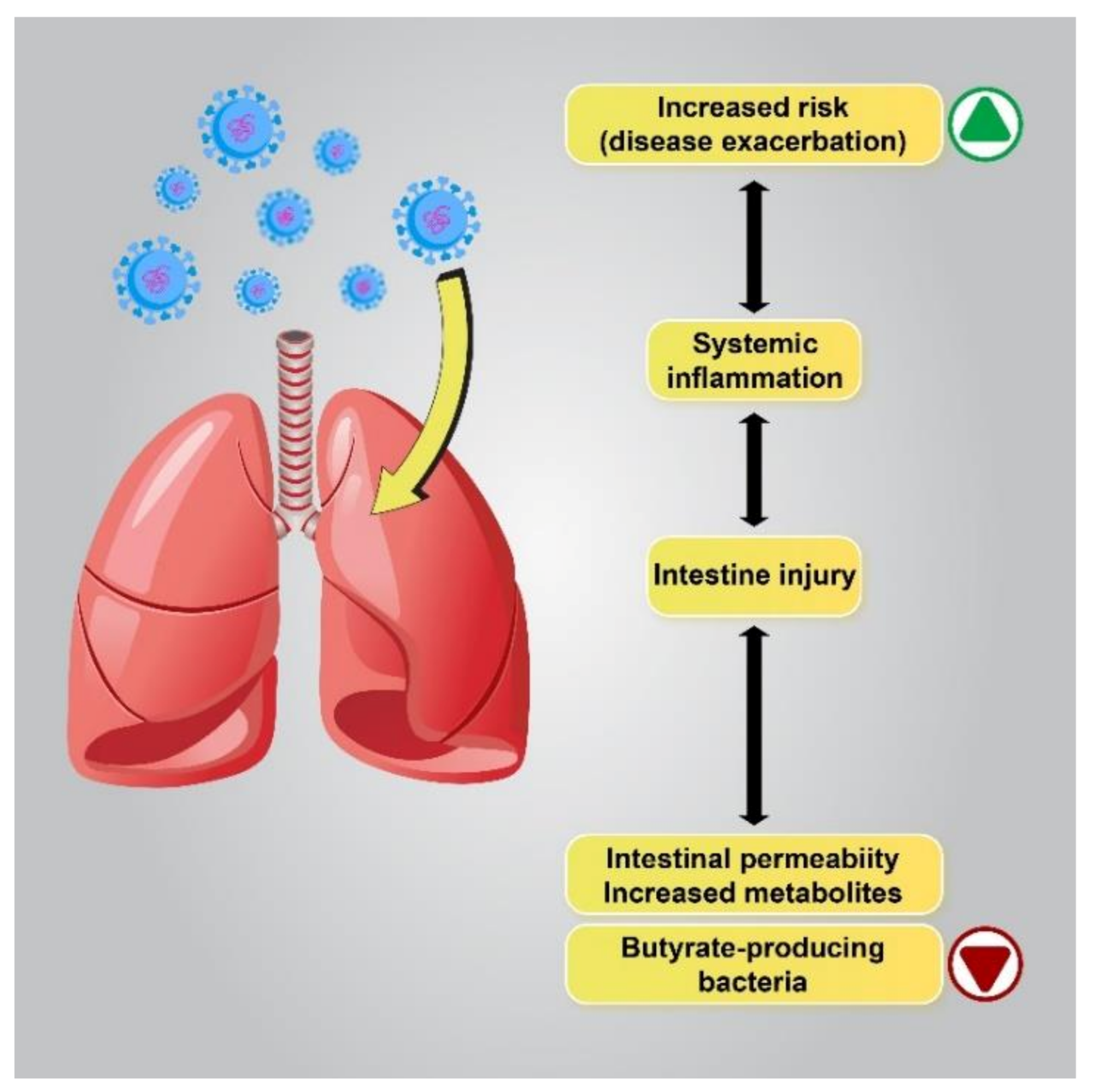
2.1. Microbiome Alterations, a Putative Mechanism of Gastrointestinal Manifestations in COVID-19 Patients
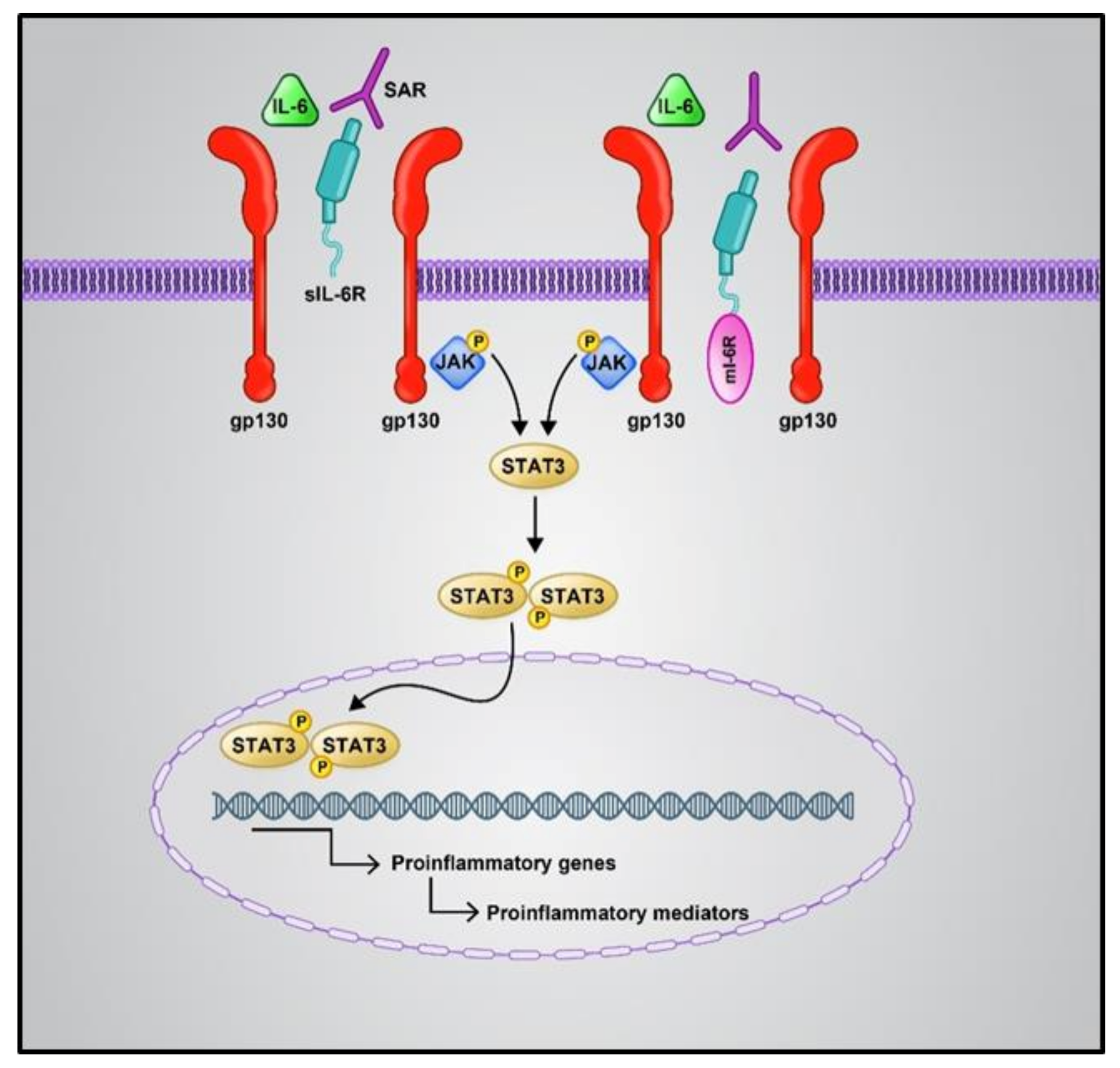
2.1.1. Pro-Inflammatory Cytokines
2.1.2. Antimicrobial Medications
2.1.3. Lung Flora Changes and Changes in the Ratio of Pathogenic Organisms
2.1.4. Enteral Nutrition
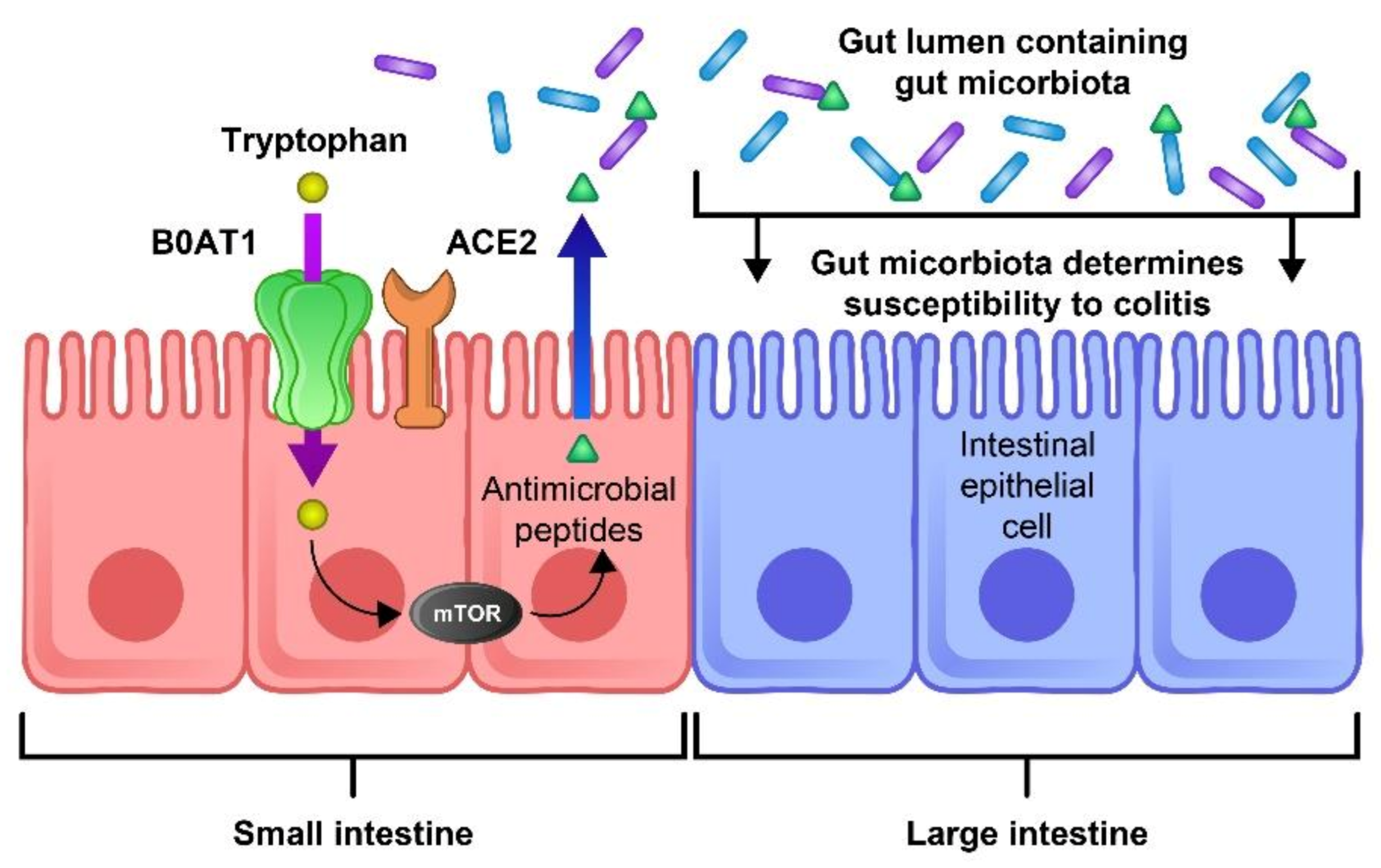
2.1.5. Aberrant mTOR Activity and Deficit of ACE2
3. Gastrointestinal Manifestations in SARS-CoV-2 and Outcome
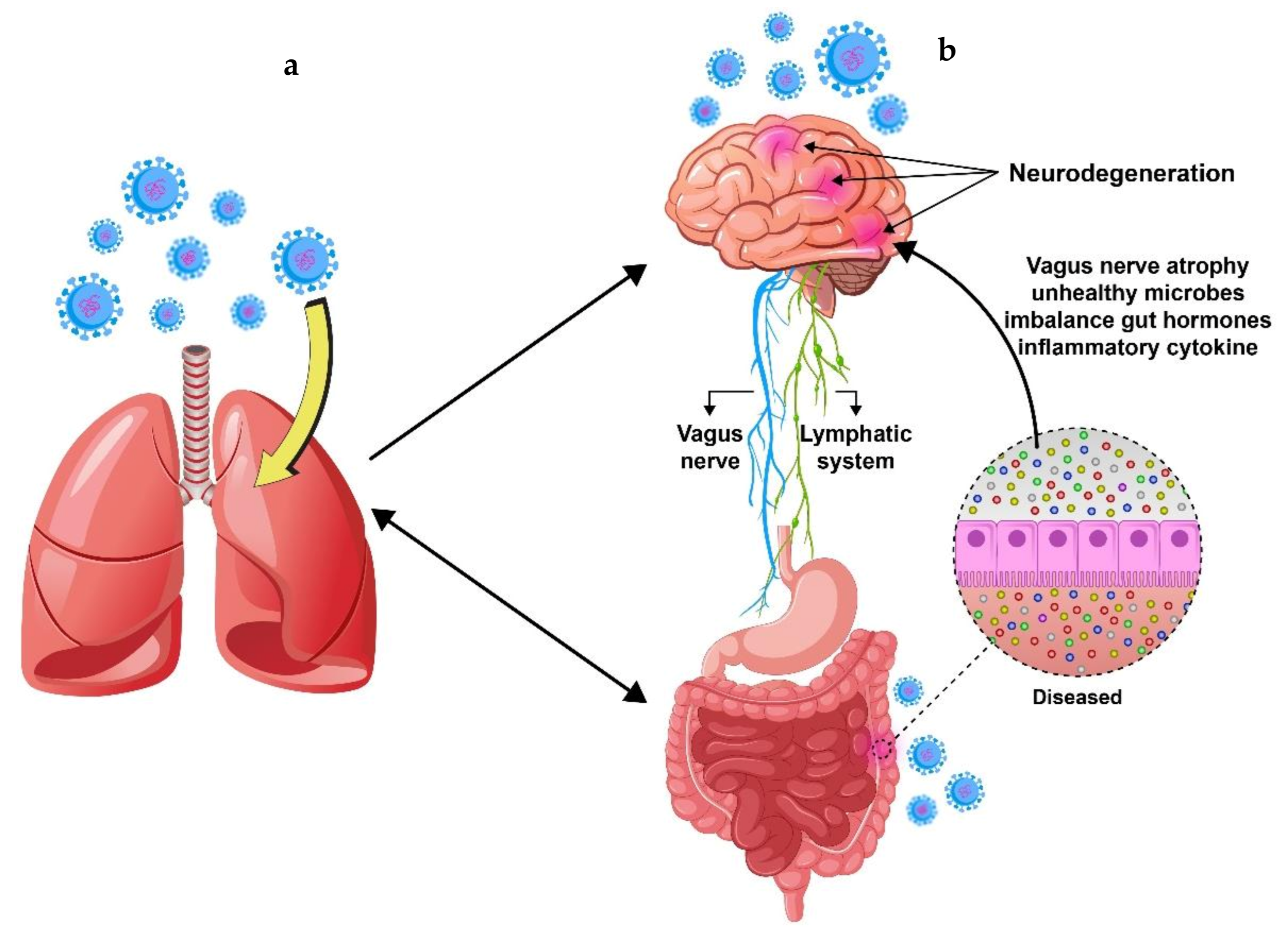
4. The Correlation between Gastrointestinal and Neurological Symptoms Induced by SARS-CoV-2
5. Effect of SARS-CoV-2 Spike Protein on Endothelial Cells and Blood–Brain Barrier
6. Hepatic Derangements, Viral Hepatitis, and COVID-19
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diao, K.; Han, P.; Pang, T.; Li, Y.; Yang, Z. HRCT imaging features in representative imported cases of 2019 novel coronavirus pneumonia. Precis. Clin. Med. 2020, 3, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.who.int/data#reports (accessed on 23 August 2021).
- Moradian, N.; Ochs, H.D.; Sedikies, C.; Hamblin, M.R.; Camargo, C.A.; Martinez, J.A.; Biamonte, J.D.; Abdollahi, M.; Torres, P.J.; Nieto, J.J.; et al. The urgent need for integrated science to fight COVID-19 pandemic and beyond. J. Transl. Med. 2020, 18, 205. [Google Scholar] [CrossRef]
- Hasan, A.; Paray, B.A.; Hussain, A.; Qadir, F.A.; Attar, F.; Aziz, F.M.; Sharifi, M.; Derakhshankhah, H.; Rasti, B.; Mehrabi, M.; et al. A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. J. Biomol. Struct. Dyn. 2021, 39, 3025–3033. [Google Scholar] [CrossRef] [Green Version]
- Tazikeh-Lemeski, E.; Moradi, S.; Raoufi, R.; Shahlaei, M.; Janlou, M.A.M.; Zolghadri, S. Targeting SARS-COV-2 non-structural protein 16: A virtual drug repurposing study. J. Biomol. Struct. Dyn. 2020, 1–14. [Google Scholar] [CrossRef]
- Zhong, P.; Xu, J.; Yang, D.; Shen, Y.; Wang, L.; Feng, Y.; Du, C.; Song, Y.; Wu, C.; Hu, X.; et al. COVID-19-associated gastrointestinal and liver injury: Clinical features and potential mechanisms. Signal Transduct. Target. Ther. 2020, 5, 256. [Google Scholar] [CrossRef]
- Bostanciklioglu, M. Temporal Correlation Between Neurological and Gastrointestinal Symptoms of SARS-CoV-2. Inflamm. Bowel Dis. 2020, 26, e89–e91. [Google Scholar] [CrossRef] [PubMed]
- Jiehao, C.; Jin, X.; Daojiong, L.; Zhi, Y.; Lei, X.; Zhenghai, Q.; Yuehua, Z.; Hua, Z.; Ran, J.; Pengcheng, L.; et al. A Case Series of Children With 2019 Novel Coronavirus Infection: Clinical and Epidemiological Features. Clin. Infect. Dis. 2020, 71, 1547–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Li, J.; Shen, L.; Zou, Y.; Hou, L.; Zhu, L.; Faden, H.S.; Tang, Z.; Shi, M.; Jiao, N.; et al. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet 2020, 5, 534–535. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.; Fu, Y.; Yue, L.; Chen, G.-d.; Cai, X.; Shuai, M.; Xu, F.; Yi, X.; Chen, H.; Zhu, Y.; et al. Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Marasco, G.; Lenti, M.V.; Cremon, C.; Barbaro, M.R.; Stanghellini, V.; Di Sabatino, A.; Barbara, G. Implications of SARS-CoV-2 infection for neurogastroenterology. Neurogastroenterol. Motil. 2021, 33, e14104. [Google Scholar] [CrossRef]
- Chu, C.M.; Cheng, V.C.C.; Hung, I.F.N.; Wong, M.M.L.; Chan, K.H.; Chan, K.S.; Kao, R.Y.T.; Poon, L.L.M.; Wong, C.L.P.; Guan, Y.; et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004, 59, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Young, B.E.; Ong, S.W.X.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.; Ng, O.-T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020, 323, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, C.; Sun, Y.; Sui, X.; Zhu, T.; Wang, Q.; Wang, S.; Yang, J.; Yang, W.; Liu, F.; et al. A novel screening strategy of anti-SARS-CoV-2 drugs via blocking interaction between Spike RBD and ACE2. Environ. Int. 2020, 147, 106361. [Google Scholar] [CrossRef]
