Factors Associated with Decisions for Initial Dosing, Up-Titration of Propiverine and Treatment Outcomes in Overactive Bladder Syndrome Patients in a Non-Interventional Setting
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Flow and Baseline Data
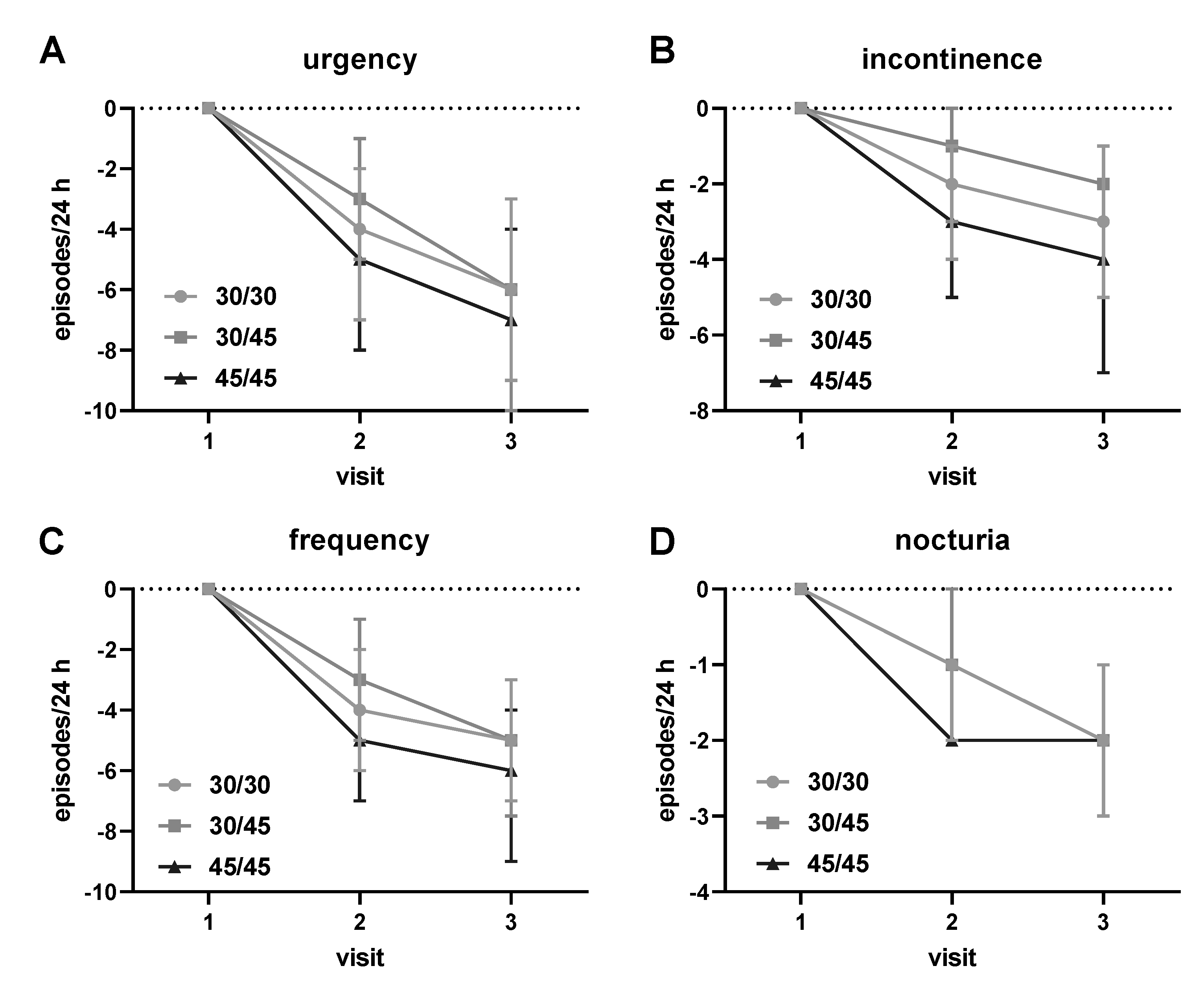
3.2. Descriptive Analysis of Treatment Outcomes
3.3. Factors Associated with Dosing Decision at Visit 1
3.4. Factors Associated with Dosing Increase at Visit 2
3.5. Factors Associated with Treatment Outcomes
3.6. Safety and Tolerability
4. Discussion
4.1. Critique of Methods
4.2. Factors Associated with Initial Dosing
4.3. Factors Associated with Dose Escalation
4.4. Factors Associated with Treatment Outcomes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novara, G.; Galfano, A.; Secco, S.; D’Elia, C.; Cavalleri, S.; Ficarra, V.; Artibani, W. A systematic review and meta-analysis of randomized controlled trials with antimuscarinic drugs for overactive bladder. Eur. Urol. 2008, 54, 740–764. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, W.S.; McPheeters, M.; Blume, J.; Surawicz, T.; Worley, K.; Wang, L.; Hartmann, K. Comparative effectiveness of anticholinergic therapy for overactive bladder in women. A systematic review and meta-analysis. Obstet. Gynecol. 2015, 125, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Steers, W.D.; Corcos, J.; Foote, J.; Kralidis, G. An investigation of dose titration with darifenacin, an M3-selective receptor antagonist. BJU Int. 2005, 95, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Khullar, V.; Rovner, E.S.; Dmochowski, R.; Nitti, V.; Wang, J.; Guan, E. Fesoterodine dose response in subjects with overactive bladder syndrome. Urology 2008, 71, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Jünemann, K.P.; Hessdörfer, E.; Unamba-Oparah, I.; Berse, M.; Brünjes, R.; Madersbacher, H.; Gramatté, T. Propiverine hydrochloride immediate and extended release: Comparison of efficacy and tolerability in patients with overactive bladder. Urol. Int. 2006, 77, 334–339. [Google Scholar] [CrossRef]
- Cardozo, L.; Lisec, M.; Millard, R.; van Vierssen Trip, O.B.; Kuzmin, I.; Drogendijk, T.E.; Huang, M.; Ridder, A.M. Randomized, double-blind placebo-controlled trial of the once-daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J. Urol. 2004, 172, 1919–1924. [Google Scholar] [CrossRef]
- Ruhe, H.G.; Booij, J.; van Weert, H.C.; Reitsma, J.B.; Fransen, E.J.F.; Michel, M.C.; Schene, A.H. Evidence why paroxetine dose-escalation is not effective in major depressive disorder: A randomized-controlled trial with assessment of serotonin transporter occupancy. Neuropsychopharmacology 2009, 34, 999–1010. [Google Scholar] [CrossRef]
- Michel, M.C.; Staskin, D. Understanding dose titration: Overactive bladder treatment with fesoterodine as an example. Eur. Urol. Suppl. 2011, 10, 8–13. [Google Scholar] [CrossRef]
