Long-Term Efficacy and Impact on Mortality of Remote Magnetic Navigation Guided Catheter Ablation of Ventricular Arrhythmias
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Magnetic Navigation System
2.3. Periprocedural Management
2.4. Ablation Procedure
2.5. Follow-Up
2.6. Endpoint
2.7. Data Collection
2.8. Statistical Analysis
3. Results
3.1. Patients’ Baseline Characteristics
3.2. Baseline Characteristics (PVC vs. VT)
3.3. Baseline Characteristics (Idiopathic VA vs. Cardiomyopathies (DCM, ICM))
3.4. Baseline Characteristics (DCM vs. ICM)
3.5. Baseline Symptoms
3.6. Procedural Data and Acute Procedural Success
3.7. Impact of RMN Ablation on PVC Burden
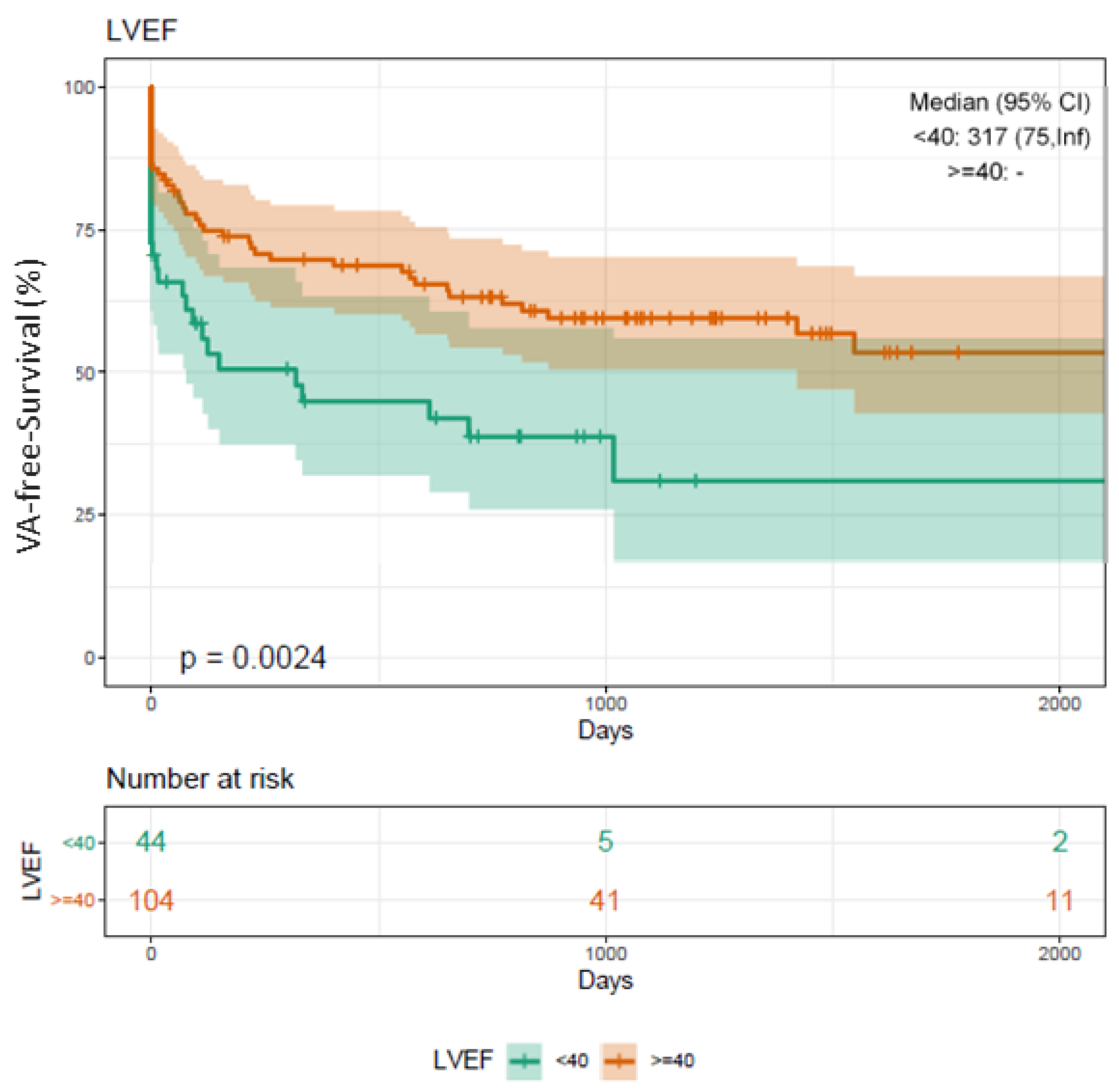
3.8. Long-Term Clinical Outcome
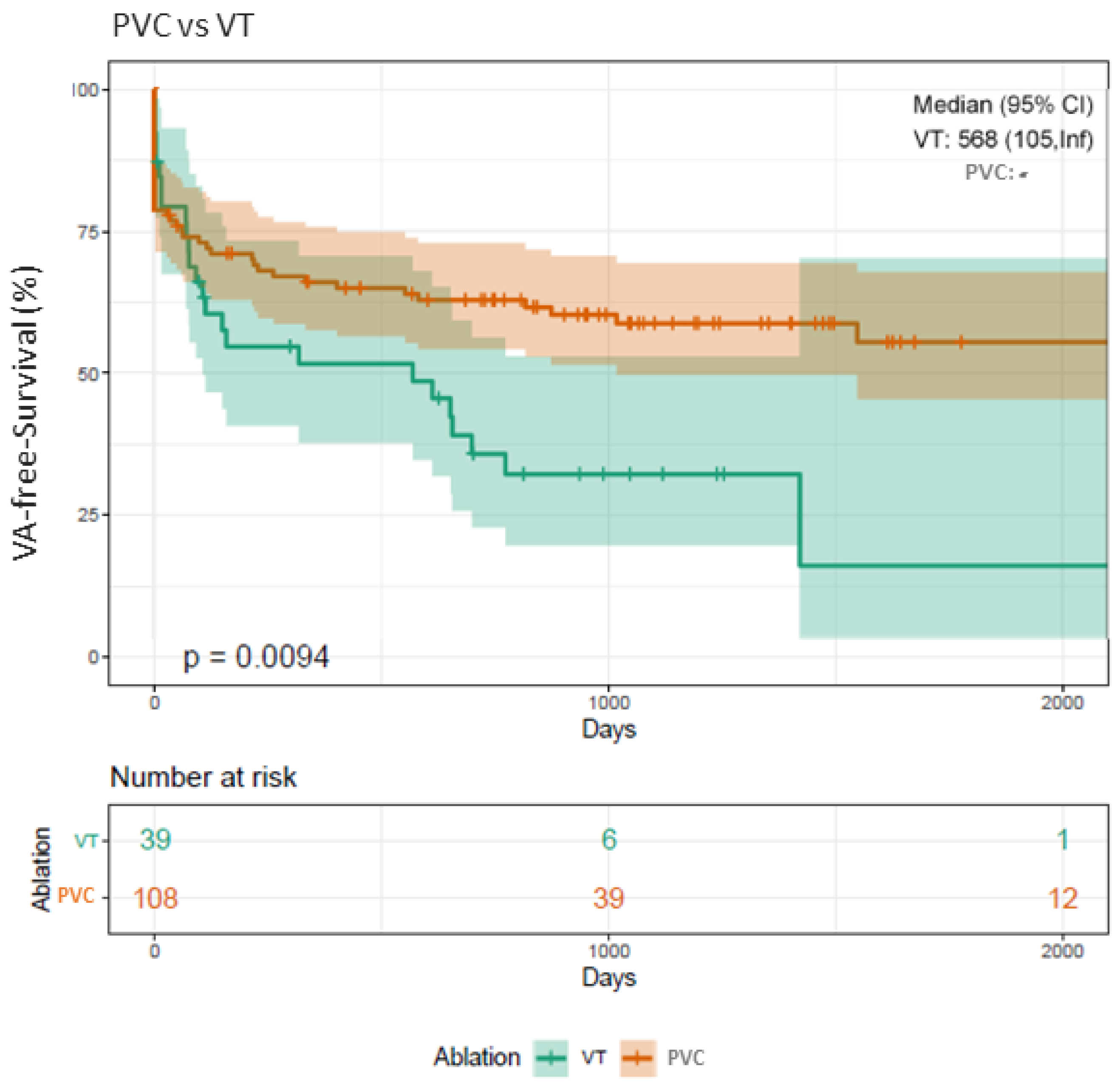
3.9. Clinical Outcome Depending on PVC Origin
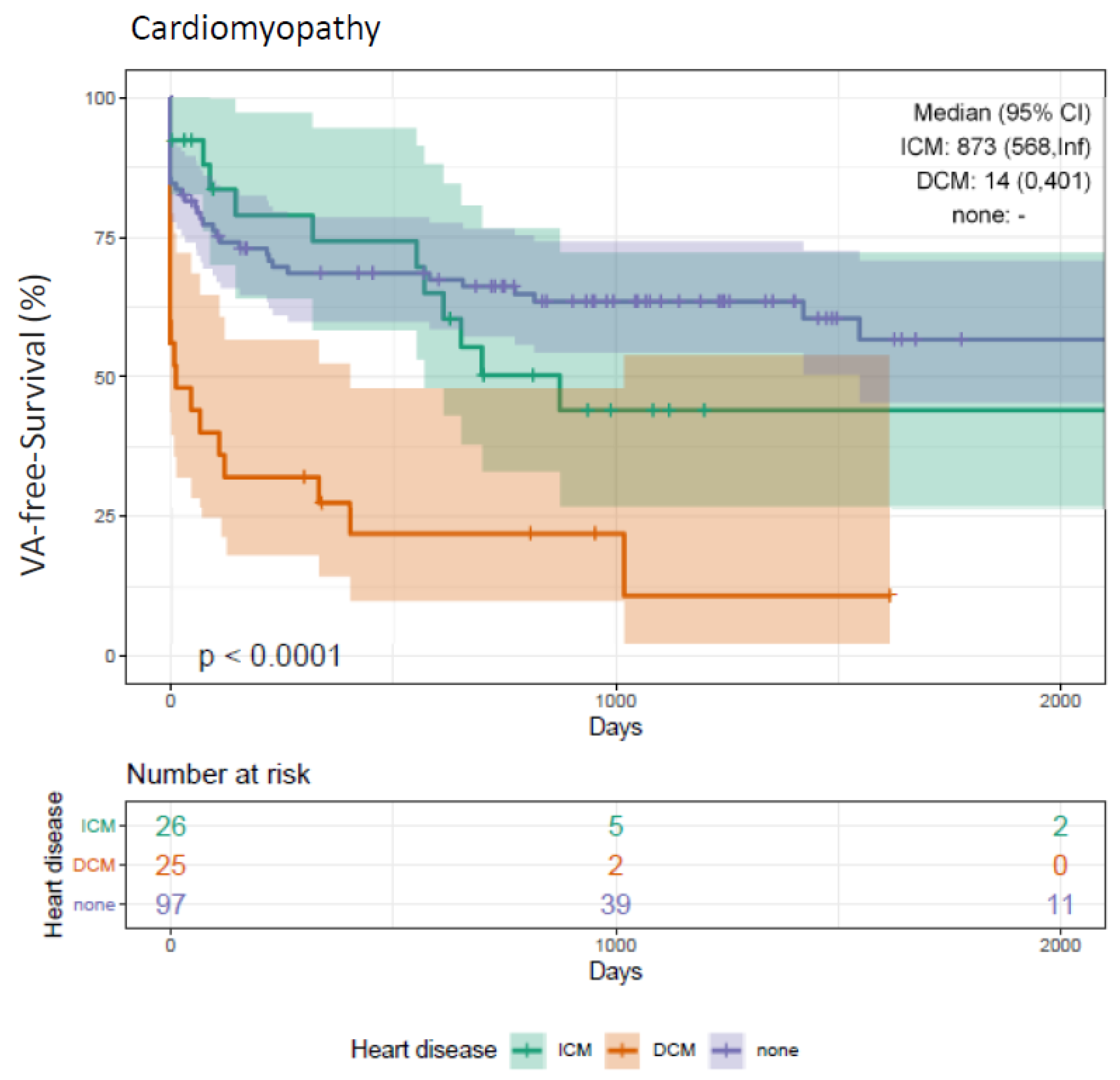
3.10. Clinical Outcome Depending on Cardiomyopathies (DCM, ICM)
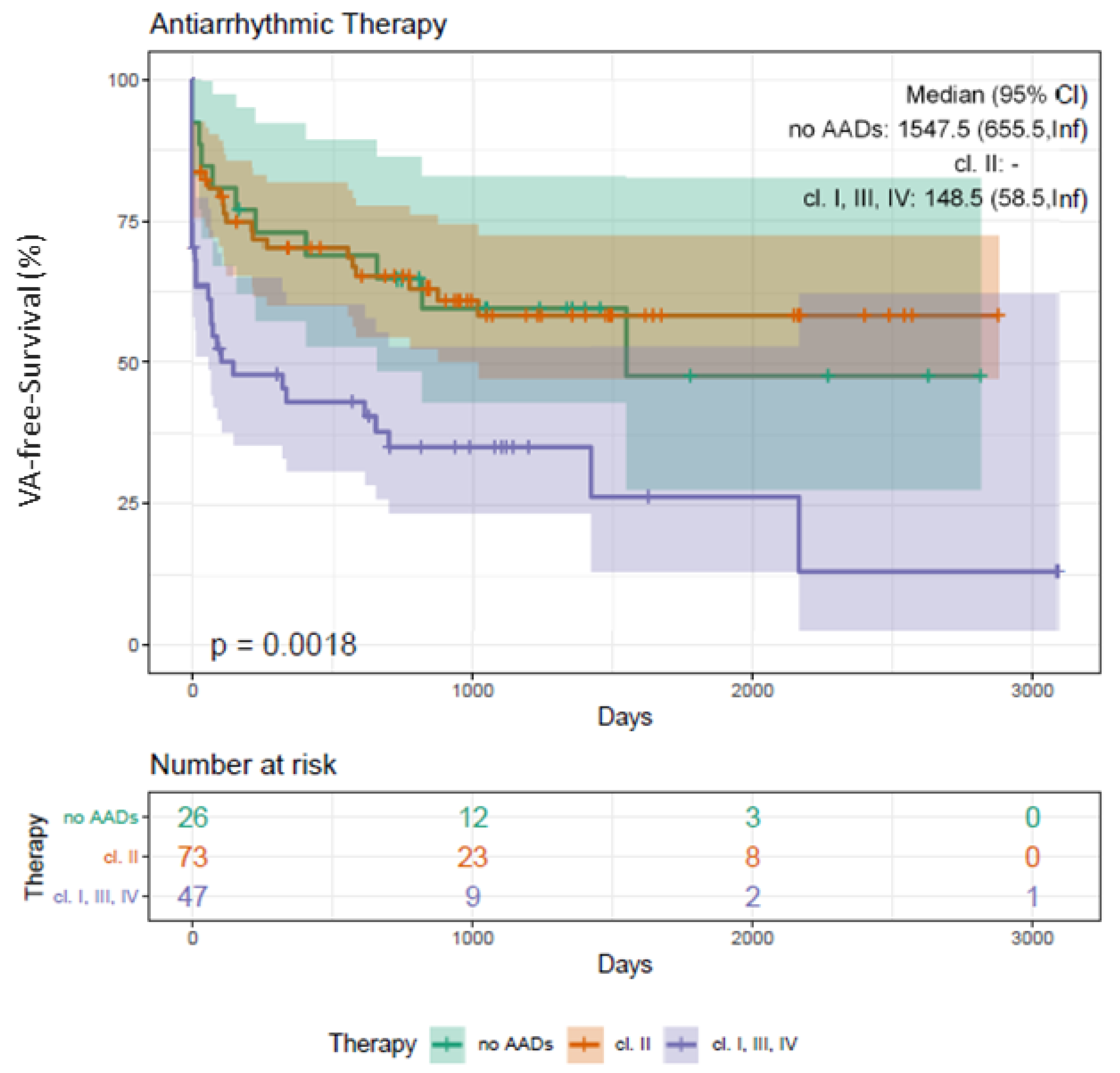
3.11. Clinical Outcome Depending on Beta Blocker and AADs Class I/III/IV
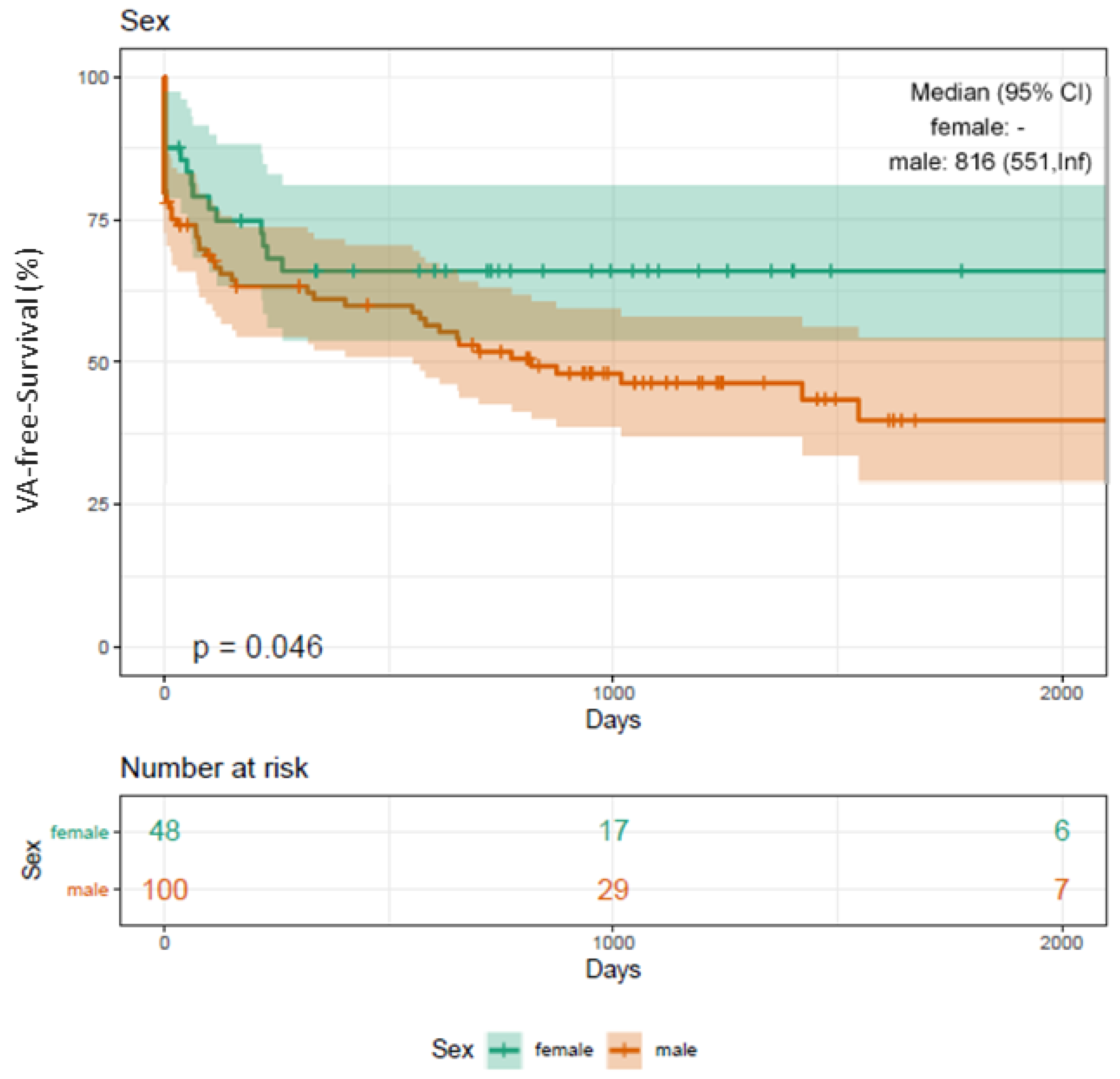
3.12. Clinical Outcome and Sex Disparities
3.13. Complications
4. Discussion
4.1. Main Findings
4.2. VA-Free Survival Using RMN
4.3. VA-Free Survival (PVC vs. VT)
4.4. VA-Free Survival (DCM vs. ICM)
4.5. VA-Free Survival (Idiopathic VT)
4.6. Additional Predictors of VA Recurrence
