RRM1 Expression as a Prognostic Biomarker for Unresectable or Recurrent Biliary Tract Cancer Treated with Gemcitabine plus Cisplatin
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Preparation and Immunostaining of Specimens
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
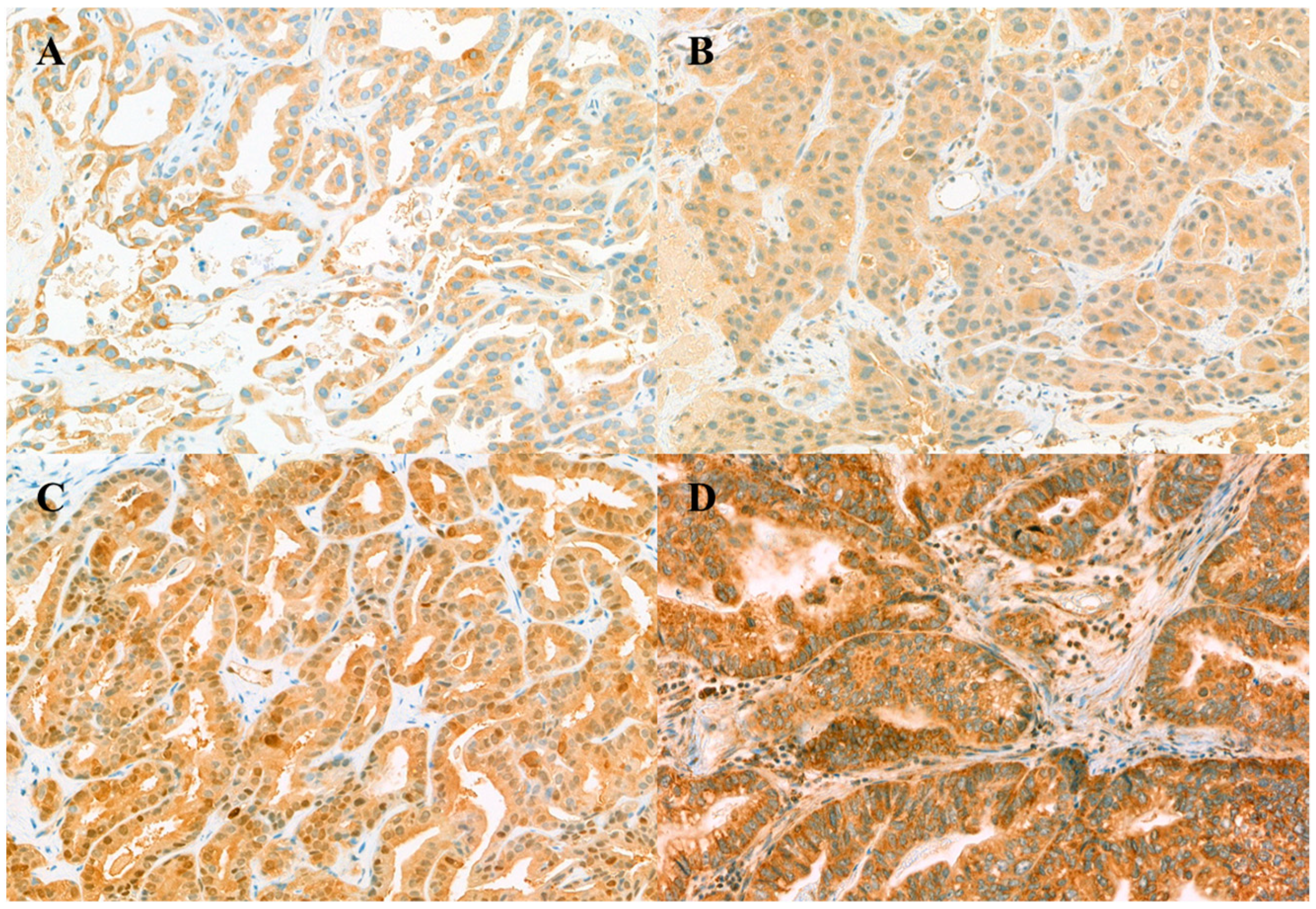
3.2. Immunohistochemistry Staining of Biomarker Expression
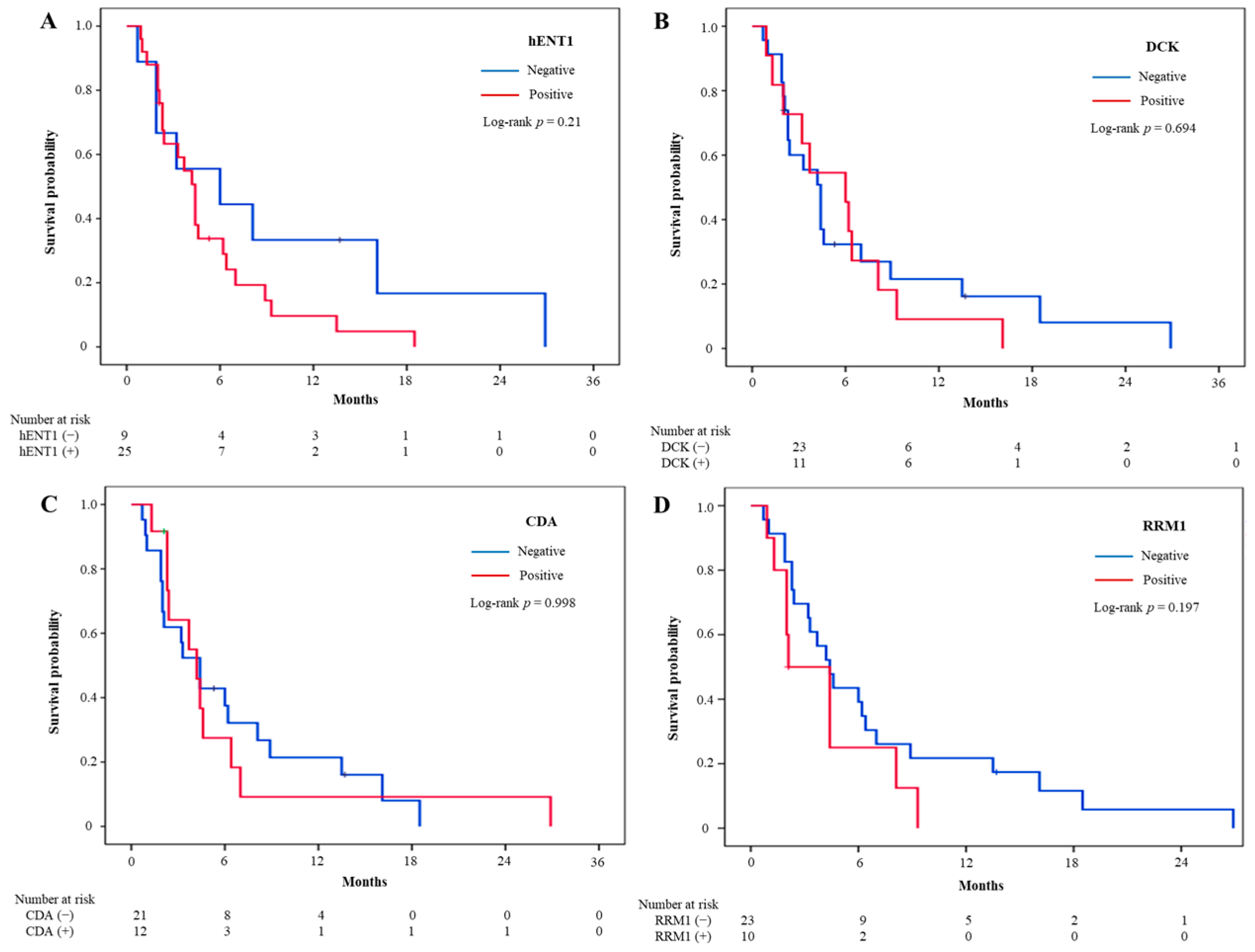
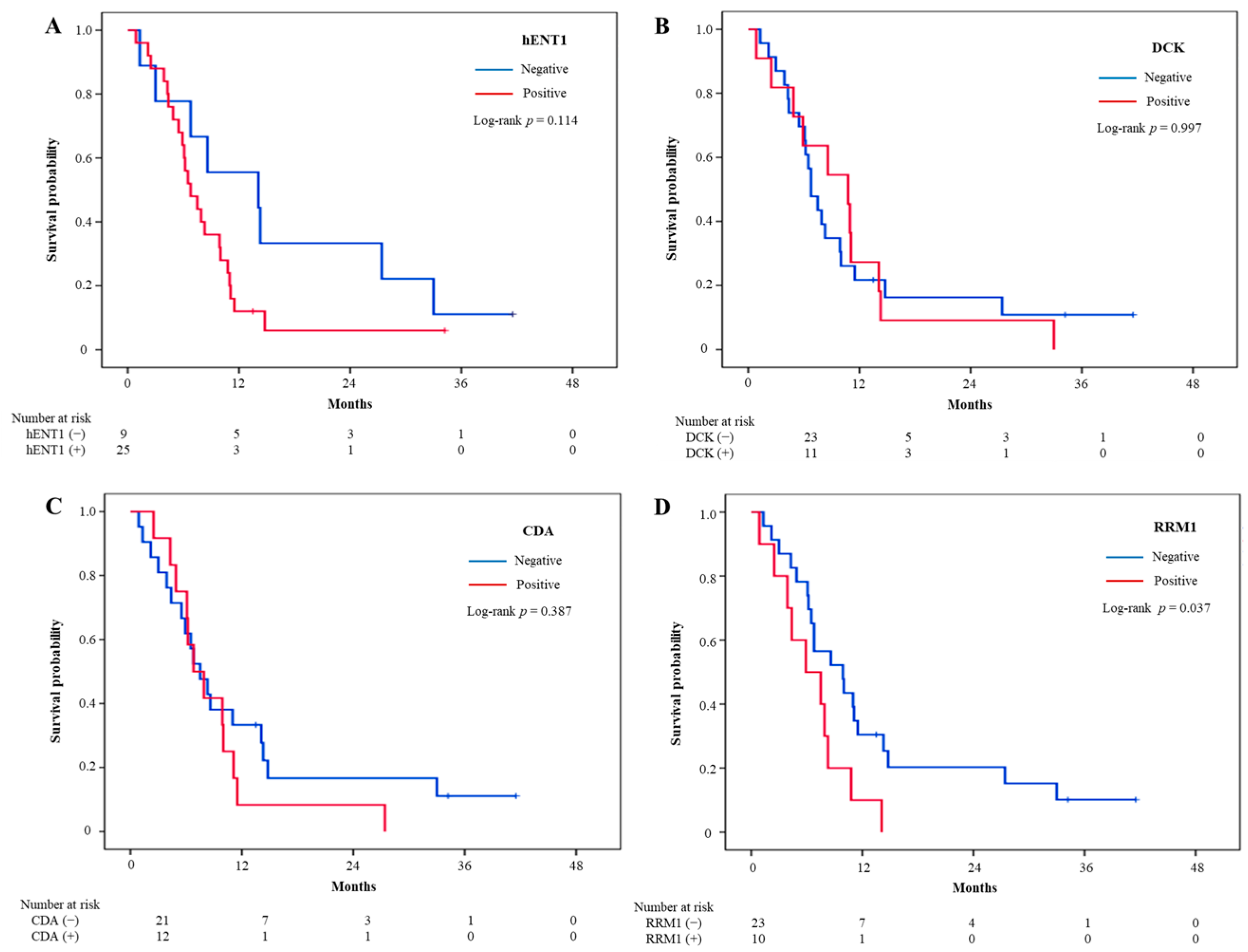
3.3. Treatment Outcomes and Intratumoral Biomarker Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Groen, P.C.; Gores, G.J.; LaRusso, N.F.; Gunderson, L.L.; Nagorney, D.M. Biliary Tract Cancers. N. Engl. J. Med. 1999, 341, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Park, J.O.; Oh, D.-Y.; Hsu, C.; Chen, J.-S.; Chen, L.-T.; Orlando, M.; Kim, J.S.; Lim, H.Y. Gemcitabine Plus Cisplatin for Advanced Biliary Tract Cancer: A Systematic Review. Cancer Res. Treat. 2015, 47, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Tariq, N.-U.-A.; McNamara, M.G.; Valle, J.W. Biliary tract cancers: Current knowledge, clinical candidates and future challenges. Cancer Manag. Res. 2019, 11, 2623–2642. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Reyes-Vidal, J.M.; Fartoux, L.; Ross, P.; Leslie, M.; Rosmorduc, O.; Clemens, M.R.; Louvet, C.; Perez, N.; Mehmud, F.; et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: A phase II study. Br. J. Cancer 2008, 99, 862–867. [Google Scholar] [CrossRef]
- Knox, J.J.; Hedley, D.; Oza, A.; Feld, R.; Siu, L.L.; Chen, E.; Nematollahi, M.; Pond, G.R.; Zhang, J.; Moore, M.J. Combining Gemcitabine and Capecitabine in Patients With Advanced Biliary Cancer: A Phase II Trial. J. Clin. Oncol. 2005, 23, 2332–2338. [Google Scholar] [CrossRef]