- Michel, M.C.; Barendrecht, M.M. Physiological and pathological regulation of the autonomic control of urinary bladder contractility. Pharmacol. Ther. 2008, 117, 297–312. [Google Scholar] [CrossRef]
- Fenech, A.G.; Billington, C.K.; Swan, C.; Richards, S.; Hunter, T.; Ebejer, M.J.; Felice, A.E.; Ellul-Micallef, R.; Hall, I.P. Novel polymorphisms influencing transcription of the human CHRM2 gene in airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 2004, 30, 678–686. [Google Scholar] [CrossRef]
- Ancelin, M.L.; Artero, S.; Portet, F.; Dupuy, A.M.; Touchon, J.; Ritchie, K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: Longitudinal cohort study. Br. Med. J. 2006, 332, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Witte, L.P.W.; Mulder, W.M.C.; de la Rosette, J.J.M.C.H.; Michel, M.C. Muscarinic receptor antagonists for overactive bladder treatment: Does one fit all? Curr. Opin. Urol. 2009, 19, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wyndaele, J.J.; Goldfischer, E.R.; Morrow, J.D.; Gong, J.; Tseng, L.J.; Choo, M.S. Patient-optimized doses of fesoterodine improve bladder symptoms in an open-label, flexible-dose study. BJU Int. 2011, 107, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, L.; Hall, T.; Ryan, J.; Bitoun, C.E.; Kausar, I.; Darekar, A.; Wagg, A. Safety and efficacy of flexible-dose fesoterodine in British subjects with overactive bladder: Insights into factors associated with dose escalation. Int. Urogynecol. J. 2012, 23, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Wagg, A.; Darekar, A.; Arumi, D.; Khullar, V.; Oelke, M. Factors associated with dose escalation of fesoterodine for treatment of overactive bladder in people >65 years of age: A post hoc analysis of data from the SOFIA study. Neurourol. Urodyn. 2015, 34, 438–443. [Google Scholar] [CrossRef]
- Wyndaele, J.J.; Schneider, T.; MacDiarmid, S.; Scholfield, D.; Arumi, D. Flexible dosing with fesoterodine 4 and 8 mg: A systematic review of data from clinical trials. Int. J. Clin. Pract. 2014, 68, 830–840. [Google Scholar] [CrossRef]
- Goldman, H.B.; Oelke, M.; Kaplan, S.A.; Kitta, T.; Russell, D.; Carlsson, M.; Arumi, D.; Mangan, E.; Ntanios, F. Do patient characteristics predict which patients with overactive bladder benefit from a higher fesoterodine dose? Int. Urogynecol. J. 2018, 30, 239–244. [Google Scholar] [CrossRef]
- Cardozo, L.; Amarenco, G.; Pushkar, D.; Mikulas, J.; Drogendijk, T.; Wright, M.; Compion, G.; Group, S.S. Severity of overactive bladder symptoms and response to dose escalation in a randomized, double-blind trial of solifenacin (SUNRISE). BJU Int. 2013, 111, 804–810. [Google Scholar] [CrossRef]