4.7. Clinical Perspective and Translational Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. EP Eur. 2019, 21, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- van Berlo, J.H.; de Voogt, W.G.; van der Kooi, A.J.; van Tintelen, J.P.; Bonne, G.; Yaou, R.B.; Duboc, D.; Rossenbacker, T.; Heidbüchel, H.; de Visser, M.; et al. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: Do lamin A/C mutations portend a high risk of sudden death? J. Mol. Med. 2005, 83, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar]
- Aagaard, P.; Natale, A.; Briceno, D.; Nakagawa, H.; Mohanty, S.; Gianni, C.; Burkhardt, J.D.; Di Biase, L.; Aagaard, P. Remote Magnetic Navigation: A Focus on Catheter Ablation of Ventricular Arrhythmias. J. Cardiovac. Electrophysiol. 2016, 27 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef]
- Piers, S.R.; Leong, D.P.; van Huls van Taxis, C.F.; Tayyebi, M.; Trines, S.A.; Pijnappels, D.A.; Delgado, V.; Schalij, M.J.; Zeppenfeld, K.; Piers, S.R.; et al. Outcome of ventricular tachycardia ablation in patients with nonischemic cardiomyopathy: The impact of noninducibility. Circ. Arrhythm. Electrophysiol. 2013, 6, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Dinov, B.; Fiedler, L.; Schönbauer, R.; Bollmann, A.; Rolf, S.; Piorkowski, C.; Hindricks, G.; Arya, A.; Dinov, B. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: Results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation 2014, 129, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Hsia, H.H.; Marchlinski, F.E.; Hsia, H.H. Characterization of the electroanatomic substrate for monomorphic ventricular tachycardia in patients with nonischemic cardiomyopathy. Pacing Clin. Electrophysiol. 2002, 25, 1114–1127. [Google Scholar] [CrossRef]