- Marjot, T.; Webb, G.J.; Barritt, A.S.; Moon, A.M.; Stamataki, Z.; Wong, V.W.; Barnes, E. COVID-19 and liver disease: Mechanistic and clinical perspectives. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 348–364. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Ochab-Jakubiak, J.; Cieślar, G.; Stanek, A. Gastrointestinal symptoms in the course of COVID-19. Postepy Hig. Med. Dosw. 2020, 74, 498–503. [Google Scholar] [CrossRef]
- Chu, D.K.W.; Pan, Y.; Cheng, S.M.S.; Hui, K.P.Y.; Krishnan, P.; Liu, Y.; Ng, D.Y.M.; Wan, C.K.C.; Yang, P.; Wang, Q.; et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin. Chem. 2020, 66, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Galanopoulos, M.; Doukatas, A.; Gazouli, M. Origin and genomic characteristics of SARS-CoV-2 and its interaction with angiotensin converting enzyme type 2 receptors, focusing on the gastrointestinal tract. World J. Gastroenterol. 2020, 26, 6335–6345. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, G.R.; Daniel, S.; Millet, J.K. Coronavirus entry: How we arrived at SARS-CoV-2. Curr. Opin. Virol. 2021, 47, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-W.; Wen, H.-L. Research progress on coronavirus S proteins and their receptors. Arch. Virol. 2021, 1–7. [Google Scholar] [CrossRef]
- Yesudhas, D.; Srivastava, A.; Sekijima, M.; Gromiha, M.M. Tackling Covid-19 using disordered-to-order transition of residues in the spike protein upon angiotensin-converting enzyme 2 binding. Proteins 2021. [Google Scholar] [CrossRef]
- Moradian, N.; Moallemian, M.; Delavari, F.; Sedikides, C.; Camargo, C.A.; Torres, P.J.; Sorooshian, A.; Mehdiabadi, S.P.; Nieto, J.J.; Bordas, S.; et al. Interdisciplinary Approaches to COVID-19. In Coronavirus Disease—COVID-19; Rezaei, N., Ed.; Springer International Publishing: Cham, Germany, 2021; pp. 923–936. [Google Scholar] [CrossRef]
- Peron, J.P.S.; Nakaya, H. Susceptibility of the Elderly to SARS-CoV-2 Infection: ACE-2 Overexpression, Shedding, and Antibody-dependent Enhancement (ADE). Clinics 2020, 75, e1912. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010. [Google Scholar] [CrossRef]
- Xu, J.; Chu, M.; Zhong, F.; Tan, X.; Tang, G.; Mai, J.; Lai, N.; Guan, C.; Liang, Y.; Liao, G. Digestive symptoms of COVID-19 and expression of ACE2 in digestive tract organs. Cell Death Discov. 2020, 6, 76. [Google Scholar] [CrossRef]
- Shafiee, S.; Cegolon, L.; Khafaei, M.; Gholami, N.; Zhao, S.; Khalesi, N.; Moosavian, H.; Fathi, S.; Izadi, M.; Ghadian, A.; et al. Gastrointestinal cancers, ACE-2/TMPRSS2 expression and susceptibility to COVID-19. Cancer Cell Int. 2021, 21, 431. [Google Scholar] [CrossRef]
- Perisetti, A.; Goyal, H.; Gajendran, M.; Boregowda, U.; Mann, R.; Sharma, N. Prevalence, Mechanisms, and Implications of Gastrointestinal Symptoms in COVID-19. Front. Med. 2020, 7, 588711. [Google Scholar] [CrossRef]
- Penninger, J.M.; Grant, M.B.; Sung, J.J.Y. The Role of Angiotensin Converting Enzyme 2 in Modulating Gut Microbiota, Intestinal Inflammation, and Coronavirus Infection. Gastroenterology 2021, 160, 39–46. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional diversity of the gastrointestinal microbiome. Cell Host Microbe 2019, 26, 314–324. [Google Scholar] [CrossRef]
- He, L.H.; Ren, L.F.; Li, J.F.; Wu, Y.N.; Li, X.; Zhang, L. Intestinal Flora as a Potential Strategy to Fight SARS-CoV-2 Infection. Front. Microbiol. 2020, 11, 1388. [Google Scholar] [CrossRef]