- Okusaka, T.; Ishii, H.; Funakoshi, A.; Yamao, K.; Ohkawa, S.; Saito, S.; Saito, H.; Tsuyuguchi, T. Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother. Pharmacol. 2006, 57, 647–653. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. Molecular predictors of gemcitabine response in pancreatic cancer. World J. Gastrointest. Oncol. 2011, 3, 153–164. [Google Scholar] [CrossRef]
- Amrutkar, M.; Gladhaug, I.P. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers 2017, 9, 157. [Google Scholar] [CrossRef]
- Lane, J.; Martin, T.A.; McGuigan, C.; Mason, M.D.; Jiang, W. The differential expression of hCNT1 and hENT1 i n breast cancer and the possible impact on breast cancer therapy. J. Exp. Ther. Oncol. 2010, 8, 203–210. [Google Scholar]
- Achiwa, H.; Oguri, T.; Sato, S.; Maeda, H.; Niimi, T.; Ueda, R. Determinants of sensitivity and resistance to gemcitabine: The roles of human equilibrative nucleoside transporter 1 and deoxycytidine kinase in non-small cell lung cancer. Cancer Sci. 2004, 95, 753–757. [Google Scholar] [CrossRef]
- Bepler, G.; Kusmartseva, I.; Sharma, S.; Gautam, A.; Cantor, A.; Sharma, A.; Simon, G. RRM1 Modulated In Vitro and In Vivo Efficacy of Gemcitabine and Platinum in Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2006, 24, 4731–4737. [Google Scholar] [CrossRef] [PubMed]
- Tibaldi, C.; on behalf of The Italian Oncological Group of Clinical Research (GOIRC); Camerini, A.; Tiseo, M.; Mazzoni, F.; Barbieri, F.; Vittimberga, I.; Brighenti, M.; Boni, L.; Baldini, E.; et al. Cytidine deaminase enzymatic activity is a prognostic biomarker in gemcitabine/platinum-treated advanced non-small-cell lung cancer: A prospective validation study. Br. J. Cancer 2018, 119, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Borbath, I.; Verbrugghe, L.; Lai, R.; Gigot, J.; Humblet, Y.; Piessevaux, H.; Sempoux, C. Human equilibrative nucleoside transporter 1 (hENT1) expression is a potential predictive tool for response to gemcitabine in patients with advanced cholangiocarcinoma. Eur. J. Cancer 2012, 48, 990–996. [Google Scholar] [CrossRef]