- Soejima, K.; Stevenson, W.G.; Sapp, J.L.; Selwyn, A.P.; Couper, G.; Epstein, L.M.; Soejima, K. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: The importance of low-voltage scars. J. Am. Coll. Cardiol. 2004, 43, 1834–1842. [Google Scholar] [CrossRef]
- Nakahara, S.; Tung, R.; Ramirez, R.J.; Michowitz, Y.; Vaeghi, M.; Buch, E.; Gima, J.; Wiener, I.; Mahajan, A.; Boyle, N.G.; et al. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia. J. Am. Coll. Cardiol. 2010, 55, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H.; Schaumann, A.; Eckardt, L.; Willems, S.; Ventura, R.; Delacrétaz, E.; Pitschner, H.F.; Kautzner, J.; Schumacher, B.; Hansen, P.S.; et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): A multicentre randomised controlled trial. Lancet 2010, 375, 31–40. [Google Scholar] [CrossRef]
- Turagam, M.K.; Atkins, D.; Tung, R.; Mansour, M.; Ruskin, J.; Cheng, J.; Di Biase, L.; Natale, A.; Lakkireddy, D.; Turagam, M.K.; et al. A meta-analysis of manual versus remote magnetic navigation for ventricular tachycardia ablation. J. Interv. Card. Electrophysiol. 2017, 49, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Zaker-Shahrak, R.; Sommer, P.; Bollmann, A.; Wetzel, U.; Gaspar, T.; Richter, S.; Husser, D.; Piorkowski, C.; Hindricks, G.; et al. Catheter ablation of atrial fibrillation using remote magnetic catheter navigation: A case-control study. Europace 2011, 13, 45–50. [Google Scholar] [CrossRef]
