Sleep Disturbances and Phenoconversion in Patients with REM Sleep Behavior Disorder
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Clinical Assessment
2.3. Subject Questionnaires
2.4. Polysomnography
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics in iRBD vs. sRBD
3.2. Polysomnographic Findings and RSWA in iRBD vs. sRBD
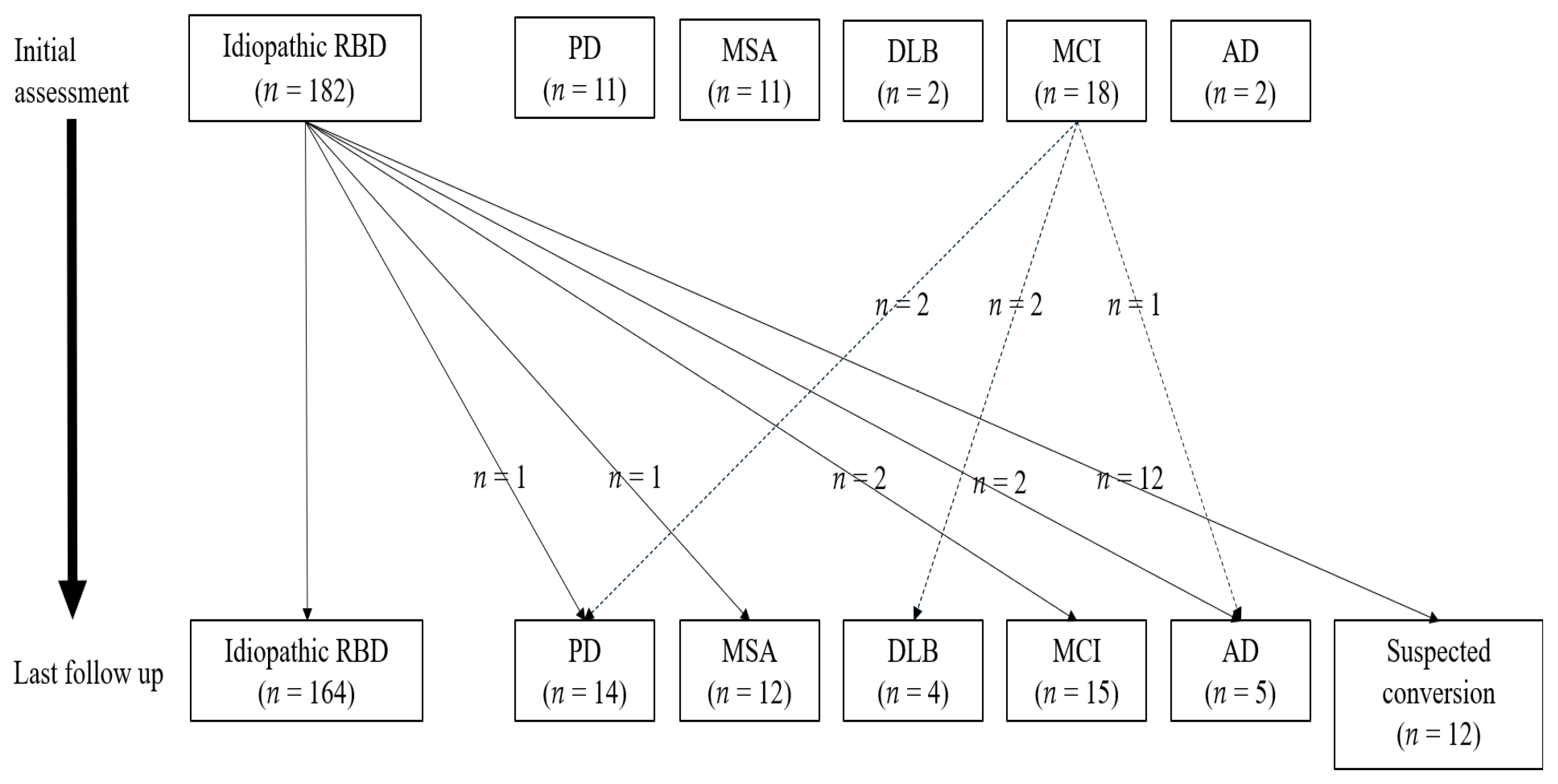
3.3. Converters vs. Non-Converters
4. Discussion
4.1. Subjective Sleep Complaints (Self-Reported Questionnaires)
4.2. Sleep Architecture
4.3. Comorbid Sleep Disorders
4.3.1. Periodic Limb Movements during Sleep
4.3.2. Obstructive Sleep Apnea
5. Limitations and Strengths
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schenck, C.H.; Mahowald, M.W. REM sleep behavior disorder: Clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 2002, 25, 120–138. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Niu, L.; Liu, X.; Liu, Y.; Li, S.; Yu, H.; Le, W. Rapid Eye Movement Sleep Behavior Disorder and Neurodegenerative Diseases: An Update. Aging Dis. 2020, 11, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Roguski, A.; Rayment, D.; Whone, A.L.; Jones, M.W.; Rolinski, M. A Neurologist’s Guide to REM Sleep Behavior Disorder. Front. Neurol. 2020, 11, 610. [Google Scholar] [CrossRef]
- Sasai-Sakuma, T.; Takeuchi, N.; Asai, Y.; Inoue, Y.; Inoue, Y. Prevalence and clinical characteristics of REM sleep behavior disorder in Japanese elderly people. Sleep 2020, 43, zsaa024. [Google Scholar] [CrossRef] [PubMed]
- Högl, B.; Stefani, A.; Videnovic, A. Idiopathic REM sleep behaviour disorder and neurodegeneration—An update. Nat. Rev. Neurol. 2018, 14, 40–55. [Google Scholar] [CrossRef] [PubMed]
- McCarter, S.J.; St Louis, E.K.; Boeve, B.F. REM sleep behavior disorder and REM sleep without atonia as an early manifestation of degenerative neurological disease. Curr. Neurol. Neurosci. Rep. 2012, 12, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, L.; Liu, J.H.; Zhan, S.Q. Relationships between Rapid Eye Movement Sleep Behavior Disorder and Neurodegenerative Diseases: Clinical Assessments, Biomarkers, and Treatment. Chin. Med. J. 2018, 131, 966–973. [Google Scholar] [CrossRef]
- Reichmann, H. Premotor Diagnosis of Parkinson’s Disease. Neurosci. Bull. 2017, 33, 526–534. [Google Scholar] [CrossRef]
- Abbott, S.M.; Videnovic, A. Sleep Disorders in Atypical Parkinsonism. Mov. Disord. Clin. Pract. 2014, 1, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Iranzo, A.; Hu, M.; Högl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain J. Neurol. 2019, 142, 744–759. [Google Scholar] [CrossRef]
- Boeve, B.F. Idiopathic REM sleep behaviour disorder in the development of Parkinson’s disease. Lancet Neurol. 2013, 12, 469–482. [Google Scholar] [CrossRef] [Green Version]