- Deng, T.; Pan, H.; Han, R.; Huang, D.; Li, H.; Zhou, L.; Wang, X.; Bai, M.; Li, X.; Liu, R.; et al. Gemcitabine sensitivity factors, hENT1 and RRM1 as potential prognostic biomarker for advanced biliary tract cancer. Int. J. Clin. Exp. Med. 2014, 7, 5041–5049. [Google Scholar]
- Kim, J.; Kim, H.; Lee, J.-C.; Kim, J.W.; Paik, W.H.; Lee, S.H.; Hwang, J.-H.; Ryu, J.K.; Kim, Y.-T. Human equilibrative nucleoside transporter 1 (hENT1) expression as a predictive biomarker for gemcitabine chemotherapy in biliary tract cancer. PLoS ONE 2018, 13, e0209104. [Google Scholar] [CrossRef] [PubMed]
- Belkouz, A.; Labeur, T.A.; Dierks, J.; Dijk, F.; van Oijen, M.G.; Verheij, J.; van Gulik, T.M.; van de Vijver, M.J.; Wilmink, H.; Punt, C.J.; et al. Prognostic immunohistochemical biomarkers of chemotherapy efficacy in biliary tract cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. 2019, 141, 82–94. [Google Scholar] [CrossRef]
- Yoon, K.-A.; Woo, S.M.; Hong, E.K.; Jung, M.K.; Park, W.S.; Bae, K.; Han, S.-S.; Kim, T.H.; Koh, Y.H.; Park, S.-J.; et al. Cytidine Deaminase as a Molecular Predictor of Gemcitabine Response in Patients with Biliary Tract Cancer. Oncology 2015, 89, 345–350. [Google Scholar] [CrossRef]
- Woo, S.M.; Yoon, K.-A.; Hong, E.K.; Park, W.S.; Han, S.-S.; Park, S.-J.; Joo, J.; Park, E.Y.; Lee, J.H.; Kim, Y.-H.; et al. DCK expression, a potential predictive biomarker in the adjuvant gemcitabine chemotherapy for biliary tract cancer after surgical resection: Results from a phase II study. Oncotarget 2017, 8, 81394–81404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shuster, J.J. Median follow-up in clinical trials. J. Clin. Oncol. 