- Nölker, G.; Gutleben, K.J.; Muntean, B.; Vogt, J.; Horstkotte, D.; Dabiri Abkenari, L.; Akca, F.; Szili-Torok, T.; Nölker, G. Novel robotic catheter manipulation system integrated with remote magnetic navigation for fully remote ablation of atrial tachyarrhythmias: A two-centre evaluation. Europace 2012, 14, 1715–1718. [Google Scholar] [CrossRef][Green Version]
- Miyazaki, S.; Shah, A.J.; Xhaët, O.; Derval, N.; Matsuo, S.; Wright, M.; Nault, I.; Forclaz, A.; Jadidi, A.S.; Knecht, S.; et al. Remote magnetic navigation with irrigated tip catheter for ablation of paroxysmal atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 585–589. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Zeppenfeld, K. Ventricular Tachycardia Ablation in Nonischemic Cardiomyopathy. JACC Clin. Electrophysiol. 2018, 4, 1123–1140. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Reynolds, M.R.; Neuzil, P.; Richardson, A.W.; Taborsky, M.; Jongnarangsin, K.; Kralovec, S.; Sediva, L.; Ruskin, J.N.; Josephson, M.E.; et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N. Engl. J. Med. 2007, 357, 2657–2665. [Google Scholar] [CrossRef]
- Eitel, C.; Hindricks, G.; Sommer, P.; Wetzel, U.; Bollmann, A.; Gaspar, T.; Piorkowski, C.; Arya, A.; Eitel, C. Safety of remote magnetic navigation in patients with pacemakers and implanted cardioverter defibrillators. J. Cardiovac. Electrophysiol. 2010, 21, 1130–1135. [Google Scholar] [CrossRef]
