Reproductive, Obstetric and Neonatal Outcomes in Women with Congenital Uterine Anomalies: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
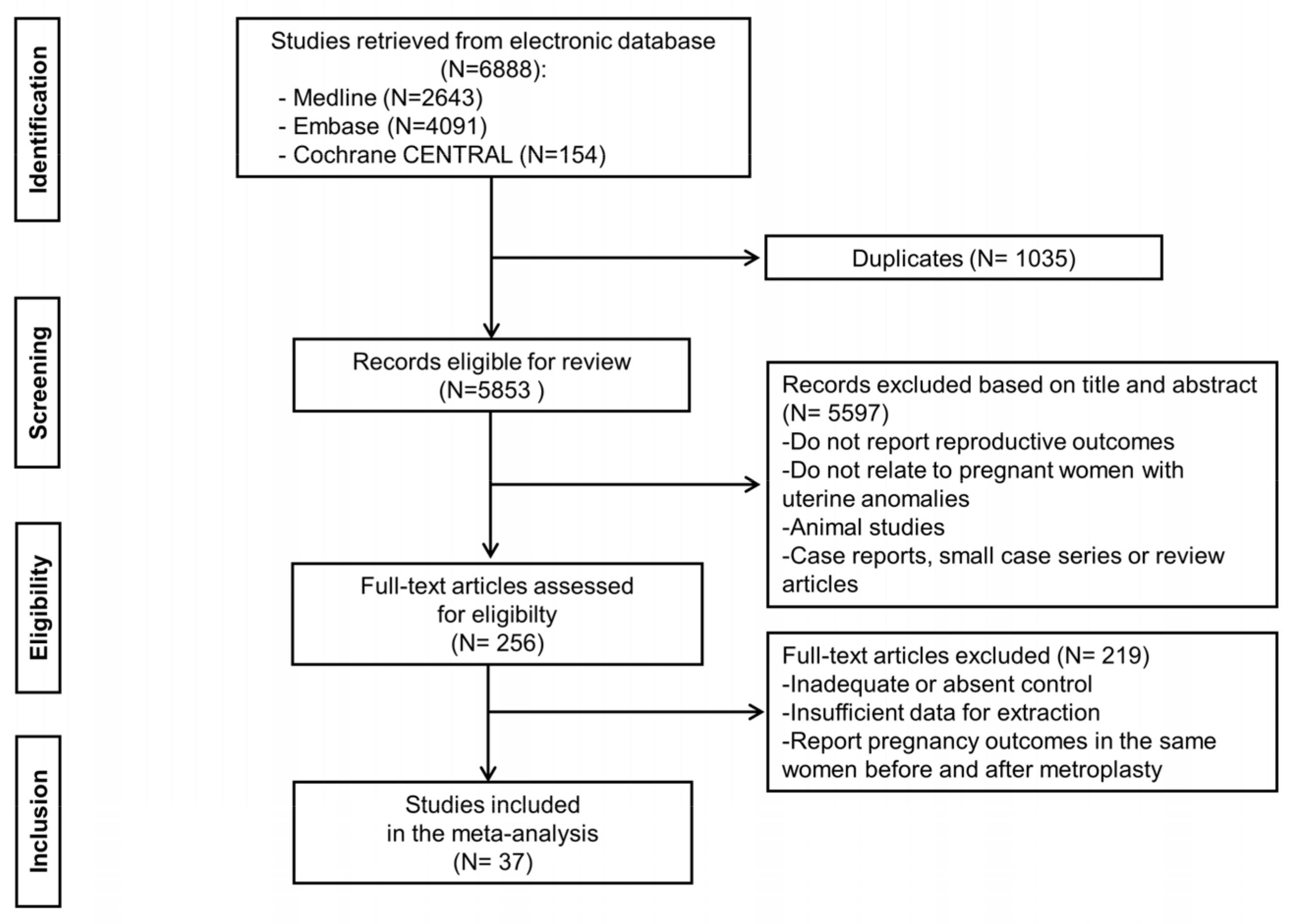
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Reproductive Outcomes
3.2.1. Clinical Pregnancy Rate
3.2.2. Live Birth
3.2.3. First Trimester Miscarriage
3.2.4. Second-Trimester Miscarriage
3.3. Obstetric Outcomes
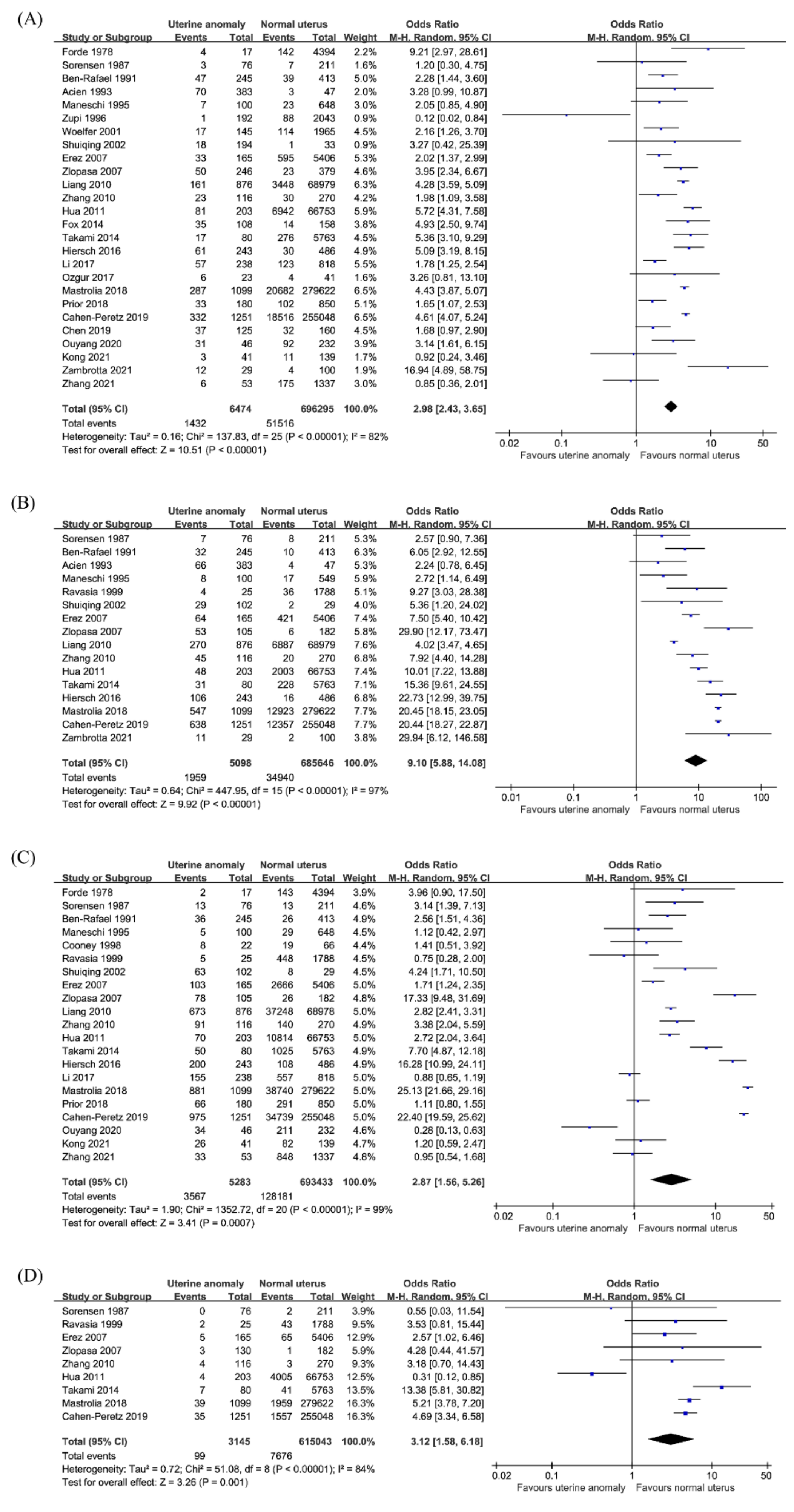
3.3.1. Preterm Birth
3.3.2. Malpresentation
3.3.3. Cesarean Section
3.3.4. Placental Abruption
3.4. Neonatal Outcomes
3.4.1. Intrauterine Growth Restriction or Small for Gestational Age
3.4.2. Low Birth Weight
3.4.3. Perinatal Mortality
3.5. Other Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simón, C.; Martinez, L.; Pardo, F.; Tortajada, M.; Pellicer, A. Müllerian defects in women with normal reproductive outcome. Fertil. Steril. 1991, 56, 1192–1193. [Google Scholar] [CrossRef]
- Makino, T.; Hara, T.; Oka, C.; Toyoshima, K.; Sugi, T.; Iwasaki, K.; Umeuchi, M.; Iizuka, R. Survey of 1120 Japanese women with a history of recurrent spontaneous abortions. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992, 44, 123–130. [Google Scholar] [CrossRef]
- Makino, T.; Umeuchi, M.; Nakada, K.I.; Nozawa, S.; Iizuka, R. Incidence of congenital uterine anomalies in repeated reproductive wastage and prognosis for pregnancy after metroplasty. Int. J. Fertil. 1992, 37, 167–170. [Google Scholar] [PubMed]
- Clifford, K.; Rai, R.; Watson, H.; Regan, L. An informative protocol for the investigation of recurrent miscarriage: Preliminary experience of 500 consecutive cases. Hum. Reprod. 1994, 9, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Acién, P. Incidence of mullerian defects in fertile and infertile women. Hum. Reprod. 1997, 12, 1372–1376. [Google Scholar] [CrossRef] [Green Version]
- Homer, H.A.; Li, T.C.; Cooke, I.D. The septate uterus: A review of management and reproductive outcome. Fertil. Steril. 2000, 73, 1–14. [Google Scholar] [CrossRef]
- Guimarães Filho, H.A.; Mattar, R.; Pires, C.R.; Araujo Júnior, E.; Moron, A.F.; Nardozza, L.M. Prevalence of uterine defects in habitual abortion patients attended on at a university health service in brazil. Arch. Gynecol. Obstet. 2006, 274, 345–348. [Google Scholar] [CrossRef]
- Guimarães Filho, H.A.; Mattar, R.; Pires, C.R.; Araujo Júnior, E.; Moron, A.F.; Nardozza, L.M. Comparison of hysterosalpingography, hysterosonography and hysteroscopy in evaluation of the uterine cavity in patients with recurrent pregnancy losses. Arch. Gynecol. Obstet. 2006, 274, 284–288. [Google Scholar] [CrossRef]
- Lin, P.C.; Bhatnagar, K.P.; Nettleton, G.S.; Nakajima, S.T. Female genital anomalies affecting reproduction. Fertil. Steril. 2002, 78, 899–915. [Google Scholar] [CrossRef]
- Motta, P.M.; Nottola, S.A.; Makabe, S. Natural history of the female germ cell from its origin to full maturation through prenatal ovarian development. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 75, 5–10. [Google Scholar] [CrossRef]
- Rackow, B.W.; Arici, A. Reproductive performance of women with müllerian anomalies. Curr. Opin. Obstet. Gynecol. 2007, 19, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Buttram, V.C., Jr.; Gomel, V.; Siegler, A.; DeCherney, A.; Gibbons, W.; March, C. The american fertility society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil. Steril. 1988, 49, 944–955. [Google Scholar]
- Salim, R.; Regan, L.; Woelfer, B.; Backos, M.; Jurkovic, D. A comparative study of the morphology of congenital uterine anomalies in women with and without a history of recurrent first trimester miscarriage. Hum. Reprod. 2003, 18, 162–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinonen, P.K.; Saarikoski, S.; Pystynen, P. Reproductive performance of women with uterine anomalies. An evaluation of 182 cases. Acta Obstet. Gynecol. Scand. 1982, 61, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Chandler, T.M.; Machan, L.S.; Cooperberg, P.L.; Harris, A.C.; Chang, S.D. Mullerian duct anomalies: From diagnosis to intervention. Br. J. Radiol. 2009, 82, 1034–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupesic, S. Clinical implications of sonographic detection of uterine anomalies for reproductive outcome. Ultrasound Obstet. Gynecol. 2001, 18, 387–400. [Google Scholar] [CrossRef] [Green Version]
- Kupesic, S.; Kurjak, A. Diagnosis and treatment outcome of the septate uterus. Croat. Med. J. 1998, 39, 185–190. [Google Scholar]
- Chan, Y.Y.; Jayaprakasan, K.; Tan, A.; Thornton, J.G.; Coomarasamy, A.; Raine-Fenning, N.J. Reproductive outcomes in women with congenital uterine anomalies: A systematic review. Ultrasound Obstet. Gynecol. 2011, 38, 371–382. [Google Scholar] [CrossRef]