- Sixel-Döring, F.; Trautmann, E.; Mollenhauer, B.; Trenkwalder, C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology 2011, 77, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Hu, M.T.M. Sleep Disturbance as Potential Risk and Progression Factor for Parkinson’s Disease. J. Parkinsons Dis. 2019, 9, 603–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhang, J.; Lam, S.P.; Tang, X.; Wing, Y.K. Clinical Biomarkers of Neurodegeneration in REM Sleep Behavior Disorder. J. Sleep Med. 2015, 12, 27–33. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Chen, Y.-T.; Tseng, C.-M.; Wu, L.-A.; Perng, D.-W.; Chen, Y.-M.; Chen, T.-J.; Chang, S.-C.; Chou, K.-T. Sleep disorders and an increased risk of Parkinson’s disease in individuals with non-apnea sleep disorders: A population-based cohort study. J. Sleep Res. 2017, 26, 623–628. [Google Scholar] [CrossRef]
- Park, J.; Koh, S.B.; Kwon, K.Y.; Kim, S.J.; Kim, J.W.; Kim, J.S.; Park, K.W.; Paik, J.S.; Sohn, Y.H.; Ahn, J.Y.; et al. Validation Study of the Official Korean Version of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale. J. Clin. Neurol. 2020, 16, 633–645. [Google Scholar] [CrossRef]
- Wenning, G.K.; Tison, F.; Seppi, K.; Sampaio, C.; Diem, A.; Yekhlef, F.; Ghorayeb, I.; Ory, F.; Galitzky, M.; Scaravilli, T.; et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 1391–1402. [Google Scholar] [CrossRef]
- Kang, Y.; Na, D.L.; Hahn, S. Seoul Neurophsychological Screening Battery: Professional Manual; Human Brain Research & Consulting Co.: Seoul, Korean, 2003. [Google Scholar]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef] [Green Version]
- Gilman, S.; Wenning, G.K.; Low, P.A.; Brooks, D.J.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.W.; Colosimo, C.; Dürr, A.; Fowler, C.J.; et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack Jr, C.R.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [Green Version]
- McCarter, S.J.; St Louis, E.K.; Boswell, C.L.; Dueffert, L.G.; Slocumb, N.; Boeve, B.F.; Silber, M.H.; Olson, E.J.; Morgenthaler, T.I.; Tippmann-Peikert, M. Factors associated with injury in REM sleep behavior disorder. Sleep Med. 2014, 15, 1332–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.H.; Jeong, Y.S.; Lee, Y.J.; Hong, S.C.; Yoon, J.H.; Kim, J.K. The Korean version of the Sniffin’ stick (KVSS) test and its validity in comparison with the cross-cultural smell identification test (CC-SIT). Auris Nasus Larynx 2009, 36, 280–286. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.W.; Lee, J.H.; Son, H.K.; Lee, S.H.; Shin, C.; Johns, M.W. The reliability and validity of the Korean version of the Epworth sleepiness scale. Sleep Breath. Schlaf Atm. 2011, 15, 377–384. [Google Scholar] [CrossRef]