- Lüthje, L.; Vollmann, D.; Seegers, J.; Sohns, C.; Hasenfuss, G.; Zabel, M.; Lüthje, L. Interference of remote magnetic catheter navigation and ablation with implanted devices for pacing and defibrillation. Europace 2010, 12, 1574–1580. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, C.J.; Sapp, J.L.; MacIntyre, C.J. Treatment of persistent ventricular tachycardia: Drugs or ablation? Trends Cardiovac. Med. 2017, 27, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Kim, H.M.; Sharaf Dabbagh, G.; Latchamsetty, R.; Stojanovska, J.; Jongnarangsin, K.; Morady, F.; Bogun, F.M.; Siontis, K.C. Association of preprocedural cardiac magnetic resonance imaging with outcomes of ventricular tachycardia ablation in patients with idiopathic dilated cardiomyopathy. Heart Rhythm. 2017, 14, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N. Engl. J. Med. 1997, 337, 1576–1583. [Google Scholar] [CrossRef]
- Poole, J.E.; Johnson, G.W.; Hellkamp, A.S.; Anderson, J.; Callans, D.J.; Raitt, M.H.; Reddy, R.K.; Marchlinski, F.E.; Yee, R.; Guarnieri, T.; et al. Prognostic importance of defibrillator shocks in patients with heart failure. N. Engl. J. Med. 2008, 359, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Dukkipati, S.R.; Choudry, S.; Koruth, J.S.; Miller, M.A.; Whang, W.; Reddy, V.Y.; Dukkipati, S.R. Catheter Ablation of Ventricular Tachycardia in Structurally Normal Hearts: Indications, Strategies, and Outcomes-Part, I. J. Am. Coll. Cardiol. 2017, 70, 2909–2923. [Google Scholar] [CrossRef]
- Baman, T.S.; Lange, D.C.; Ilg, K.J.; Gupta, S.K.; Liu, T.Y.; Alguire, C.; Armstrong, W.; Good, E.; Chugh, A.; Jongnarangsin, K.; et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010, 7, 865–869. [Google Scholar] [CrossRef]