- Chun, J.-Y.; Song, M.; Han, J.-Y.; Na, S.; Hong, B.; Choo, M.-S. Clinical factors associated with dose escalation of solifenacin for the treatment of overactive bladder in real life practice. Int. Neurourol. J. 2014, 18, 23–30. [Google Scholar] [CrossRef]
- Shim, M.; Kim, J.K.; Bang, W.J.; Lee, Y.S.; Cho, S.T.; Cho, J.S.; Joo, K.J.; Hyun, J.S.; Kim, B.H.; Lee, J.B.; et al. Efficacy and safety of dose escalation in male patients with overactive bladder showing poor efficacy after low-dose antimuscarinic treatment: A retrospective multicenter study. Investig. Clin. Urol. 2020, 61, 600–606. [Google Scholar] [CrossRef]
- Cardozo, L.; Khullar, V.; El-Tahtawy, A.; Guan, Z.; Malhotra, B.; Staskin, D. Modeling dose-response relationships of the effects of fesoterodine in patients with overactive bladder. BMC Urol. 2010, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Staskin, D.R.; Michel, M.C.; Sun, F.; Guan, Z.; Morrow, J.D. The effect of elective sham dose escalation on the palcebo response during an antimuscarinic trial for overactive bladder symptoms. J. Urol. 2012, 187, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Wuest, M.; Hecht, J.; Christ, T.; Braeter, M.; Schoeberl, C.; Hakenberg, O.W.; Wirth, M.P.; Ravens, U. Pharmacodynamics of propiverine and three of its metabolites on detrusor contraction. Br. J. Pharmacol. 2005, 145, 608–619. [Google Scholar] [CrossRef]
- Zhu, H.L.; Brain, K.L.; Aishima, M.; Shibata, A.; Young, J.S.; Sueishi, K.; Teramoto, N. Actions of two main metabolites of propiverine (M-1 and M-2) on voltage-dependent L-type Ca2+ currents and Ca2+ transients in murine urinary bladder myocytes. J. Pharmacol. Exp. Ther. 2008, 324, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Frazier, E.P.; Peters, S.L.M.; Braverman, A.S.; Ruggieri, M.R., Sr.; Michel, M.C. Signal transduction underlying control of urinary bladder smooth muscle tone by muscarinic receptors and ß-adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 377, 449–462. [Google Scholar] [CrossRef]
- Amiri, M.; Murgas, S.; Stang, A.; Michel, M.C. Do overactive bladder symptoms and their treatment-associated changes exhibit a normal distribution? Implications for analysis and reporting. Neurourol. Urodyn. 2020, 39, 754–761. [Google Scholar] [CrossRef]
- Dimpfl, T.; Kölbl, H.; Peschers, U.; Petri, E.; Gauruder-Burmester, A.; Höfner, K.; Schultz-Lampel, D.; Tamussino, K.; Heidler, H.; Schär, G. The Overactive Bladder; AWMF: Frankfurt, Germany, 2010. [Google Scholar]
- Michel, M.C.; Murphy, T.J.; Motulsky, H.J. New author guidelines for displaying data and reporting data analysis and statistical methods in experimental biology. J. Pharmacol. Exp. Ther. 2020, 372, 136–147. [Google Scholar] [CrossRef]
- van Leeuwen, J.H.S.; Castro, R.; Busse, M.; Bemelmans, B.L.H. The placebo effect in the pharmacologic treatment of patients with lower urinary tract symptoms. Eur. Urol. 2006, 50, 440–453. [Google Scholar] [CrossRef]