- Cho, Y.W.; Song, M.L.; Morin, C.M. Validation of a Korean version of the insomnia severity index. J. Clin. Neurol. 2014, 10, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Bastien, C.H.; Vallieres, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Sohn, S.I.; Kim, D.H.; Lee, M.Y.; Cho, Y.W. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. Schlaf Atm. 2012, 16, 803–812. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Sung, H.M.; Kim, J.B.; Park, Y.N.; Bai, D.S.; Lee, S.H.; Ahn, H.N. A study on the reliability and the validity of Korean version of the Beck Depression Inventory-II (BDI-II). J. Korean Soc. Biol. Ther. Psychiatry 2008, 14, 2001–2012. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the BDI-II; The Psychological Corporation: San Antoinio, TX, USA, 1996. [Google Scholar]
- Kim, J.R.; Song, P.; Joo, E.Y. Sex Differences in Obstructive Sleep Apnea by Bioelectrical Impedance Analysis. J. Clin. Neurol. 2021, 17, 283–289. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Abreu, A.R.; Bibbs, M.L.; DelRosso, L.; Harding, S.M.; Lloyd, R.M.; Marcus, C.L.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.6; American Academy of Sleep Medicine: Darien, IL, USA, 2020. [Google Scholar]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Frauscher, B.; Gschliesser, V.; Brandauer, E.; Ulmer, H.; Peralta, C.M.; Muller, J.; Poewe, W.; Hogl, B. Video analysis of motor events in REM sleep behavior disorder. Mov. Disord. Off. J. Mov. Disord. Soc. 2007, 22, 1464–1470. [Google Scholar] [CrossRef]
- Cygan, F.; Oudiette, D.; Leclair-Visonneau, L.; Leu-Semenescu, S.; Arnulf, I. Night-to-Night Variability of Muscle Tone, Movements, and Vocalizations in Patients with REM Sleep Behavior Disorder. J. Clin. Sleep Med. 2010, 06, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Arnulf, I. Excessive daytime sleepiness in parkinsonism. Sleep Med. Rev. 2005, 9, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Ratti, P.L.; Nègre-Pagès, L.; Pérez-Lloret, S.; Manni, R.; Damier, P.; Tison, F.; Destée, A.; Rascol, O. Subjective sleep dysfunction and insomnia symptoms in Parkinson’s disease: Insights from a cross-sectional evaluation of the French CoPark cohort. Parkinsonism Relat. Disord. 2015, 21, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-T.; Liu, F.-T.; Bi, Y.-L.; Shen, X.-N.; Xu, W.; Wang, J.; Tan, L.; Yu, J.-T. Associations of sleep characteristics with alpha-synuclein in cerebrospinal fluid in older adults. Ann. Clin. Transl. Neurol. 2020, 7, 2026–2034. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Postuma, R.B.; Gagnon, J.