- Takemoto, M.; Yoshimura, H.; Ohba, Y.; Matsumoto, Y.; Yamamoto, U.; Mohri, M.; Yamamoto, H.; Origuchi, H.; Takemoto, M. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improPVC left ventricular dilation and clinical status in patients without structural heart disease. J. Am. Coll. Cardiol. 2005, 45, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Bogun, F.; Crawford, T.; Reich, S.; Koelling, T.M.; Armstrong, W.; Good, E.; Jongnarangsin, K.; Marine, J.E.; Chugh, A.; Pelosi, F.; et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: Comparison with a control group without intervention. Heart Rhythm. 2007, 4, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Liu, Z.; Su, L.; Zipunnikov, V.; Wu, J.; Du, H.; Woo, K.; Chen, S.; Zhong, B.; Lan, X.; et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract: Prospective randomized study. Circ. Arrhythm. Electrophysiol. 2014, 7, 237–243. [Google Scholar] [CrossRef]
- Latchamsetty, R.; Yokokawa, M.; Morady, F.; Kim, H.M.; Mathew, S.; Tilz, R.; Kuck, K.H.; Nagashima, K.; Tedrow, U.; Stevenson, W.G.; et al. Multicenter Outcomes for Catheter Ablation of Idiopathic Premature Ventricular Complexes. JACC Clin. Electrophysiol. 2015, 1, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sapp, J.L.; Wells, G.A.; Parkash, R.; Stevenson, W.G.; Blier, L.; Sarrazin, J.F.; Thibault, B.; Rivard, L.; Gula, L.; Leong-Sit, P.; et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N. Engl. J. Med. 2016, 375, 111–121. [Google Scholar] [CrossRef]