1991, 9, 191–192. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Kornek, M.; Rodrigues, P.M.; Paiva, N.A.; Castro, R.E.; Urban, S.; Pereira, S.P.; Cadamuro, M.; Rupp, C.; Loosen, S.H.; et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019, 39, 108–122. [Google Scholar] [CrossRef]
- Jordheim, L.P.; Sève, P.; Tredan, O.; Dumontet, C. The ribonucleotide reductase large subunit (RRM1) as a predictive factor in patients with cancer. Lancet Oncol. 2011, 12, 693–702. [Google Scholar] [CrossRef]
- Miyazaki, K.; Nakamura, J.; Kohya, N.; Kai, K.; Ohtaka, K.; Hashiguchi, K.; Hiraki, M.; Kitajima, Y.; Tokunaga, O.; Noshiro, H. Ribonucleotide reductase subunit M1 assessed by quantitative double-fluorescence immunohistochemistry predicts the efficacy of gemcitabine in biliary tract carcinoma. Int. J. Oncol. 2010, 37, 845–852. [Google Scholar] [CrossRef]
- Sasaki, H.; Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Kondo, N.; Sueda, T. Concurrent analysis of human equilibrative nucleoside transporter 1 and ribonucleotide reductase subunit 1 expression increases predictive value for prognosis in cholangiocarcinoma patients treated with adjuvant gemcitabine-based chemotherapy. Br. J. Cancer 2014, 111, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Amano, R.; Yamada, N.; Kimura, K.; Yashiro, M.; Nakata, B.; Hirakawa, K. Prognostic predictive values of gemcitabine sensitivity-related gene products for unresectable or recurrent biliary tract cancer treated with gemcitabine alone. World J. Surg. Oncol. 2013, 11, 1–117. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Zhang, X.; Wu, J.; Chen, L.; Li, L.; Sun, J.; Lv, Y.; Wei, X.; Du, Y.; Jin, H.; et al. RRM1 expression and clinical outcome of gemcitabine-containing chemotherapy for advanced non-small-cell lung cancer: A meta-analysis. Lung Cancer 2012, 75, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Wakai, T.; Shirai, Y.; Sakata, J.; Takamura, M.; Matsuda, Y.; Korita, P.V.; Muneoka, K.; Sasaki, M.; Ajioka, Y.; Hatakeyama, K. Ribonucleotide Reductase M1 Expression in Intrahepatic Cholangiocarcinoma. Hepatogastroenterology 2011, 58, 1659–1663. [Google Scholar] [CrossRef]
- Santini, D.; Schiavon, G.; Vincenzi, B.; Cass, C.E.; Vasile, E.; Manazza, A.D.; Catalano, V.; Baldi, G.G.; Lai, R.; Rizzo, S.; et al. Human Equilibrative Nucleoside Transporter 1 (hENT1) Levels Predict Response to Gemcitabine in Patients With Biliary Tract Cancer (BTC). Curr. Cancer Drug Targets 2011, 11, 123–129. [Google Scholar] [CrossRef][Green Version]
- Chiang, N.-J.; Hsu, C.; Chen, J.-S.; Tsou, H.-H.; Shen, Y.-Y.; Chao, Y.; Chen, M.-H.; Yeh, T.-S.; Shan, Y.-S.; Huang, S.-F.; et al. Expression levels of ROS1/ALK/c-MET and therapeutic efficacy of cetuximab plus chemotherapy in advanced biliary tract cancer. Sci. Rep. 2016, 6, 25369. [Google Scholar] [CrossRef]
| Variables | N (%) | Univariate (Overall Survival) | Univariate (Progression-Free Survival) | ||||
|---|---|---|---|---|---|---|---|
| n | HR (95% CI) | p | n | HR (95% CI) | p | ||
| Age, median (range) | 65.5 (45–81) | 31 | 1.00 (0.96–1.05) | 0.859 | 31 | 1.01 (0.96–1.06) | 0.653 |
| Sex | |||||||
| Male | 20 (58.8) | 19 | 1 | 20 | 1 | ||
| Female | 14 (41.2) | 12 | 0.78 (0.38–1.61) | 0.498 | 11 | 0.62 (0.29–1.33) | 0.215 |
| Primary tumor site | |||||||
| Extrahepatic | 8 (23.5) | 8 | 1 | 0.566 | 7 | 1 | 0.632 |
| Gallbladder | 16 (47.1) | 13 | 0.78 (0.32–1.89) | 0.574 | 15 | 1.10 (0.42–2.85) | 0.852 |
| Intrahepatic | 10 (29.4) | 10 | 1.21 (0.47–3.11) | 0.690 | 19 | 1.56 (0.55–1.46) | 0.404 |