- Cornu, J.N.; Abrams, P.; Chapple, C.R.; Dmochowski, R.R.; Lemack, G.E.; Michel, M.C.; Tubaro, A.; Madersbacher, S. A contemporary assessment of nocturia: Definitions, epidemiology, pathophysiology and management. A systematic review and meta-analysis. Eur. Urol. 2012, 62, 877–890. [Google Scholar] [CrossRef]
- Michel, M.C.; de la Rosette, J.J.M.C.H.; Piro, M.; Schneider, T. Comparison of symptom severity and treatment response in patients with incontinent and continent overactive bladder. Eur. Urol. 2005, 48, 110–115. [Google Scholar] [CrossRef]
- Schneider, T.; Marschall-Kehrel, D.; Hanisch, J.U.; Michel, M.C. Do gender, age or life style factors affect responses to anti-muscarinic treatment in overactive bladder patients? Int. J. Clin. Pract. 2010, 64, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; de la Rosette, J.J.M.C.H.; Michel, M.C. Nocturia—A non-specific but important symptom of urological disease. Int. J. Urol. 2009, 16, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Schumacher, H.; Mehlburger, L.; de la Rosette, J.J.M.C.H. Factors associated with nocturia-related quality of life in men with lower urinary tract symptoms and treated with tamsulosin oral controlled absorption system in a noniInterventional study. Front. Pharmacol. 2020, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Oelke, M.; Murgas, S.; Baumann, I.; Schnabel, F.; Michel, M.C. Efficacy of propiverine ER with or without α-blockers related to maximum urinary flow rate in adult men with OAB: Results of a 12-week, multicenter, non-interventional study. World J. Urol. 2011, 29, 217–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schneider, T.; Arumi, D.; Crook, T.J.; Sun, F.; Michel, M.C. An observational study of patient satisfaction with fesoterodine in the treatment of overactive bladder: Effects of additional educational material. Int. J. Clin. Pract. 2014, 68, 1074–1080. [Google Scholar] [CrossRef]
- Michel, M.C.; Wetterauer, U.; Vogel, M.; de la Rosette, J.J.M.C.H. Cardiovascular safety and overall tolerability of solifenacin in routine clinical use. Drug Saf. 2008, 31, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.F.; Kuchel, G.A.; Smith, P.P. Overactive bladder in the vulnerable elderly. Res. Rep. Urol. 2014, 6, 131–138. [Google Scholar] [CrossRef][Green Version]
- Michel, M.C.; Oelke, M.; Goepel, M.; Beck, E.; Burkart, M. Relationships among symptoms, bother, and treatment satisfaction in overactive bladder patients. Neurourol. Urodyn. 2007, 26, 190–195. [Google Scholar] [CrossRef]
| Initial 30 mg | Initial 45 mg | p-Value | |
|---|---|---|---|
| n | 1021 | 239 | |
| Demographic parameters | |||
| Gender, % female/male | 64.3/32.6 | 64.3/32.9 | |
| Previous OAB treatment, % | 33.7 | 45.0 | |