-F.; Pelletier, A.; Montplaisir, J.Y. Insomnia and somnolence in idiopathic RBD: A prospective cohort study. NPJ Parkinsons Dis. 2017, 3, 9. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, R.; Yang, L.; Sanford, L.D.; Tang, X. Polysomnographically measured sleep changes in idiopathic REM sleep behavior disorder: A systematic review and meta-analysis. Sleep Med. Rev. 2020, 54, 101362. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A. Sleep pattern in Parkinson’s disease. Acta Med. Pol. 1980, 21, 193–199. [Google Scholar] [PubMed]
- Poewe, W.; Högl, B. Parkinson’s disease and sleep. Curr. Opin. Neurol. 2000, 13, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Dauvilliers, Y.; Tafti, M.; Landolt, H.P. Catechol-O-methyltransferase, dopamine, and sleep-wake regulation. Sleep Med. Rev. 2015, 22, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Monti, D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med. Rev. 2007, 11, 113–133. [Google Scholar] [CrossRef]
- Olson, E.J.; Boeve, B.F.; Silber, M.H. Rapid eye movement sleep behaviour disorder: Demographic, clinical and laboratory findings in 93 cases. Brain 2000, 123 Pt 2, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Schenck, C.H.; Hurwitz, T.D.; Mahowald, M.W. Symposium: Normal and abnormal REM sleep regulation: REM sleep behaviour disorder: An update on a series of 96 patients and a review of the world literature. J. Sleep Res. 1993, 2, 224–231. [Google Scholar] [CrossRef]
- Fantini, M.L.; Michaud, M.; Gosselin, N.; Lavigne, G.; Montplaisir, J. Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology 2002, 59, 1889–1894. [Google Scholar] [CrossRef]
- Manconi, M.; Ferri, R.; Zucconi, M.; Fantini, M.L.; Plazzi, G.; Ferini-Strambi, L. Time structure analysis of leg movements during sleep in REM sleep behavior disorder. Sleep 2007, 30, 1779–1785. [Google Scholar] [CrossRef] [Green Version]
- Mahowald, M.W.; Schenck, C.H. Rem sleep without atonia-from cats to humans. Arch. Ital. Biol. 2004, 142, 469–478. [Google Scholar] [PubMed]
- Schenck, C.H.; Bundlie, S.R.; Mahowald, M.W. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology 1996, 46, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Schenck, C.H.; Boeve, B.F. The strong presence of REM sleep behavior disorder in PD. Clin. Res. Implic. 2011, 77, 1030–1032. [Google Scholar] [CrossRef]
- Sasai, T.; Inoue, Y.; Matsuura, M. Clinical significance of periodic leg movements during sleep in rapid eye movement sleep behavior disorder. J. Neurol. 2011, 258, 1971–1978. [Google Scholar] [CrossRef]
- Malhotra, A.; White, D.P. Obstructive sleep apnoea. Lancet 2002, 360, 237–245. [Google Scholar] [CrossRef]