| Pathological differentiation | |||||||
| Well | 2 (6.5) | 2 | 1 | 0.441 | 2 | 1 | 0.193 |
| Moderately | 16 (51.6) | 14 | 0.74 (0.16–3.34) | 0.691 | 14 | 0.63 (0.14–2.94) | 0.556 |
| Poorly | 13 (41.9) | 12 | 0.46 (0.10–2.13) | 0.317 | 12 | 0.32 (0.06–1.57) | 0.159 |
| Stage | |||||||
| Metastatic disease | 24 (70.6) | 21 | 1 | 22 | 1 | ||
| Recurrent disease | 10 (29.4) | 10 | 0.87 (0.40–1.85) | 0.708 | 9 | 0.62 (0.27–1.41) | 0.252 |
| Metastatic sites | |||||||
| Liver metastasis | 16 (47.1) | 14 | 0.81 (0.45–1.87) | 0.807 | 16 | 1.28 (0.62–2.64) | 0.502 |
| Lung metastasis | 5 (14.7) | 5 | 1.96 (0.74–5.20) | 0.179 | 5 | 5.95 (1.92–18.5) | 0.002 |
| Peritoneal seeding | 6 (17.7) | 6 | 3.07 (1.21–7.82) | 0.018 | 6 | 2.53 (0.99–6.49) | 0.054 |
| Lymph node metastasis | 20 (58.8) | 18 | 0.97 (0.47–1.99) | 0.929 | 17 | 0.80 (0.39–1.64) | 0.538 |
| Number of metastatic sites | 31 | 1.73 (1.04–2.86) | 0.035 | 31 | 3.01 (1.59–5.68) | <0.001 | |
| 1 | 19 (55.9) | 17 | 16 | ||||
| 2 | 12 (35.3) | 11 | 12 | ||||
| 3 or more | 3 (8.8) | 3 | 3 | ||||
| IHC expression | |||||||
| hENT1 | 25 (73.5) | 23 | 1.95 (0.84–4.52) | 0.119 | 23 | 1.73 (0.72–4.13) | 0.219 |
| DCK | 11 (32.4) | 11 | 1.00 (0.48–2.10) | 0.997 | 11 | 1.16 (0.55–2.47) | 0.697 |
| CDA | 12 (36.4) | 12 | 1.39 (0.66–2.94) | 0.390 | 11 | 1.00 (0.46–2.18) | 0.998 |
| RRM1 | 10 (30.3) | 10 | 2.30 (1.03–5.14) | 0.043 | 9 | 1.68 (0.75–3.77) | 0.207 |
| Variables | Chemotherapy Response | ||
|---|---|---|---|
| PR + SD (%) | PD (%) | p Value | |
| hENT1 | 1 | ||
| (−) | 4 (44.4) | 5 (55.6) | |
| (+) | 10 (40) | 15 (60) | |
| DCK | 0.257 | ||
| (−) | 10 (43.5) | 13 (56.5) | |
| (+) | 4 (36.4) | 7 (63.6) | |
| CDA | 1 | ||
| (−) | 9 (42.9) | 12 (57.1) | |
| (+) | 5 (41.7) | 7 (58.3) | |
| RRM1 | 0.257 | ||
| (−) | 8 (34.8) | 15 (65.2) | |