- Venetis, C.A.; Papadopoulos, S.P.; Campo, R.; Gordts, S.; Tarlatzis, B.C.; Grimbizis, G.F. Clinical implications of congenital uterine anomalies: A meta-analysis of comparative studies. Reprod. Biomed. Online 2014, 29, 665–683. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Reprint--preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef]
- Davey, J.; Turner, R.M.; Clarke, M.J.; Higgins, J.P. Characteristics of meta-analyses and their component studies in the cochrane database of systematic reviews: A cross-sectional, descriptive analysis. BMC Med. Res. Methodol. 2011, 11, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X. Clinical outcomes analysis of infertile women with unicornuate uterus in ivf-et. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102111. [Google Scholar] [CrossRef]
- Zambrotta, E.; Di Gregorio, L.M.; Di Guardo, F.; Agliozzo, R.; Maugeri, G.C.; Gulino, F.A.; Cutello, S.; Cerana, M.C.; Palumbo, M. Congenital uterine anomalies and perinatal outcomes: A retrospective single-center cohort study. Clin. Exp. Obstet. Gynecol. 2021, 48, 161–164. [Google Scholar]
- Kong, W.Y.; Zhao, S.R.; Deng, K.; Zhang, Q.; Liu, W.; Yan, L. Effects of bicornuate uterus on pregnancy and obstetric outcomes of in vitro fertilization / intracytoplasmic sperm injection. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Cai, P.; Gong, F.; Lin, G.; Qin, J.; Li, X. The risk of twin pregnancies should be minimized in patients with a unicornuate uterus undergoing ivf-et. Sci. Rep. 2020, 10, 5571. [Google Scholar] [CrossRef] [PubMed]
- Neal, S.A.; Morin, S.J.; Werner, M.D.; Gueye, N.A.; Pirtea, P.; Scott, R.T., Jr.; Goodman, L.R. Three-dimensional ultrasound diagnosis of t-shaped uterus is associated with adverse pregnancy outcomes after embryo transfer. Reprod. Biomed. Online 2019, 39, 777–783. [Google Scholar] [CrossRef]
- Chen, X.; Liu, P.; Sheng, Y.; Li, W.; Tang, R.; Ding, L.; Qin, Y.; Chen, Z.J. The impact of unicornuate uterus on perinatal outcomes after ivf/icsi cycles: A matched retrospective cohort study. J. Matern. Fetal Neonatal Med. 2019, 32, 2469–2474. [Google Scholar] [CrossRef]
- Cahen-Peretz, A.; Sheiner, E.; Friger, M.; Walfisch, A. The association between müllerian anomalies and perinatal outcome. J. Matern. Fetal Neonatal Med. 2019, 32, 51–57. [Google Scholar] [CrossRef]
- Prior, M.; Richardson, A.; Asif, S.; Polanski, L.; Parris-Larkin, M.; Chandler, J.; Fogg, L.; Jassal, P.; Thornton, J.G.; Raine-Fenning, N.J. Outcome of assisted reproduction in women with congenital uterine anomalies: A prospective observational study. Ultrasound Obstet. Gynecol. 2018, 51, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Pleş, L.; Alexandrescu, C.; Ionescu, C.A.; Arvătescu, C.A.; Vladareanu, S.; Moga, M.A. Three-dimensional scan of the uterine cavity of infertile women before assisted reproductive technology use. Medicine 2018, 97, e12764. [Google Scholar] [CrossRef]
- Mastrolia, S.A.; Baumfeld, Y.; Hershkovitz, R.; Loverro, G.; Di Naro, E.; Yohai, D.; Schwarzman, P.; Weintraub, A.Y. Bicornuate uterus is an independent risk factor for cervical os insufficiency: A retrospective population based cohort study. J. Matern. Fetal Neonatal Med. 2017, 30, 2705–2710. [Google Scholar] [CrossRef]
- Mastrolia, S.A.; Baumfeld, Y.; Hershkovitz, R.; Yohay, D.; Trojano, G.; Weintraub, A.Y. Independent association between uterine malformations and cervical insufficiency: A retrospective population-based cohort study. Arch. Gynecol. Obstet. 2018, 297, 919–926. [Google Scholar] [CrossRef]
- Chen, Y.; Nisenblat, V.; Yang, P.; Zhang, X.; Ma, C. Reproductive outcomes in women with unicornuate uterus undergoing in vitro fertilization: A nested case-control retrospective study. Reprod. Biol. Endocrinol. 2018, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, K.; Bulut, H.; Berkkanoglu, M.; Coetzee, K. Reproductive outcomes of ivf patients with unicornuate uteri. Reprod. Biomed. Online 2017, 34, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ouyang, Y.; Yi, Y.; Lin, G.; Lu, G.; Gong, F. Pregnancy outcomes of women with a congenital unicornuate uterus after ivf-embryo transfer. Reprod. Biomed. Online 2017, 35, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Hiersch, L.; Yeoshoua, E.; Miremberg, H.; Krissi, H.; Aviram, A.; Yogev, Y.; Ashwal, E. The association between mullerian anomalies and short-term pregnancy outcome. J. Matern. Fetal Neonatal Med. 2016, 29, 2573–2578. [Google Scholar] [CrossRef]
- Takami, M.; Aoki, S.; Kurasawa, K.; Okuda, M.; Takahashi, T.; Hirahara, F. A classification of congenital uterine anomalies predicting pregnancy outcomes. Acta Obstet. Gynecol. Scand. 2014, 93, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.S.; Roman, A.S.; Stern, E.M.; Gerber, R.S.; Saltzman, D.H.; Rebarber, A. Type of congenital uterine anomaly and adverse pregnancy outcomes. J. Matern. Fetal Neonatal Med. 2014, 27, 949–953. [Google Scholar] [CrossRef]