- White, C.; Hill, E.A.; Morrison, I.; Riha, R.L. Diagnostic delay in REM sleep behavior disorder (RBD). J. Clin. Sleep Med. 2012, 8, 133–136. [Google Scholar] [CrossRef]
- Gabryelska, A.; Roguski, A.; Simpson, G.; Maschauer, E.L.; Morrison, I.; Riha, R.L. Prevalence of obstructive sleep apnoea in REM behaviour disorder: Response to continuous positive airway pressure therapy. Sleep Breath. 2018, 22, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iranzo, A.; Santamaría, J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep 2005, 28, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Alster, P.; Madetko, N.; Koziorowski, D.; Friedman, A. Progressive Supranuclear Palsy-Parkinsonism Predominant (PSP-P)-A Clinical Challenge at the Boundaries of PSP and Parkinson’s Disease (PD). Front. Neurol. 2020, 11, 180. [Google Scholar] [CrossRef]

| Variables | Total (n = 226) | IRBD (n = 182) | SRBD (n = 44) | p-Value (IRBD vs. SRBD) |
|---|---|---|---|---|
| Male, n (%) | 139 (61.5) | 110 (60.4) | 29 (65.9) | 0.605 † |
| Education, n (%) | ||||
| ≤12 years | 52 (23.0) | 40 (22.0) | 12 (27.3) | 0.428 † |
| ≥13 years | 159 (70.4) | 130 (71.4) | 29 (65.9) | |
| Alcohol, n (%) | 97 (42.9) | 81 (46.8) | 16 (38.1) | 0.388 † |
| Smoking, n (%) | 20 (8.8) | 17 (9.7) | 3 (7.1) | 0.771 † |
| Age at RBD onset, years | 59.4 ± 10.5 | 59.4 ± 11.1 | 59.0 ± 7.9 | 0.834 |
| Age at RBD diagnosis, years | 65.5 ± 9.9 | 65.0 ± 10.3 | 67.6 ± 7.7 | 0.107 |
| RBD duration, years | 5.9 ± 5.6 | 5.5 ± 5.6 | 7.5 ± 5.4 | 0.050 |
| Follow-up duration, years | 2.3 ± 2.6 | 2.0 ± 2.1 | 3.7 ± 3.9 | 0.010 * |
| Presence of DEB, n (%) | 162 (71.7) | 136 (74.7) | 26 (59.1) | 0.417 † |
| Frequency of DEB, days/month | ||||
| before treatment | 12.3 ± 11.1 | 11.5 ± 10.6 | 16.5 ± 12.8 | 0.130 |
| after treatment | 4.1 ± 5.3 | 3.6 ± 3.6 | 6.0 ± 9.3 | 0.435 |
| DEB related patient injury, n (%) | 85 (37.6) | 72 (39.6) | 13 (29.5) | 0.279 † |
| DEB related bed-partner injury, n (%) | 39 (17.3) | 35 (19.2) | 4 (9.1) | 0.402 † |
| Injury type, n, (% of injured patients) | ||||
| Mild | 16 (18.8) | |||
| Moderate | 59 (69.4) | |||
| Marked | 10 (11.8) | |||
| KVSS I score | 4.9 ± 1.7 | 4.8 ± 1.7 | 5.6 ± 0.9 | 0.337 |
| KVSS I ≤ 6, n (%) | 34 (85.0%) | 30 (85.7%) | 4 (80.0%) | 1.000 † |
| Medical treatment | ||||
| Melatonin, number of patients | 32 | 22 | 10 | |
| dose | 2.0 ± 0.3 | 1.9 ± 0.4 | 1.9 ± 0.4 | 0.654 |
| Clonazepam, number of patients | 92 | 77 | 15 | |
| dose | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.7 ± 0.2 | 0.232 |
| Family history, n (%) | ||||
| RBD | 8 (3.5) | 5 (2.7) | 3 (6.8) | |
| Dementia | 5 (2.0) | 5 (2.7) | 0 | |
| PD | 5 (2.0) | 4 (2.2) | 1 (2.3) | |
| BMI, kg/m2 | 24.4 ± 3.1 | 24.4 ± 3.1 | 24.3 ± 3.3 | 0.936 |