- Villapol, S. Gastrointestinal symptoms associated with COVID-19: Impact on the gut microbiome. Transl. Res. 2020, 226, 57–69. [Google Scholar] [CrossRef]
- Rajput, S.; Paliwal, D.; Naithani, M.; Kothari, A.; Meena, K.; Rana, S. COVID-19 and Gut Microbiota: A Potential Connection. Indian J. Clin. Biochem. 2021, 36, 1–12. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e948. [Google Scholar] [CrossRef]
- Sandhu, A.; Tillotson, G.; Polistico, J.; Salimnia, H.; Cranis, M.; Moshos, J.; Cullen, L.; Jabbo, L.; Diebel, L.; Chopra, T. Clostridioides difficile in COVID-19 Patients, Detroit, Michigan, USA, March–April 2020. Emerg. Infect. Dis. 2020, 26. [Google Scholar] [CrossRef]
- Dhar, D.; Mohanty, A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020, 285, 198018. [Google Scholar] [CrossRef]
- Syed, A.; Khan, A.; Gosai, F.; Asif, A.; Dhillon, S. Gastrointestinal pathophysiology of SARS-CoV2—A literature review. J. Community Hosp. Intern. Med. Perspect 2020, 10, 523–528. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373. [Google Scholar] [CrossRef]
- Pawlik, M.W.; Kwiecien, S.; Ptak-Belowska, A.; Pajdo, R.; Olszanecki, R.; Suski, M.; Madej, J.; Targosz, A.; Konturek, S.J.; Korbut, R. The renin-angiotensin system and its vasoactive metabolite angiotensin-(1-7) in the mechanism of the healing of preexisting gastric ulcers. The involvement of Mas receptors, nitric oxide, prostaglandins and proinflammatory cytokines. J. Physiol. Pharm. 2016, 67, 75–91. [Google Scholar]
- Fujita, M.; Hayashi, I.; Yamashina, S.; Fukamizu, A.; Itoman, M.; Majima, M. Angiotensin type 1a receptor signaling-dependent induction of vascular endothelial growth factor in stroma is relevant to tumor-associated angiogenesis and tumor growth. Carcinogenesis 2005, 26, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Rahban, M.; Habibi-Rezaei, M.; Mazaheri, M.; Saso, L.; Moosavi-Movahedi, A.A. Anti-Viral. Potential and Modulation of Nrf2 by Curcumin: Pharmacological Implications. Antioxidants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Raimondo, M.G.; Biggioggero, M.; Crotti, C.; Becciolini, A.; Favalli, E.G. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des. Dev. 2017, 11, 1593–1603. [Google Scholar] [CrossRef] [Green Version]
- Lowery, S.A.; Sariol, A.; Perlman, S. Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19. Cell Host Microbe 2021, 29, 1052–1062. [Google Scholar] [CrossRef]
- Garg, M.; Royce, S.; Tikellis, C.; Shallue, C.; Batu, D.; Velkoska, E.; Burrell, L.; Patel, S.; Beswick, L.; Jackson, A.; et al. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: A novel therapeutic target? Gut 2019, 69, 841–851. [Google Scholar] [CrossRef]
- Liang, W.; Feng, Z.; Rao, S.; Xiao, C.; Xue, X.; Lin, Z.; Zhang, Q.; Qi, W. Diarrhoea may be underestimated: A missing link in 2019 novel coronavirus. Gut 2020, 69, 1141. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, F.; Baumgart, D.C.; Danese, S.; Peyrin-Biroulet, L. Diarrhea during COVID-19 infection: Pathogenesis, epidemiology, prevention and management. Clin. Gastroenterol. Hepatol. 2020, 18, 1663–1672. [Google Scholar] [CrossRef]
- Tang, W.; Cao, Z.; Han, M.; Wang, Z.; Chen, J.; Sun, W.; Wu, Y.; Xiao, W.; Liu, S.; Chen, E.; et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ 2020, 369, m1849. [Google Scholar] [CrossRef]
- Chedid, M.; Waked, R.; Haddad, E.; Chetata, N.; Saliba, G.; Choucair, J. Antibiotics in treatment of COVID-19 complications: A review of frequency, indications, and efficacy. J. Infect. Public Health 2021, 14, 570. [Google Scholar] [CrossRef]