- Jayaprakasan, K.; Chan, Y.Y.; Sur, S.; Deb, S.; Clewes, J.S.; Raine-Fenning, N.J. Prevalence of uterine anomalies and their impact on early pregnancy in women conceiving after assisted reproduction treatment. Ultrasound Obstet. Gynecol. 2011, 37, 727–732. [Google Scholar] [CrossRef]
- Hua, M.; Odibo, A.; Longman, R.; Roehl, K.A.; Macones, G.; Cahill, A. Congenital uterine anomalies and adverse pregnancy outcomes. Am. J. Obstet. Gynecol. 2011, 204, S334–S335. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.Y.; Qiao, J. Obstetric outcome of women with uterine anomalies in china. Chin. Med. J. 2010, 123, 418–422. [Google Scholar]
- Tomaževič, T.; Ban-Frangež, H.; Virant-Klun, I.; Verdenik, I.; Požlep, B.; Vrtačnik-Bokal, E. Septate, subseptate and arcuate uterus decrease pregnancy and live birth rates in ivf/icsi. Reprod. Biomed. Online 2010, 21, 700–705. [Google Scholar] [CrossRef] [Green Version]
- Sugiura-Ogasawara, M.; Ozaki, Y.; Kitaori, T.; Kumagai, K.; Suzuki, S. Midline uterine defect size is correlated with miscarriage of euploid embryos in recurrent cases. Fertil. Steril. 2010, 93, 1983–1988. [Google Scholar] [CrossRef]
- Saravelos, S.H.; Cocksedge, K.A.; Li, T.C. The pattern of pregnancy loss in women with congenital uterine anomalies and recurrent miscarriage. Reprod. Biomed. Online 2010, 20, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.; Hu, W. Pregnancy complications and obstetric outcomes among women with congenital uterine malformations. Int. J. Gynecol. Obstet. 2010, 109, 159–160. [Google Scholar] [CrossRef]
- Ban-Frangez, H.; Tomazevic, T.; Virant-Klun, I.; Verdenik, I.; Ribic-Pucelj, M.; Bokal, E.V. The outcome of singleton pregnancies after ivf/icsi in women before and after hysteroscopic resection of a uterine septum compared to normal controls. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 146, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Zlopasa, G.; Skrablin, S.; Kalafatić, D.; Banović, V.; Lesin, J. Uterine anomalies and pregnancy outcome following resectoscope metroplasty. Int. J. Gynaecol. Obstet. 2007, 98, 129–133. [Google Scholar] [CrossRef]
- Erez, O.; Dukler, D.; Novack, L.; Rozen, A.; Zolotnik, L.; Bashiri, A.; Koifman, A.; Mazor, M. Trial of labor and vaginal birth after cesarean section in patients with uterine müllerian anomalies: A population-based study. Am. J. Obstet. Gynecol. 2007, 196, 537.e1–537.e11. [Google Scholar] [CrossRef] [PubMed]
- Woelfer, B.; Salim, R.; Banerjee, S.; Elson, J.; Regan, L.; Jurkovic, D. Reproductive outcomes in women with congenital uterine anomalies detected by three-dimensional ultrasound screening. Obstet. Gynecol. 2001, 98, 1099–1103. [Google Scholar] [PubMed]
- Ravasia, D.J.; Brain, P.H.; Pollard, J.K. Incidence of uterine rupture among women with mullerian duct anomalies who attempt vaginal birth after cesarean delivery. Am. J. Obstet. Gynecol. 1999, 181, 877–881. [Google Scholar] [CrossRef]
- Cooney, M.J.; Benson, C.B.; Doubilet, P.M. Outcome of pregnancies in women with uterine duplication anomalies. J. Clin. Ultrasound 1998, 26, 3–6. [Google Scholar] [CrossRef]
- Acién, P. Reproductive performance of women with uterine malformations. Hum. Reprod. 1993, 8, 122–126. [Google Scholar] [CrossRef]
- Shuiqing, M.; Xuming, B.; Jinghe, L. Pregnancy and its outcome in women with malformed uterus. Chin. Med. Sci. J. 2002, 17, 242–245. [Google Scholar]
- Zupi, E.; Solima, E.; Marconi, D.; Valli, E.; Romanini, C. Uterine anomalies prevalence and reproductive outcome in women undergoing diagnostic hysteroscopy. Gynaecol. Endosc. 1996, 5, 147–150. [Google Scholar]
- Maneschi, F.; Zupi, E.; Marconi, D.; Valli, E.; Romanini, C.; Mancuso, S. Hysteroscopically detected asymptomatic mullerian anomalies: Prevalence and reproductive implications. J. Reprod. Med. Obstet. Gynecol. 1995, 40, 684–688. [Google Scholar]
- Ben-Rafael, Z.; Seidman, D.S.; Recabi, K.; Bider, D.; Mashiach, S. Uterine anomalies: A retrospective, matched-control study. J. Reprod. Med. Obstet. Gynecol. 1991, 36, 723–727. [Google Scholar]
- Sorensen, S.S.; Trauelsen, A.G.H. Obstetric implications of minor mullerian anomalies in oligomenorrheic women. Am. J. Obstet. Gynecol. 1987, 156, 1112–1118. [Google Scholar] [CrossRef]
- Forde, P.; O’Driscoll, D.; Murphy, H. Pregnancy associated with uterine abnormality. Ir. Med. J. 1978, 71, 164–165. [Google Scholar]
- Simón, C.; Moreno, C.; Remohí, J.; Pellicer, A. Cytokines and embryo implantation. J. Reprod. Immunol. 1998, 39, 117–131. [Google Scholar] [CrossRef]
- Candiani, G.B.; Fedele, L.; Zamberletti, D.; De Virgiliis, D.; Carinelli, S. Endometrial patterns in malformed uteri. Acta Eur. Fertil. 1983, 14, 311–318. [Google Scholar] [PubMed]