| NC, cm | 37.1 ± 4.0 | 36.9 ± 3.4 | 36.1 ± 3.0 | 0.213 |
| ESS | 7.2 ± 4.4 | 7.3 ± 4.5 | 7.1 ± 4.0 | 0.766 |
| ESS > 10, n (%) | 43 (19.0%) | 34 (18.7) | 9 (20.5) | 0.773 † |
| ISI | 9.1 ± 6.3 | 9.0 ± 6.2 | 9.7 ± 6.8 | 0.491 |
| ISI > 14, n (%) | 37 (16.4%) | 28 (15.4 | 9 (20.5%) | 0.415 † |
| PSQI | 6.6 ± 3.6 | 6.6 ± 3.5 | 6.8 ± 4.0 | 0.713 |
| PSQI > 5, n (%) | 114 (50.4%) | 96 (52.7) | 18 (40.9) | 0.259 † |
| K-BDI-II | 13.7 ± 9.5 | 13.4 ± 9.4 | 15.0 ± 9.8 | 0.326 |
| Variables | Total (n = 226) | IRBD (n = 182) | SRBD (n = 44) | p-Value (IRBD vs. SRBD) |
|---|---|---|---|---|
| Total sleep time, min | 356.2 ± 65.4 | 359.6 ± 63.2 | 342.2 ± 72.6 | 0.111 |
| Sleep latency, min | 20.3 ± 30.0 | 20.8 ± 32.0 | 18.2 ± 19.2 | 0.606 |
| REM latency, min | 108.6 ± 64.4 | 104.1 ± 59.9 | 127.1 ± 78.7 | 0.075 |
| WASO, % | 16.5 ± 11.4 | 15.7 ± 10.3 | 19.7 ± 14.9 | 0.138 |
| Sleep efficiency, % | 79.6 ± 12.8 | 80.3 ± 11.9 | 76.9 ± 16.0 | 0.186 |
| Sleep stages | ||||
| N1 sleep, % | 20.4 ± 10.6 | 20.0 ± 10.5 | 22.3 ± 11.0 | 0.197 |
| N2 sleep, % | 55.1 ± 11.1 | 54.9 ± 11.2 | 56.1 ± 10.7 | 0.490 |
| N3 sleep, % | 3.0 ± 4.7 | 3.3 ± 5.0 | 1.6 ± 3.1 | 0.004 * |
| REM sleep, % | 21.5 ± 6.9 | 21.9 ± 6.5 | 20.0 ± 8.0 | 0.097 |
| Arousal Index, /h | 20.0 ± 9.3 | 20.1 ± 9.4 | 19.9 ± 9.1 | 0.897 |
| REM arousal index, /h | 16.1 ± 9.8 | 16.1 ± 10.0 | 16.2 ± 9.0 | 0.938 |
| AHI, /h | 16.5 ± 16.3 | 15.3 ± 14.4 | 21.7 ± 21.9 | 0.071 |
| REM AHI, /h | 16.9 ± 18.2 | 15.7 ± 17.1 | 22.0 ± 21.8 | 0.063 |
| RDI, /h | 18.8 ± 16.1 | 17.7 ± 14.4 | 23.5 ± 21.4 | 0.090 |
| RDI ≥ 15, n (%) | 110 (48.7) | 86 (47.3) | 24 (54.5) | 0.405 † |
| REM RDI, /h | 18.8 ± 18.0 | 17.7 ± 17.0 | 23.7 ± 21.2 | 0.069 |
| PLMI | 40.8 ± 36.7 | 36.3 ± 31.8 | 56.9 ± 47.5 | 0.021 * |
| PLMI ≥ 15, n (%) | 111 (49.1) | 83 (45.6) | 28 (63.6) | 0.136 † |
| MAI | 2.1 ± 3.7 | 2.1 ± 3.9 | 1.9 ± 2.4 | 0.729 |
| RSWA (%) | 14.0 ± 13.3 | 12.8 ± 12.7 | 19.1 ± 14.8 | 0.003 * |
| Tonic activity | 3.2 ± 6.5 | 3.1 ± 7.0 | 3.9 ± 5.4 | 0.019 * |
| Phasic activity | 10.5 ± 9.8 | 9.7 ± 8.8 | 15.2 ± 13.2 | 0.005 * |
| Non-Converters (n = 164) | Converters (n = 18) | p-Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Male, n (%) | 101 (63.1) | 10 (55.6) | 0.610 † |
| Age at RBD onset, years | 59.6 ± 10.7 | 61.3 ± 8.0 | 0.848 |
| Age at RBD diagnosis, years | 65.3 ± 9.4 | 62.3 ± 12.8 | 0.410 |
| RBD duration, years | 5.7 ± 5.8 | 3.7 ± 2.4 | 0.433 |
| Follow-up duration, years | 2.4 ± 2.2 | 2.5 ± 1.6 | 0.544 |
| KVSS I score | 5.2 ± 1.5 | 5.5 ± 2.1 | 0.821 |
| KVSS I ≤ 6, n (%) | 26 (66.7%) | 4 (80.0%) | 0.342 † |
| Medical treatment | |||
| Melatonin, number of patients | 18 | 4 | |
| dose | 1.9 ± 0.5 | 2.0 ± 0.0 | 0.758 |
| Clonazepam, number of patients | 69 | 8 | |