- Bagheri, A.; Moezzi, S.M.I.; Mosaddeghi, P.; Parashkouhi, S.N.; Hoseini, S.M.F.; Badakhshan, F.; Negahdaripour, M. Interferon-inducer antivirals: Potential candidates to combat COVID-19. Int. Immunopharmacol. 2021, 91, 107245. [Google Scholar] [CrossRef]
- Frediansyah, A.; Tiwari, R.; Sharun, K.; Dhama, K.; Harapan, H. Antivirals for COVID-19: A critical review. Clin. Epidemiol. Glob. Health 2021, 9, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selber-Hnatiw, S.; Rukundo, B.; Ahmadi, M.; Akoubi, H.; Al-Bizri, H.; Aliu, A.F.; Ambeaghen, T.U.; Avetisyan, L.; Bahar, I.; Baird, A.; et al. Human Gut Microbiota: Toward an Ecology of Disease. Front. Microbiol. 2017, 8, 1265. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Lan, T.; Zeng, L.; Luo, H.; Yang, X.; Li, N.; Chen, X.; Liu, Z.; Li, R.; Win, S.; et al. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J. Hepatol. 2018, 69, 51–59. [Google Scholar] [CrossRef]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Dickson, R.P.; Singer, B.H.; Newstead, M.W.; Falkowski, N.R.; Erb-Downward, J.R.; Standiford, T.J.; Huffnagle, G.B. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat. Microbiol. 2016, 1, 16113. [Google Scholar] [CrossRef]
- Kim, H.S. Do an altered gut microbiota and an associated leaky gut affect COVID-19 severity? Mbio 2021, 12, e03022-20. [Google Scholar] [CrossRef]
- Li, J.; Richards, E.M.; Handberg, E.M.; Pepine, C.J.; Raizada, M.K. Butyrate regulates COVID-19–relevant genes in gut epithelial organoids from normotensive rats. Hypertension 2021, 77, e13–e16. [Google Scholar] [CrossRef]
- Bradley, C.P.; Teng, F.; Felix, K.M.; Sano, T.; Naskar, D.; Block, K.E.; Huang, H.; Knox, K.S.; Littman, D.R.; Wu, H.J. Segmented Filamentous Bacteria Provoke Lung Autoimmunity by Inducing Gut-Lung Axis Th17 Cells Expressing Dual TCRs. Cell Host Microbe 2017, 22, 697–704.e694. [Google Scholar] [CrossRef]
- Ye, L.; Yang, Z.; Liu, J.; Liao, L.; Wang, F. Digestive system manifestations and clinical significance of coronavirus disease 2019: A systematic literature review. J. Gastroenterol. Hepatol. 2021, 36, 1414–1422. [Google Scholar] [CrossRef]
- Liu, S.; Tang, M.-M.; Du, J.; Gong, Z.-C.; Sun, S.-S. COVID-19 in gastroenterology and hepatology: Lessons learned and questions to be answered. World J. Clin. Cases 2021, 9, 4199. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Manosso, L.M.; Arent, C.O.; Borba, L.A.; Ceretta, L.B.; Quevedo, J.; Réus, G.Z. Microbiota-Gut-Brain Communication in the SARS-CoV-2 Infection. Cells 2021, 10, 1993. [Google Scholar] [CrossRef]
- Tariq, R.; Saha, S.; Furqan, F.; Hassett, L.; Pardi, D.; Khanna, S. Prevalence and mortality of COVID-19 patients with gastrointestinal symptoms: A systematic review and meta-analysis. Mayo Clin. Proc. 2020, 95, 1632–1648. [Google Scholar] [CrossRef]
- Parasa, S.; Desai, M.; Chandrasekar, V.T.; Patel, H.K.; Kennedy, K.F.; Roesch, T.; Spadaccini, M.; Colombo, M.; Gabbiadini, R.; Artifon, E.L. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: A systematic review and meta-analysis. JAMA Netw. Open 2020, 3, e2011335. [Google Scholar] [CrossRef]
- Zheng, T.; Yang, C.; Wang, H.Y.; Chen, X.; Yu, L.; Wu, Z.L.; Sun, H. Clinical characteristics and outcomes of COVID-19 patients with gastrointestinal symptoms admitted to Jianghan Fangcang Shelter Hospital in Wuhan, China. J. Med. Virol. 2020, 92, 2735–2741. [Google Scholar] [CrossRef]
- Chen, R.; Yu, Y.L.; Li, W.; Liu, Y.; Lu, J.X.; Chen, F.; Zhou, Q.; Xia, Z.Y.; Gao, L.; Meng, Q.T.; et al. Gastrointestinal Symptoms Associated with Unfavorable Prognosis of COVID-19 Patients: A Retrospective Study. Front. Med. 2020, 7, 608259. [Google Scholar] [CrossRef]