- Fedele, L.; Bianchi, S.; Marchini, M.; Franchi, D.; Tozzi, L.; Dorta, M. Ultrastructural aspects of endometrium in infertile women with septate uterus. Fertil. Steril. 1996, 65, 750–752. [Google Scholar] [CrossRef]
- Dabirashrafi, H.; Bahadori, M.; Mohammad, K.; Alavi, M.; Moghadami-Tabrizi, N.; Zandinejad, K.; Ghafari, V. Septate uterus: New idea on the histologic features of the septum in this abnormal uterus. Am. J. Obstet. Gynecol. 1995, 172, 105–107. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Jayaprakasan, K.; Zamora, J.; Thornton, J.G.; Raine-Fenning, N.; Coomarasamy, A. The prevalence of congenital uterine anomalies in unselected and high-risk populations: A systematic review. Hum. Reprod. Update 2011, 17, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravelos, S.H.; Cocksedge, K.A.; Li, T.C. Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: A critical appraisal. Hum. Reprod. Update 2008, 14, 415–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcal, L.; Nothaft, M.A.; Coelho, F.; Volpato, R.; Iyer, R. Mullerian duct anomalies: Mr imaging. Abdom. Imaging 2011, 36, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Grimbizis, G.F.; Di Spiezio Sardo, A.; Saravelos, S.H.; Gordts, S.; Exacoustos, C.; Van Schoubroeck, D.; Bermejo, C.; Amso, N.N.; Nargund, G.; Timmerman, D.; et al. The thessaloniki eshre/esge consensus on diagnosis of female genital anomalies. Hum. Reprod. 2016, 31, 2–7. [Google Scholar] [CrossRef]
- Valle, R.F.; Ekpo, G.E. Hysteroscopic metroplasty for the septate uterus: Review and meta-analysis. J. Minim. Invasive Gynecol. 2013, 20, 22–42. [Google Scholar] [CrossRef]
- Kowalik, C.R.; Goddijn, M.; Emanuel, M.H.; Bongers, M.Y.; Spinder, T.; de Kruif, J.H.; Mol, B.W.; Heineman, M.J. Metroplasty versus expectant management for women with recurrent miscarriage and a septate uterus. Cochrane Database Syst. Rev. 2011, 6, Cd008576. [Google Scholar] [CrossRef]
- Rikken, J.F.; Kowalik, C.R.; Emanuel, M.H.; Mol, B.W.; Van der Veen, F.; van Wely, M.; Goddijn, M. Septum resection for women of reproductive age with a septate uterus. Cochrane Database Syst. Rev. 2017, 1, Cd008576. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.A.; Saravelos, S.H.; Li, T.C.; Jayaprakasan, K. Reproductive implications and management of congenital uterine anomalies: Scientific impact paper no. 62 november 2019. BJORG 2020, 127, e1–e13. [Google Scholar] [CrossRef]
- Esteban Manchado, B.; Lopez-Yarto, M.; Fernandez-Parra, J.; Rodriguez-Oliver, A.; Gonzalez-Paredes, A.; Laganà, A.S.; Garzon, S.; Haimovich, S. Office hysteroscopic metroplasty with diode laser for septate uterus: A multicenter cohort study. Minim. Invasive Ther. Allied Technol. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bosteels, J.; Weyers, S.; Kasius, J.; Broekmans, F.J.; Mol, B.W.; D’Hooghe, T.M. Anti-adhesion therapy following operative hysteroscopy for treatment of female subfertility. Cochrane Database Syst. Rev. 2015, 11, Cd011110. [Google Scholar] [CrossRef] [PubMed]
- Nappi, L.; Falagario, M.; Angioni, S.; De Feo, V.; Bollino, M.; Sorrentino, F. The use of hysteroscopic metroplasty with diode laser to increase endometrial volume in women with septate uterus: Preliminary results. Gynecol. Surg. 2021, 18, 11. [Google Scholar] [CrossRef]
- Nappi, L.; Sorrentino, F.; Angioni, S.; Pontis, A.; Greco, P. The use of laser in hysteroscopic surgery. Minerva Ginecol. 2016, 68, 722–726. [Google Scholar]


| Author & Year | Type of Study | Population | Exclusion Criteria | Mode of Conception | Women with CUA | Women with a Normal Uterus | CUA Types | Mode of Diagnosis | Method of Classification of Anomalies |
|---|---|---|---|---|---|---|---|---|---|
| Forde, 1978 | Retrospective | All deliveries | Not reported | Not reported | n = 17 deliveries | n = 4394 deliveries | Arcuate, bicornuate, didelphys | Hysterography | Jarcho (1946) |
| Sorensen and Trauelsen, 1987 | Retrospective | Infertile women | Women that could not be traced and women with major uterine anomalies | Not reported | n = 50 | n = 141 | Arcuate | HSG | According to the degrees of fundal excavation, if H/L ratio ≥ 0.1, minor Müllerian anomalies were diagnosed. (H: the distance from the nadir of the fundal indentation to the line connecting the summits of the uterine horns L: the length of this line) |
| Ben-Rafael, 1991 | Retrospective | (A) Infertile women or (B) women who have experienced recurrent fetal loss | Women with arcuate uterus or subseptate uterus | Not reported | (A) n = 27 (B) n = 40 | (A) n = 89 (B) n = 41 | Unicornuate, bicornuate, didelphys | HSG | Not reported |
| Acien, 1993 | Retrospective and prospective | Women with uterine or genitourinary anomalies | Patients with Rokitansky syndrome, hypoplastic uterus, and incomplete case studies | Not reported | Uterine or uterovaginal malformations, n = 176 | Other genital and/or urinary anomalies, but normal uterus, n = 28 | Arcuate, unicornuate, bicornuate, septate, didelphys | Clinical examination, ultrasound, HSG, pyelography | The criteria of Jarcho (1946), Buttram and Gibbons (1979), American Fertility Society (1988) |