| dose | 0.6 ± 0.3 | 0.7 ± 0.4 | 0.381 |
| Self-reported sleep measures | |||
| ESS | 8.0 ± 4.9 | 6.7 ± 5.2 | 0.134 |
| ESS > 10, n (%) | 43 (26.2) | 3 (16.7) | 0.757 † |
| ISI | 9.0 ± 6.1 | 12.1 ± 6.9 | 0.066 |
| ISI > 14, n (%) | 24 (14.6) | 4 (22.2) | 0.006 † |
| PSQI | 6.5 ± 3.424 (14.6) | 7.6 ± 3.4 | 0.100 |
| PSQI > 5, n (%) | 84 (51.2) | 12 (66.7) | 0.341 † |
| K-BDI-II | 13.8 ± 9.3 | 15.2 ± 8.6 | 0.412 |
| Anthropometric and Polysomnographic data | |||
| BMI, kg/m2 | 24.1 ± 3.1 | 25.5 ± 3.5 | 0.412 |
| NC, cm | 36.9 ± 4.1 | 36.6 ± 2.8 | 0.469 |
| Total sleep time, min | 360.5 ± 65.1 | 351.5 ± 66.5 | 0.527 |
| Sleep latency, min | 16.8 ± 21.9 | 16.3 ± 17.6 | 0.804 |
| REM latency, min | 109.9 ± 68.1 | 91.6 ± 46.7 | 0.581 |
| WASO, % | 16.9 ± 12.3 | 14.2 ± 8.1 | 0.651 |
| Sleep efficiency, % | 79.8 ± 12.9 | 80.8 ± 11.6 | 0.830 |
| Sleep stages | |||
| N1 sleep, % | 22.0 ± 11.5 | 19.8 ± 7.4 | 0.612 |
| N2 sleep, % | 54.0 ± 11.5 | 54.8 ± 12.0 | 0.657 |
| N3 sleep, % | 2.7 ± 4.1 | 2.5 ± 6.2 | 0.914 |
| REM sleep, % | 21.3 ± 6.9 | 23.0 ± 8.7 | 0.499 |
| Arousal Index, /h | 21.1 ± 9.9 | 20.2 ± 8.3 | 0.734 |
| REM arousal index, /h | 17.1 ± 10.0 | 11.2 ± 4.9 | 0.031 * |
| AHI, /h | 17.3 ± 17.0 | 15.0 ± 12.1 | 0.836 |
| REM AHI, /h | 17.7 ± 18.6 | 16.1 ± 15.6 | 0.886 |
| RDI, /h | 19.5 ± 16.6 | 17.9 ± 11.6 | 0.885 |
| RDI ≥ 15, n (%) | 76 (46.3) | 10 (55.6) | 0.352 † |
| REM RDI, /h | 19.6 ± 18.5 | 17.6 ± 15.4 | 0.823 |
| PLMI | 40.1 ± 37.0 | 39.9 ± 28.6 | 0.803 |
| PLMI ≥ 15, n (%) | 74 (45.1) | 9 (50.0) | 1.000 † |
| MAI | 2.1 ± 3.9 | 1.6 ± 2.5 | 0.716 |
| RSWA (%) | 12.4 ± 12.4 | 16.1 ± 14.9 | 0.299 |
| Tonic activity | 2.8 ± 6.5 | 5.8 ± 10.4 | 0.121 |
| Phasic activity | 9.6 ± 8.9 | 10.3 ± 8.8 | 0.683 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Kim, D.; Song, J.; Choi, S.; Joo, E. Sleep Disturbances and Phenoconversion in Patients with REM Sleep Behavior Disorder. J. Clin. Med. 2021, 10, 4709. https://doi.org/10.3390/jcm10204709
Jo H, Kim D, Song J, Choi S, Joo E. Sleep Disturbances and Phenoconversion in Patients with REM Sleep Behavior Disorder. Journal of Clinical Medicine. 2021; 10(20):4709. https://doi.org/10.3390/jcm10204709
Chicago/Turabian StyleJo, Hyunjin, Dongyeop Kim, Jooyeon Song, Sujung Choi, and Eunyeon Joo. 2021. "Sleep Disturbances and Phenoconversion in Patients with REM Sleep Behavior Disorder" Journal of Clinical Medicine 10, no. 20: 4709. https://doi.org/10.3390/jcm10204709
APA StyleJo, H., Kim, D., Song, J., Choi, S., & Joo, E. (2021). Sleep Disturbances and Phenoconversion in Patients with REM Sleep Behavior Disorder. Journal of Clinical Medicine, 10(20), 4709. https://doi.org/10.3390/jcm10204709