- Cha, M.H.; Regueiro, M.; Sandhu, D.S. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J. Gastroenterol. 2020, 26, 2323–2332. [Google Scholar] [CrossRef]
- Papa, A.; Covino, M.; Pizzolante, F.; Miele, L.; Lopetuso, L.R.; Bove, V.; Iorio, R.; Simeoni, B.; Vetrone, L.M.; Tricoli, L.; et al. Gastrointestinal symptoms and digestive comorbidities in an Italian cohort of patients with COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7506–7511. [Google Scholar] [CrossRef]
- Velev, V.; Popov, M.; Velikov, P.; Dinkova, M.; Ilieva, V.; Gospodinova, G.; Tcherveniakova, T.; Pavlova, M. COVID-19 and gastrointestinal injury: A brief systematic review and data from Bulgaria. Le Infez. Med. 2020, 28, 37–41. [Google Scholar]
- Ghoshal, U.C.; Ghoshal, U.; Mathur, A.; Singh, R.K.; Nath, A.; Garg, A.; Singh, D.; Singh, S.; Singh, J.; Pandey, A.; et al. The Spectrum of Gastrointestinal Symptoms in Patients With Coronavirus Disease-19: Predictors, Relationship With Disease Severity, and Outcome. Clin. Transl. Gastroenterol. 2020, 11, e00259. [Google Scholar] [CrossRef]
- Hatami, N.; Ahi, S.; Sadeghinikoo, A.; Foroughian, M.; Javdani, F.; Kalani, N.; Fereydoni, M.; Keshavarz, P. Worldwide ACE (I/D) Polymorphism May Affect COVID-19 Recovery Rate: An Ecological Meta-Regression; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Manganotti, P.; Bellavita, G.; D’Acunto, L.; Tommasini, V.; Fabris, M.; Sartori, A.; Bonzi, L.; Buoite Stella, A.; Pesavento, V. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barre syndrome and polyneuritis cranialis in COVID-19 patients: A case series. J. Med. Virol. 2021, 93, 766–774. [Google Scholar] [CrossRef]
- Yadav, R.; Srivastava, D.K.; Bajpai, P.K.; Kumar, R. Neurological Involvement in COVID-19 Patients: A Narrative Review. J. Neurosci. Rural Pract. 2020, 11, 526–529. [Google Scholar] [CrossRef]
- Roman, G.C.; Spencer, P.S.; Reis, J.; Buguet, A.; Faris, M.E.A.; Katrak, S.M.; Lainez, M.; Medina, M.T.; Meshram, C.; Mizusawa, H.; et al. The neurology of COVID-19 revisited: A proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J. Neurol. Sci. 2020, 414, 116884. [Google Scholar] [CrossRef]
- Li, H.; Xue, Q.; Xu, X. Involvement of the Nervous System in SARS-CoV-2 Infection. Neurotox Res. 2020, 38, 1–7. [Google Scholar] [CrossRef]
- Deffner, F.; Scharr, M.; Klingenstein, S.; Klingenstein, M.; Milazzo, A.; Scherer, S.; Wagner, A.; Hirt, B.; Mack, A.F.; Neckel, P.H. Histological Evidence for the Enteric Nervous System and the Choroid Plexus as Alternative Routes of Neuroinvasion by SARS-CoV2. Front. Neuroanat. 2020, 14, 596439. [Google Scholar] [CrossRef]
- Guo, M.; Tao, W.; Flavell, R.A.; Zhu, S. Potential intestinal infection and faecal–oral transmission of SARS-CoV-2. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 269–283. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Li, M.; Chuang, H.; Ye, Y.; Zhao, H.; Wang, H. Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J. Neurol. 2020, 267, 2777–2789. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Simani, L. Central nervous system manifestations of COVID-19: A systematic review. J. Neurol. Sci. 2020, 413, 116832. [Google Scholar] [CrossRef] [PubMed]
- Groiss, S.J.; Balloff, C.; Elben, S.; Brandenburger, T.; Muttel, T.; Kindgen-Milles, D.; Vollmer, C.; Feldt, T.; Kunstein, A.; Ole Jensen, B.E.; et al. Prolonged Neuropsychological Deficits, Central Nervous System Involvement, and Brain Stem Affection After COVID-19-A Case Series. Front. Neurol. 2020, 11, 574004. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Sarnelli, G. Can the enteric nervous system be an alternative entrance door in SARS-CoV2 neuroinvasion? Brain Behav. Immun. 2020, 87, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Barrantes, F.J. Central Nervous System Targets and Routes for SARS-CoV-2: Current Views and New Hypotheses. ACS Chem. Neurosci. 2020, 11, 2793–2803. [Google Scholar] [CrossRef]