| Maneschi, 1995 | Prospective | All women with abnormal uterine bleeding | Women who had never attempted to conceive or filled the incomplete questionnaire, women with a submucosal myoma or unknown shape of the uterine cavity at the time of pregnancy | Not reported | n = 33 (arcuate:21, sepate/bicornuate:12) | n = 216 | Arcuate, septate/bicornuate (not differentiated) | Hysteroscopy | Arcuate uterus: fundal protrusion of <20% of the uterine cavity |
| Zupi, 1996 | Retrospective | Patients who underwent outpatient hysteroscopy for reasons other than infertility | Women who did not know the state of their uterine cavity at conception, women who had undergone voluntary abortion, women with submucous fibroids or synechiae | Not reported | n = 64 (arcuate:49, sepate/bicornuate:13, unicornuate: 2) | n = 763 | Arcuate, septate/bicornuate, unicornuate | Hysteroscopy | Septate or bicornuate uterus: a double cavity, separated by a mid-cavity septum that covered at least one third of the uterine cavity Arcuate uterus: a uterus with a fundal notch consisting of less than one third of the cavity (Fedele et al. 1990) |
| Cooney, 1998 | Retrospective | All pregnancies with fetal cardiac activity in the first trimester | Equivocal images and a lack of independent confirmation | Not reported | n = 22 | n = 66 | Uterine duplication anomaly (bicornuate, septate, didelphys) | Surgical procedure before or after the sonogram, hysteroscopy | Not reported |
| Ravasia, 1999 | Retrospective | Women undergoing trial of labor after previous caesarean section | Not reported. | Not reported. | n = 25 | n = 1788 | Unicornuate, bicornuate, septate, didelphys | Laparoscopy, hysteroscopy after an abnormal result of US or HSG, during first cesarean delivery | Not reported |
| Woelfer, 2001 | Prospective | Women referred for gynecologic examination | Ongoing pregnancy, history of infertility or recurrent miscarriage, presence of uterine fibroids that distorted the uterine cavity, and previous hysterectomy or myomectomy. | Not reported. | n = 106 | n = 983 | Arcuate, subseptate, bicornuate | 2D- & 3D-US | American Fertility Society (1988) |
| Shuiqing, 2002 | Retrospective | Women with CUA and women with other urinary or genital anomalies but with normal uterus | Not reported | Not reported | n = 153 | n = 27 | Unicornuate, bicornuate, septate, didelphys | History, clinical examination, high resolution US, HSG, hysteroscopy, laparoscopy, laparotomy | Buttram (1983) |
| Erez, 2007 | Retrospective | All patients attempted vaginal birth after cesarean section after previous cesarean section | Multiple pregnancies, more than one previous cesarean section and known congenital and/or chromosomal fetal anomalies | Not reported | n = 165 | n = 5406 | Arcuate, unicornuate, bicornuate, septate, didelphys | All the surgery reports of the primary cesarean section | American Fertility Society (1988) |
| Zlopasa, 2007 | Retrospective | All pregnant women | Twin gestation, chorioamnionitis, presence of submucosal myomas, fetal chromosomopathy, maternal diabetes, or fertilization in vitro | Exclude IVF | n = 130 | n = 182 | Arcuate, unicornuate, bicornuate, subseptate, septate, didelphys | Previous surgery, sonohysterographic evaluation, laparoscopy with hysterography, or hysteroscopy | American Fertility Society (1988) |
| Ban-Frangez, 2009 | Retrospective | Women with a singleton intrauterine pregnancy with fetal heart beat after IVF or ICSI | Extrauterine pregnancies, multiple pregnancies and cases with an empty gestational sac | IVF/ICSI | n = 31 | n = 62 | Arcuate, septate | 2D-US without intrauterine saline infusion, hysteroscopy | American Fertility Society (1988) |
| Liang, 2010 | Retrospective | All pregnant women (from week 28 of pregnancy to day 7 postdelivery) | Women with medical complications and history of cesarean delivery | Not reported | n = 715 | n = 68,979 | Unicornuate, bicornuate, incomplete septate, complete septate, didelphys | Patient’s history of illness, physical examination, high resolution US, HSG, hysteroscopy, laparoscopy, laparotomy | Buttram and Gibbons (1979) |
| Saravelos, 2010 | Retrospective | Women who have experienced recurrent miscarriages | Patients who had received medical treatment (e.g., low molecular weight heparin, acetyl-salicylic acid, steroids) or surgery (e.g., septotomy, Strassman’s metroplasty, cervical cerclage) | Not reported | n = 56 | n = 107 | Arcuate, unicornuate, bicornuate, septate, didelphys | 2D-US, HSG, hysteroscopy, laparoscopy | American Fertility Society (1988) |