- Sapp, J.L.; Parkash, R.; Wells, G.A.; Yetisir, E.; Gardner, M.J.; Healey, J.S.; Thibault, B.; Sterns, L.D.; Birnie, D.; Nery, P.B.; et al. Cardiac Resynchronization Therapy Reduces Ventricular Arrhythmias in Primary but Not Secondary Prophylactic Implantable Cardioverter Defibrillator Patients: Insight From the Resynchronization in Ambulatory Heart Failure Trial. Circ. Arrhythm. Electrophysiol. 2017, 10, e004875. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n = 176 |
|---|---|
| Age (years) | 53.23 ± 17.55 |
| Sex, female | 65 (37%) |
| BMI (kg/m2) | 26.71 ± 4.25 |
| LVEF (%) | 48.22 ± 10.8 |
| Cardiomyopathy | 57 (32%) |
| ICM | 31 (18%) |
| DCM | 26 (15%) |
| Valve disease | 43 (24%) |
| Hypertension | 98 (56%) |
| Diabetes mellitus | 22 (13%) |
| Beta blocker BL | 125 (71%) |
| AADs class I/III/IV BL | 56 (32%) |
| ICD | 24 (14%) |
| CRT-D | 24 (14%) |
| Renal failure | 23 (13%) |
| History of stroke | 11 (6%) |
| Risk Factor | Hazard Ratio | p-Value |
|---|---|---|
| ICM | 1.49 (0.79–2.81) | 0.221 |
| DCM | 3.80 (2.18–6.64) | >0.001 * |
| LVEF < 40% | 2.06 (1.26–3.35) | 0.004 * |
| AADs class I/III/IV BL | 2.27 (1.14–4.52) | 0.020 * |
| Beta blocker BL | 0.97 (0.48–1.96) | 0.942 |
| Sex (female) | 0.58 (0.33–1.01) | 0.055 |
| VT ablation | 1.9 (1.15–3.12) | 0.012 * |
| GFR <60 mg/dl/min | 1.45 (0.8–2.62) | 0.221 |
| Risk Factor | Hazard Ratio | 95% Lower | 95% Upper | p-Value |
|---|---|---|---|---|
| LVEF < 40% | 1.11 | 0.45 | 2.76 | 0.821 |
| DCM | 3.74 | 1.58 | 8.88 | <0.01 * |
| AADs class I/III/IV BL | 2.48 | 1.11 | 5.68 | 0.03 * |