- Follmer, C. Viral. Infection-Induced Gut Dysbiosis, Neuroinflammation, and alpha-Synuclein Aggregation: Updates and Perspectives on COVID-19 and Neurodegenerative Disorders. ACS Chem. Neurosci. 2020, 11, 4012–4016. [Google Scholar] [CrossRef]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef]
- Idrees, D.; Kumar, V. SARS-CoV-2 spike protein interactions with amyloidogenic proteins: Potential clues to neurodegeneration. Biochem. Biophys. Res. Commun. 2021, 554, 94–98. [Google Scholar] [CrossRef]
- Zanyatkin, I.; Stroylova, Y.; Tishina, S.; Stroylov, V.; Melnikova, A.; Haertle, T.; Muronetz, V. Inhibition of Prion Propagation by 3,4-Dimethoxycinnamic Acid. Phytother. Res. 2017, 31, 1046–1055. [Google Scholar] [CrossRef]
- Medvedeva, M.; Barinova, K.; Melnikova, A.; Semenyuk, P.; Kolmogorov, V.; Gorelkin, P.; Erofeev, A.; Muronetz, V. Naturally occurring cinnamic acid derivatives prevent amyloid transformation of alpha-synuclein. Biochimie 2020, 170, 128–139. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L.; et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.v.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Cheung, O.; Chan, J.; Ng, C.; Koo, C. The spectrum of pathological changes in severe acute respiratory syndrome (SARS). Histopathology 2004, 45, 119–124. [Google Scholar] [CrossRef]
- Chen, J.; Subbarao, K. The immunobiology of SARS. Annu. Rev. Immunol. 2007, 25, 443–472. [Google Scholar] [CrossRef]
- Henrion, J.; Schapira, M.; Luwaert, R.; Colin, L.; Delannoy, A.; Heller, F.R. Hypoxic hepatitis: Clinical and hemodynamic study in 142 consecutive cases. Medicine 2003, 82, 392–406. [Google Scholar] [CrossRef]
- Seeto, R.K.; Fenn, B.; Rockey, D.C. Ischemic hepatitis: Clinical presentation and pathogenesis. Am. J. Med. 2000, 109, 109–113. [Google Scholar] [CrossRef]
- Bessone, F.; Dirchwolf, M.; Rodil, M.A.; Razori, M.V.; Roma, M.G. Review article: Drug-induced liver injury in the context of nonalcoholic fatty liver disease—A physiopathological and clinical integrated view. Aliment. Pharm. Ther. 2018, 48, 892–913. [Google Scholar] [CrossRef]
- Gordon, A.; McLean, C.A.; Pedersen, J.S.; Bailey, M.J.; Roberts, S.K. Hepatic steatosis in chronic hepatitis B and C: Predictors, distribution and effect on fibrosis. J. Hepatol. 2005, 43, 38–44. [Google Scholar] [CrossRef]
- Nishida, N.; Chiba, T.; Ohtani, M.; Yoshioka, N. Sudden unexpected death of a 17-year-old male infected with the influenza virus. Leg. Med. 2005, 7, 51–57. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-W.; Wu, X.-X.; Jiang, X.-G.; Xu, K.-J.; Ying, L.-J.; Ma, C.-L.; Li, S.-B.; Wang, H.-Y.; Zhang, S.; Gao, H.-N. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: Retrospective case series. BMJ 2020, 368, m606. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Fang, M.; Li, S.; Wu, L.; Gao, B.; Gao, H.; Ran, X.; Bian, Y.; Li, R.; Yu, S.; et al. Characteristics of Liver Function in Patients With SARS-CoV-2 and Chronic HBV Coinfection. Clin. Gastroenterol. Hep. 2021, 19, 597–603. [Google Scholar] [CrossRef]
- Chen, L.; Huang, S.; Yang, J.; Cheng, X.; Shang, Z.; Lu, H.; Cheng, J. Clinical characteristics in patients with SARS-CoV-2 /HBV co-infection. J. Viral. Hepat. 2020, 27, 1504–1507. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, L.; Cheng, X.; Han, H.; Li, C.; Li, D.; Liu, A.; Gao, G.; Zhou, F.; Liu, F. Clinical characteristics of COVID-19 patients with hepatitis B virus infection—A retrospective study. Liver Int. 2021, 41, 720–730. [Google Scholar] [CrossRef]