| Sugiura-Ogasawara, 2010 | Retrospective | Patients with a history of two or more consecutive miscarriages | Patients with structural chromosome abnormalities. | Not reported | n = 42 | n = 1528 | Unicornuate, bicornuate, septate, didelphys | Laparoscopy, laparotomy and/or magnetic resonance imaging | American Fertility Society (1988) & Tompkin’s index |
| Tomazevic, 2010 | Retrospective | Infertile women who underwent embryo transfers in IVF/ICSI cycles | Not reported | IVF/ICSI | Embryo transfers before hysteroscopic resection, n = 289 | Embryo transfers, n = 578 | Arcuate, subseptate, septate | 2D-US without intrauterine saline infusion, hysteroscopy | American Fertility Society (1988) If the uterine septum measured 1.3~1.5 cm in length, it was defined as arcuate uterus. |
| Zhang, 2010 | Retrospective | All patients referred for delivery | Multiple pregnancies, congenital and/or chromosomal fetal anomalies | Infertility treatment or natural cycle | n = 116 | n = 270 | Arcuate, unicornuate, bicornuate, septate, didelphys | Previous surgery or sonohysterography, laparoscopy with hysterography, hysteroscopy | American Fertility Society (1988) |
| Hua, 2011 | Retrospective | all consecutive singleton pregnancies undergoing routine anatomic survey | Patients with incomplete follow-up data | Not reported | n = 203 | n = 66,753 | Unicornuate, bicornuate, septate, didelphys | Not reported | Not reported |
| Jayaprakasan, 2011 | Prospective | Women who have undergone IVF/ICSI | Women who have one or more uterine fibroids or polyps distorting the endometrial cavity or if the ultrasound view was unclear and not good enough to allow a definitive diagnosis to be made | IVF/ICSI | n = 76 | n = 364 | Arcuate, unicornuate, bicornuate, subseptate, septate, T-shaped uterus | 2D-, 3D-US | Modified American Fertility Society classification proposed by Salim et al. (2003) |
| Fox, 2014 | Retrospective | Women with singleton pregnancies ≥ 22 weeks delivered | Not reported | ART & natural cycle | n = 158 | n = 158 | Arcuate, unicornuate, bicornuate, septate, didelphys, T-shaped uterus | Saline infusionsonohysterogram, MRI, hysteroscopy, laparoscopy | American Fertility Society (1988) |
| Takami, 2014 | Retrospective | Women who delivered a live singleton baby after 22 gestational weeks | Women whose fetuses had congenital anomalies or with a history of surgery | Infertility treatment or natural cycle | n = 80 | n = 5763 | Unicornuate, bicornuate, subseptate, incomplete septate, complete septate, didelphys | US, internal examinations during pregnancy, findings at cesarean section, MRI, HSG | American Fertility Society (1988) |
| Hiersch, 2016 | Retrospective | Women who delivered at or beyond 24 weeks of gestation | Women who underwent any surgical treatment of Müllerian anomalies, pregnancies with uncertain pregnancy dating or those complicated by stillbirth or major fetal anomalies | IVF, IUI or natural cycle | n = 243 | n = 486 | Unicornuate, bicornuate, septate, didelphys | HSG, US, hysterosonography, CT, MRI, hysteroscopy, laparoscopy, laparotomy | American Fertility Society (1988) |
| Li, 2017 | Retrospective | Infertile women who achieved pregnancy with IVF cycles | Patients with donor oocytes, preimplantation genetic diagnosis, preimplantation genetic screening, parental chromosomal abnormalities, spontaneous/selective reduction, triplet pregnancies, induced labor for congenital fetal structural or chromosomal abnormalities, uterine fibroids or polyps distorting the endometrial cavity | IVF-ET | n = 238 | n = 818 | Unicornuate | 3D-US, HSG, hysteroscopy, laparoscopy | ESHRE/ESGE classification system |
| Mastrolia, 2017 | Retrospective | All women carrying a singleton pregnancy who delivered | Patients with multiple pregnancies or missing data | ART & natural cycle | n = 444 | n = 279,662 | Bicornuate | Workup for infertility or recurrent pregnancy loss, during pregnancy, or at the time of cesarean delivery | American Fertility Society (1988) |
| Ozgur, 2017 | Retrospective | Patients who had first infertility consultations/ICSI with fresh and cryopreserved embryo transfer | Patients with intrauterine abnormalities or who underwent therapeutic surgery | IVF/ICSI | n = 50 | n = 100 | Unicornuate | 2D-US, HSG, saline-infused sonography, hysteroscopy, laparoscopy | American Fertility Society (1988) |
| Chen, 2018 | Retrospective | Women who underwent IVF/ICSI cycles | Other uterine malformations (septum, unicornuate uterus class IVa, bicornuate uterus), endometrial lesions (polyps, endometrial hyperplasia, intrauterine adhesions), uterine fibroids distorting uterine cavity diagnosed by TVS or hysteroscopy, sonographic features of adenomyosis, chromosomal abnormality of male or female Partner, patients who undertook a donor oocyte program or had preimplantation genetic diagnosis/preimplantation genetic screening, patients who had cancelled IVF cycle that did not result in embryo transfer | IVF/ICSI | n = 342 | n = 1026 | Unicornuate | 3D-US, hysteroscopy, laparoscopy, MRI | ESHRE/ESGE classification system |