| VT ablation | 1.02 | 0.53 | 1.99 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guckel, D.; Niemann, S.; Ditzhaus, M.; Molatta, S.; Bergau, L.; Fink, T.; Sciacca, V.; El Hamriti, M.; Imnadze, G.; Steinhauer, P.; et al. Long-Term Efficacy and Impact on Mortality of Remote Magnetic Navigation Guided Catheter Ablation of Ventricular Arrhythmias. J. Clin. Med. 2021, 10, 4695. https://doi.org/10.3390/jcm10204695
Guckel D, Niemann S, Ditzhaus M, Molatta S, Bergau L, Fink T, Sciacca V, El Hamriti M, Imnadze G, Steinhauer P, et al. Long-Term Efficacy and Impact on Mortality of Remote Magnetic Navigation Guided Catheter Ablation of Ventricular Arrhythmias. Journal of Clinical Medicine. 2021; 10(20):4695. https://doi.org/10.3390/jcm10204695
Chicago/Turabian StyleGuckel, Denise, Sarah Niemann, Marc Ditzhaus, Stephan Molatta, Leonard Bergau, Thomas Fink, Vanessa Sciacca, Mustapha El Hamriti, Guram Imnadze, Philipp Steinhauer, and et al. 2021. "Long-Term Efficacy and Impact on Mortality of Remote Magnetic Navigation Guided Catheter Ablation of Ventricular Arrhythmias" Journal of Clinical Medicine 10, no. 20: 4695. https://doi.org/10.3390/jcm10204695
APA StyleGuckel, D., Niemann, S., Ditzhaus, M., Molatta, S., Bergau, L., Fink, T., Sciacca, V., El Hamriti, M., Imnadze, G., Steinhauer, P., Braun, M., Khalaph, M., Nölker, G., Sommer, P., & Sohns, C. (2021). Long-Term Efficacy and Impact on Mortality of Remote Magnetic Navigation Guided Catheter Ablation of Ventricular Arrhythmias. Journal of Clinical Medicine, 10(20), 4695. https://doi.org/10.3390/jcm10204695