- Yu, R.; Tan, S.; Dan, Y.; Lu, Y.; Zhang, J.; Tan, Z.; He, X.; Xiang, X.; Zhou, Y.; Guo, Y. Effect of SARS-CoV-2 coinfection was not apparent on the dynamics of chronic hepatitis B infection. Virology 2021, 553, 131–134. [Google Scholar] [CrossRef]
- Lin, Y.; Yuan, J.; Long, Q.; Hu, J.; Deng, H.; Zhao, Z.; Chen, J.; Lu, M.; Huang, A. Patients with SARS-CoV-2 and HBV co-infection are at risk of greater liver injury. Genes Dis. 2020. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Cai, Q.; Sun, L.; Huang, D.; Zhou, G.; He, Q.; Wang, F.-S.; Liu, L.; Chen, J. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol. Res. 2020, 50, 1211–1221. [Google Scholar] [CrossRef]
- Rodríguez-Tajes, S.; Miralpeix, A.; Costa, J.; López-Suñé, E.; Laguno, M.; Pocurull, A.; Lens, S.; Mariño, Z.; Forns, X. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J. Viral. Hepat. 2021, 28, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Beavers, K.L.; Hammond, S.P.; Lim, J.K.; Falck-Ytter, Y.T. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015, 148, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.F.; Mo, Y.Q.; Jing, J.; Ma, J.D.; Zheng, D.H.; Dai, L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: A prospective clinical observation. Int. J. Rheum. Dis. 2017, 20, 859–869. [Google Scholar] [CrossRef]
| Drug/Drug Class | Therapeutic Category | Reference |
|---|---|---|
| Aminoglycosides | Antibiotic | Bagheri 2021 |
| Azithromycin | Antibiotic | Bagheri 2021, Chedid 2021 |
| Moxifloxacin | Antibiotic | Chedid 2021 |
| Ceftriaxone | Antibiotic | Chedid 2021 |
| Cephalosporin | Antibiotic | Chedid 2021 |
| Quinolones | Antibiotic | Chedid 2021 |
| Clarithromycin | Antibiotic | Chedid 2021 |
| Ceftriaxone | Antibiotic | Chedid 2021 |
| Tigecycline | Antibiotic | Chedid 2021 |
| Cefoperazone | Antibiotic | Chedid 2021 |
| Umifenovir | Antiviral | Frediansyah 2021 |
| Lopinavir | Antiviral | Frediansyah 2021 |
| Darunavir | Antiviral | Frediansyah 2021 |
| Atazanavir | Antiviral | Frediansyah 2021 |
| Saquinavir | Antiviral | Frediansyah 2021 |
| Emtricitabine | Antiviral | Frediansyah 2021 |
| Azvudine | Antiviral | Frediansyah 2021 |
| Remdesivir | Antiviral | Frediansyah 2021 |
| Favipiravir | Antiviral | Frediansyah 2021 |
| Ribavirin | Antiviral | Frediansyah 2021 |
| Sofosbuvir | Antiviral | Frediansyah 2021 |
| Oseltamivir | Antiviral | Frediansyah 2021 |
| Authors | Study Design | Salient Findings/Observations | Whether Associated with the Final Outcome of COVID-19 |
|---|---|---|---|
| Zou X. et al. [105] | 105 patients with SARS-CoV-2 and chronic HBV co-infection were studied to determine their biochemical and clinical outcomes. | Biochemical parameters (ALT, AST, total bilirubin, AFP) increased significantly during hospitalization. 4 of 14 patients who developed liver injury rapidly progressed to acute-on-chronic liver failure. The proportion of severe COVID-19 was higher in patients with liver injury (n = 14) (p = 0.42) including acute-on-chronic liver failure and, acute cardiac injury (p < 0.05). Mortality higher among patients with liver injury (p = 0.004) | Yes |
| Chen L. et al. [106] | Clinical study to evaluate whether SARS-CoV-2/HBV co-infection could influence liver function and disease outcome among 20 patients with HBV co-infection vs. 306 patients without HBV co-infection. | No differences in the level of liver functions parameter. No significant differences in terms for the discharge rate and length of stay between the two groups. | No |