| Mastrolia, 2018 | Retrospective | All women who have delivered | Patients with multiple pregnancies or missing data | ART & natural cycle | n = 1099 | n = 279,662 | All CUA | Workup for infertility or recurrent pregnancy loss, during pregnancy or at the time of cesarean delivery | American Fertility Society (1988) |
| Ples, 2018 | Retrospective | Patients with infertility, who underwent ART | Patients with associated uterine pathology one or more polyps, synechiae, or submucosal myoma, patients in whom the ultrasound image was not sufficient for a definitive diagnosis | ART | n = 52 | n = 148 | Dysmorphic uterus, septate, Bicorporeal uterus, aplastic uterus | 2D & 3D-US | ESHRE/ESGE classification system |
| Prior, 2018 | Prospective | Women with subfertility, defined as failure to conceive after regular unprotected sexual intercourse for 2 years, who underwent ART | The presence of fibroids, intrauterine device or polyps distorting the cavity, Asherman’s syndrome, previous hysteroscopic surgery and/or poor quality of images | IVF/ICSI | n = 432 | n = 1943 | Arcuate, unicornuate, bicornuate, subseptate, septate, didelphys | 2D & 3D-US | Modified American Fertility Society classification proposed by Salim et al. (2003) |
| Cahen-Peretz, 2019 | Retrospective | All women who delivered | Multifetal pregnancies, unknown gestational age, gestational age of less than 24 weeks upon delivery, fetuses with congenital malformations | ART & natural cycle | n = 1251 | n = 255,048 | Arcuate, unicornuate, bicornuate, septate, didelphys | US, HSG, hysterosonography, MRI, hysteroscopy, laparoscopy, laparotomy | Not reported |
| Chen, 2019 | Retrospective | Patients receiving IVF/ICSI | Oocyte donor treatment cycles, abnormal uterine bleeding, endometrial fibroids or polyps, intrauterine adhesion, premature ovary insufficiency, polycystic ovary syndrome, consecutive spontaneous abortion history ≥ three times | IVF/ICSI | n = 160 | n = 160 | Unicornuate | TVS, HSG, hysteroscopy, laparoscopy | Not reported |
| Neal, 2019 | Prospective | Infertile patients planning to undergo a single thawed euploid blastocyst transfer | Use of a gestational carrier, body mass index 40 kg/m2 or over, previously diagnosed uterine anomalies of the Class U2–U5 variety, history of myomectomy, communicating hydrosalpinx | IVF-ET | n = 10 | n = 472 | T-shaped uterus | 3D-US | ESHRE/ESGE classification system |
| Ouyang, 2020 | Retrospective | All patients successfully achieved first pregnancies and delivered at ≥22 weeks after IVF-ET | Maternal age ≥ 40 years old, body mass index outside the range of 18–28, only one ovary detected, uterine fibroids or polyps distorting the endometrial cavity, received donor oocytes, preimplantation genetic diagnosis, preimplantation genetic screening, parental chromosomal abnormalities, spontaneous reduction, monochorionic twin or triplet pregnancies, early or late miscarriage, ectopic pregnancy, induced labour, abnormal chromosomal karyotypes, other urinary tract malformations | IVF/ICSI | n = 206 | n = 314 | Unicornuate | 3D-US, HSG, hysteroscopy, laparoscopy, laparotomy | ESHRE/ESGE classification system |
| Kong, 2021 | Retrospective | Women who underwent first IVF/ICSI cycles | Severe systemic disease, presence of uterine or pelvic disease, such as severe intrauterine adhesions, uterine adenomyosis, or untreated hydrosalpinx, presence of a chromosomal abnormality in the male or female partner, participation in a donor oocyte program or presence of a preimplantation genetic test, giving up treatment midway or no follow up information availability | IVF/ICSI | n = 58 | n = 174 | Bicornuate | Hysteroscopy combined with laparoscopy surgery or cesarean section, MRI, 3D-US, 2D-US combined with hysteroscopy and/or HSG under X-ray | American Fertility Society (1988) |
| Zambrotta, 2021 | Retrospective | Women with a history of one or more pregnancies or current pregnancy with diagnosis of uterine anomalies | Women who have never had confirmed pregnancy by beta-HGC with first trimester ultrasound and who did not have specific diagnosis of uterine malformations, who underwent ART cycles and who experienced at least one miscarriage | Natural cycle | n = 29 | n = 100 | Arcuate, unicornuate, bicornuate, incomplete septate, complete septate, didelphys | US | American Fertility Society (1988) |
| Zhang, 2021 | Retrospective | Infertile women who underwent IVF/ICSI | Other types of uterine malformations (mediastinal uterus, bicorne uterus, etc., diminished ovarian reserve, endometrial lesions, uterine fibroids, adenomyosis, polycystic ovary syndrome, recurrent miscarriage, chromosomal abnormalities | IVF/ICSI | n = 109 | n = 2390 | Unicornuate | TVS, HSG, hysteroscopy, laparoscopy | Not reported |