| (+) | 6 (60) | 4 (40) | |
| HR (95%CI) | p Value | |
|---|---|---|
| Model 1 including peritoneal seeding | ||
| RRM1 expression | 3.33 (1.37–8.07) | 0.008 |
| Peritoneal seeding | 4.99 (1.76–14.2) | 0.003 |
| Model 2 including number of metastatic sites | ||
| RRM1 expression | 3.44 (1.40–8.45) | 0.007 |
| Number of metastatic sites | 2.30 (1.30–4.05) | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, J.W.; Lee, B.; Park, W.S.; Han, N.; Hong, E.K.; Park, E.Y.; Han, S.S.; Park, S.-J.; Kim, T.H.; Lee, W.J.; et al. RRM1 Expression as a Prognostic Biomarker for Unresectable or Recurrent Biliary Tract Cancer Treated with Gemcitabine plus Cisplatin. J. Clin. Med. 2021, 10, 4652. https://doi.org/10.3390/jcm10204652
Chun JW, Lee B, Park WS, Han N, Hong EK, Park EY, Han SS, Park S-J, Kim TH, Lee WJ, et al. RRM1 Expression as a Prognostic Biomarker for Unresectable or Recurrent Biliary Tract Cancer Treated with Gemcitabine plus Cisplatin. Journal of Clinical Medicine. 2021; 10(20):4652. https://doi.org/10.3390/jcm10204652
Chicago/Turabian StyleChun, Jung Won, Boyoung Lee, Weon Seo Park, Nayoung Han, Eun Kyung Hong, Eun Young Park, Sung Sik Han, Sang-Jae Park, Tae Hyun Kim, Woo Jin Lee, and et al. 2021. "RRM1 Expression as a Prognostic Biomarker for Unresectable or Recurrent Biliary Tract Cancer Treated with Gemcitabine plus Cisplatin" Journal of Clinical Medicine 10, no. 20: 4652. https://doi.org/10.3390/jcm10204652
APA StyleChun, J. W., Lee, B., Park, W. S., Han, N., Hong, E. K., Park, E. Y., Han, S. S., Park, S.-J., Kim, T. H., Lee, W. J., & Woo, S. M. (2021). RRM1 Expression as a Prognostic Biomarker for Unresectable or Recurrent Biliary Tract Cancer Treated with Gemcitabine plus Cisplatin. Journal of Clinical Medicine, 10(20), 4652. https://doi.org/10.3390/jcm10204652