| Liu R. et al. [107] | 50 SARS-CoV-2 and HBV co-infected patients, 56 SARS-CoV-2 mono-infected patients, 57 HBeAg negative chronic HBV patient controls and 57 healthy controls. Serum biochemical parameters and cytokines were assessed. T cell, B cell and NK cell counts were measured. | SARS-CoV-2 and HBV co-infection did not significantly affect the outcome of COVID-19. Most of the disarrangement including severe monocytopenia and thrombocytopenia as well as disturbed hepatic function with respect to albumin production and lipid metabolism was reversed after recovery from COVID-19. | No |
| Yu R. et al. [108] | SARS-CoV-2 infected patients with HBsAg +ve serology (n = 7) and with HbsAg –ve serology (n = 60) were studied. | SARS-CoV-2 did not affect the dynamics of chronic HBV infection and was not found to be the source of HBV reactivation. Markers of HBV replication did not extensively fluctuate during SARS-CoV-2 infection. | No |
| Lin Y. et al. [109] | 116 COVID-19 patients with HBV negative serology and 17 COVID-19 patients with HBV serology but as inactive carriers were studied. | Though there were significant differences for the discharge rate or duration of hospitalization, SARS-CoV-2 and HBV co-infection among 17 patients were found to have exacerbated liver function. COVID-19 with inactive HBV carriers with SARS-CoV-2 co-infection were at higher risk of abnormal liver functions. | Undecided whether the final outcome of COVID-19 was influenced. |
| Liu J. et al. [110] | Included 21 vs. 326 as with vs. without chronic HBV infection. However, with the help of the Propensity Score Method (PSM) final inclusion was restricted to 20 vs. 57 for the HBV group and non-HBV group, respectively. | All the 71 patients included after applying PSM, achieved SARS-CoV-2 clearance. No significant difference between the two groups showing progression to severe COVID-19. Longitudinal changes in biochemical parameters between the two groups were not significantly different. However, 3 patients in the HBV group experienced reactivation of HBV. | No (however, the authors suspect HBV reactivation and suggest monitoring the liver functions as well as HBV DNA levels of COVID-19 patients during the whole disease course.) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahban, M.; Stanek, A.; Hooshmand, A.; Khamineh, Y.; Ahi, S.; Kazim, S.N.; Ahmad, F.; Muronetz, V.; Samy Abousenna, M.; Zolghadri, S.; et al. Infection of Human Cells by SARS-CoV-2 and Molecular Overview of Gastrointestinal, Neurological, and Hepatic Problems in COVID-19 Patients. J. Clin. Med. 2021, 10, 4802. https://doi.org/10.3390/jcm10214802
Rahban M, Stanek A, Hooshmand A, Khamineh Y, Ahi S, Kazim SN, Ahmad F, Muronetz V, Samy Abousenna M, Zolghadri S, et al. Infection of Human Cells by SARS-CoV-2 and Molecular Overview of Gastrointestinal, Neurological, and Hepatic Problems in COVID-19 Patients. Journal of Clinical Medicine. 2021; 10(21):4802. https://doi.org/10.3390/jcm10214802
Chicago/Turabian StyleRahban, Mahdie, Agata Stanek, Amirreza Hooshmand, Yasaman Khamineh, Salma Ahi, Syed Naqui Kazim, Faizan Ahmad, Vladimir Muronetz, Mohamed Samy Abousenna, Samaneh Zolghadri, and et al. 2021. "Infection of Human Cells by SARS-CoV-2 and Molecular Overview of Gastrointestinal, Neurological, and Hepatic Problems in COVID-19 Patients" Journal of Clinical Medicine 10, no. 21: 4802. https://doi.org/10.3390/jcm10214802
APA StyleRahban, M., Stanek, A., Hooshmand, A., Khamineh, Y., Ahi, S., Kazim, S. N., Ahmad, F., Muronetz, V., Samy Abousenna, M., Zolghadri, S., & Saboury, A. A. (2021). Infection of Human Cells by SARS-CoV-2 and Molecular Overview of Gastrointestinal, Neurological, and Hepatic Problems in COVID-19 Patients. Journal of Clinical Medicine, 10(21), 4802. https://doi.org/10.3390/jcm10214802