| All CUA | Arcuate Uterus | Canalization Defects | Unification Defects | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Subseptate | Septate | All | Unicornuate | Bicornuate | Didelphys | All | |||
| Clinical pregnancy rate | 0.87 (0.70, 1.08) | 1.00 (0.64, 1.58) | 0.73 (0.28, 1.92) | 0.45 (0.21, 0.95) | 0.59 (0.32, 1.08) | 0.75 (0.58, 0.99) | 0.57 (0.32, 1.03) | 0.36 (0.09, 1.39) | 0.72 (0.57, 0.90) |
| Live births | 0.47 (0.33, 0.69) | 0.45 (0.22, 0.92) | 0.18 (0.02, 1.27) * | 0.25 (0.09, 0.75) | 0.24 (0.10, 0.57) | 0.57 (0.34, 0.96) | 0.61 (0.36, 1.02) | 0.15 (0.01, 2.56) * | 0.60 (0.40, 0.90) |
| First trimester miscarriage | 1.79 (1.34, 2.40) | 1.38 (0.88, 2.17) | 4.36 (2.64, 7.21) | 2.55 (1.33, 4.91) | 3.32 (1.96, 5.60) | 1.45 (0.85, 2.48) | 2.59 (1.25, 5.35) | 1.26 (0.33, 4.76) | 1.77 (1.18, 2.65) |
| Second trimester miscarriage | 2.92 (1.35, 6.32) | 2.01 (1.03, 3.93) | 1.90 (0.54, 6.75) | 4.33 (2.52, 7.43) | 3.38 (1.94, 5.88) | 2.10 (0.95, 4.61) | 2.71 (1.40, 5.23) | 1.72 (0.60, 4.90) | 2.28 (1.45, 3.60) |
| Ectopic pregnancy | 1.28 (0.81, 2.02) | 0.96 (0.36, 2.57) | - | 1.69 (0.65, 4.40) | 1.69 (0.65, 4.40) | 1.61 (0.79, 3.29) | 1.01 (0.22, 4.69) | 3.75 (0.66, 21.39) | 1.60 (0.92, 2.78) |
| Preterm delivery | 2.98 (2.43, 3.65) | 1.62 (0.86, 3.04) | 3.15 (1.34, 7.40) | 2.93 (2.01, 4.28) | 3.11 (2.24, 4.32) | 2.83 (1.92, 4.19) | 3.69 (2.60, 5.22) | 4.93 (3.60, 6.75) | 3.50 (2.74, 4.46) |
| PPROM | 3.50 (2.22, 5.54) | - | - | 4.66 (1.79, 12.15) | 4.66 (1.79, 12.15) | 5.42 (1.80, 16.30) | 3.77 (1.56, 9.08) | 5.80 (1.89, 17.77) | 4.66 (2.83, 7.69) |
| Malpresentation | 9.10 (5.88, 14.08) | 3.27 (1.66, 6.44) | 11.42 (3.74, 34.86) | 11.49 (5.24, 25.17) | 11.39 (6.24, 20.78) | 8.09 (3.14, 20.84) | 10.87 (6.68, 17.68) | 7.20 (3.09, 16.74) | 8.68 (5.82, 12.95) |
| Cesarean section | 2.87 (1.56, 5.26) | 2.22 (1.07, 4.61) | 5.91 (1.59, 21.95) | 4.84 (2.33, 10.02) | 5.02 (2.77, 9.10) | 1.24 (0.76, 2.03) | 5.23 (2.11, 12.96) | 7.55 (2.40, 23.72) | 3.91 (2.14, 7.13) |
| Preeclampsia | 1.25 (1.07, 1.46) | 0.76 (0.18, 3.22) | - | 0.25 (0.03, 1.82) | 0.25 (0.03, 1.82) | 1.27 (0.29, 5.45) | 2.83 (0.29, 27.84) | 0.45 (0.06, 3.31) | 1.49 (0.52, 4.30) |
| Placental abruption | 3.12 (1.58, 6.18) | 4.56 (1.03, 20.06) | 17.45 (5.05, 60.22) * | 5.33 (1.50, 18.95) | 9.22 (3.42, 24.82) | 7.78 (1.99, 30.45) | 6.53 (1.96, 21.78) | 2.68 (0.51, 14.17) | 6.53 (3.39, 12.61) |
| Placenta previa | 1.56 (0.60, 4.07) | 1.68 (0.22, 13.06) | - | 1.21 (0.16, 9.33) * | 1.21 (0.16, 9.33) | 2.71 (0.35, 21.28) | 3.59 (1.89, 6.82) | 2.18 (0.28, 17.07) | 3.37 (1.88, 6.06) |
| Postpartum hemorrhage | 1.02 (0.67, 1.55) | - | - | - | - | - | - | - | - |
| Recurrent pregnancy loss | 2.61 (2.31, 2.94) | - | 1.65 (0.09, 32.05) * | 2.69 (2.05, 3.52) | 0.37 (0.02, 6.25) * | 2.63 (2.01, 3.44) | |||
| Cervical imcompetence | 7.94 (3.81, 16.55) | - | - | 9.13 (1.97, 42.33) * | 9.13 (1.97, 42.33) | 13.69 (2.10, 89.18) * | 10.04 (6.30, 15.99) | 5.49 (1.63, 18.44) | 9.04 (6.11, 13.37) |
| IUFD | 2.06 (1.36, 3.11) | - | - | 1.04 (0.12, 9.15) | 1.04 (0.12, 9.15) | 2.40 (1.27, 4.53) | 3.09 (0.38, 24.79) | 3.30 (0.38, 28.95) | 2.50 (1.39, 4.50) |
| IUGR or SGA | 2.53 (1.77, 3.62) | 3.77 (0.92, 15.46) | 2.40 (1.13, 5.09) | 1.90 (0.89, 4.08) | 2.14 (1.26, 3.65) | 3.50 (1.24, 9.91) | 2.84 (1.68, 4.80) | 4.03 (2.00, 8.12) | 3.30 (2.29, 4.75) |
| Perinatal mortality | 2.17 (1.46, 3.23) | 2.11 (0.79, 5.63) | 2.51 (0.82, 7.69) | 2.57 (1.08, 6.08) | 2.55 (1.29, 5.04) | 3.85 (1.61, 9.20) | 3.17 (2.08, 4.84) | 1.75 (0.77, 3.96) | 2.93 (2.15, 4.00) |
| Low birth weight | 1.59 (0.94, 2.68) | 1.50 (0.70, 3.25) | - | 1.73 (0.91, 3.29) | 1.73 (0.91, 3.29) | 1.84 (1.08, 3.14) | 1.91 (1.12, 3.27) | 2.87 (1.38, 5.97) | 1.99 (1.38, 2.87) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-A.; Kim, H.S.; Kim, Y.-H. Reproductive, Obstetric and Neonatal Outcomes in Women with Congenital Uterine Anomalies: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 4797. https://doi.org/10.3390/jcm10214797
Kim M-A, Kim HS, Kim Y-H. Reproductive, Obstetric and Neonatal Outcomes in Women with Congenital Uterine Anomalies: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(21):4797. https://doi.org/10.3390/jcm10214797
Chicago/Turabian StyleKim, Min-A, Hyo Sun Kim, and Young-Han Kim. 2021. "Reproductive, Obstetric and Neonatal Outcomes in Women with Congenital Uterine Anomalies: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 21: 4797. https://doi.org/10.3390/jcm10214797
APA StyleKim, M.-A., Kim, H. S., & Kim, Y.-H. (2021). Reproductive, Obstetric and Neonatal Outcomes in Women with Congenital Uterine Anomalies: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(21), 4797. https://doi.org/10.3390/jcm10214797