| Age, years | 65.8 ± 13.1 | 65.4 ± 12.5 | 0.3820 |
| Height, cm | 169.0 ± 7.9 | 169.9 ± 8.3 | 0.1543 |
| Weight, kg | 77.4 ± 14.2 | 80.3 ± 17.0 | 0.0184 |
| BMI, kg/m2 | 27.1 ± 4.3 | 27.9 ± 5.5 | 0.1187 |
| OAB-related parameters | |||
| OAB duration, months | 12.1 (3.9; 34.6) | 13.2 (4.7; 35.1) | 0.3343 |
| Urgency episodes/24 h | 9 (6; 13) 10.0 ± 5.8 | 10 (7; 14) 10.8 ± 5.8 | 0.0251 |
| Incontinence episodes/24 h | 6 (4; 23) 4.7 ± 3.7 | 8 (5; 30) 6.2 ± 4.4 | <0.0001 |
| Urinary frequency/24 h | 13 (11; 16) 13.3 ± 4.2 | 14 (11; 17) 14.3 ± 4.4 | 0.0004 |
| Nocturia episodes/24 h | 3 (2; 4) 3.4 ± 1.6 | 3 (3; 5) 3.7 ± 1.7 | 0.0008 |
| Parameter | Estimate ± SE | p-Value | Estimate ± SE | p-Value |
|---|---|---|---|---|
| Study I | Study II | |||
| Gender, female | −0.225 ± 0.140 | 0.1081 | - | - |
| Age, years | 0.017 ± 0.008 | 0.0335 | 0.027 ± 0.010 | 0.0067 |
| Weight, kg | 0.083 ± 0.070 | 0.2338 | −0.132 ± 0.093 | 0.1556 |
| Height, cm | −0.120 ± 0.069 | 0.0820 | 0.010 ± 0.090 | 0.2706 |
| BMI, kg/m2 | −0.261 ± 0.198 | 0.1875 | 0.347 ± 0.271 | 0.2013 |
| OAB duration, months | - | - | −0.011 ± 0.002 | <0.0001 |
| Urgency/24 h | - | - | 0.032 ± 0.023 | 0.1606 |
| Incontinence/24 h | −0.107 ± 0.024 | <0.0001 | - | - |
| Micturitions/24 h | - | - | −0.238 ± 0.071 | 0.0008 |
| Nocturia/24 h | −0.126 ± 0.060 | 0.0348 | - | - |
| Stay on 30 mg | Increase to 45 mg | p-Value | |
|---|---|---|---|
| n | 789 | 160 | |
| Demographic parameters | |||
| Gender, % female/male | 66.2/33.8 | 60.0/40.0 | |
| Previous OAB treatment, % | 31.1 | 50.6 | |
| Age, years | 65.6 ±13.1 | 67.0 ± 13.0 | 0.2313 |
| Height, cm | 169.0 ± 7.7 | 171.3 ± 7.9 | 0.0010 |
| Weight, kg | 77.0 ± 13.9 | 81.2 ± 14.4 | <0.0001 |
| BMI, kg/m2 | 27.0 ± 4.2 | 27.6 ± 4.6 | 0.1403 |
| OAB-related parameters at baseline | |||
| OAB duration, months | 11.2 (3.6; 31.3) | 13.6 (5.0; 51.9) | |
| Urgency episodes/24 h | 9 (5; 13) 9.6 ± 5.6 | 11 (6.5; 15) 11.7 ± 6.6 | <0.0001 |
| Incontinence episodes/24 h | 4 (2; 6) 4.6 ± 3.4 | 4 (2; 7) 5.4 ± 4.7 | <0.0001 |
| Urinary frequency/24 h | 13 (10; 15) 13.1 ± 4.1 | 14 (12; 16) 14.3 ± 4.1 | <0.0001 |
| Nocturia/24 h | 3 (2; 4) 3.3 ± 1.5 | 4 (3; 4.5) 3.7 ± 1.6 | <0.0001 |
| OAB-related parameters after 4 weeks | |||
| Urgency episodes/24 h | 3 (2; 6) 4.4 ± 3.6 | 7 (4; 11) 8.0± 5.4 | <0.0001 |
| Incontinence episodes/24 h | 1 (0; 2) 1.8 ± 2.1 | 2 (1; 5) 3.6 ± 3.6 | <0.0001 |
| Urinary frequency/24 h | 8 (7; 10) 8.8 ± 2.8 | 11 (9; 13) 11.2 ± 3.4 | <0.0001 |
| Nocturia/24 h | 2 (1; 2) 1.8 ± 1.1 | 2 (2; 3) 2.7 ± 1.2 | <0.0001 |
| Parameter | Estimate ± SE | p-value | Estimate ± SE | p-Value |
|---|---|---|---|---|
| Study I | Study II | |||
| Age, years | - | - | 1.420 ± 1.387 | 0.3059 |
| Weight, kg | −0.017 ± 0.009 | 0.0611 | −0.17 ± 0.012 | 0.1444 |
| Height, cm | −0.036 ± 0.018 | 0.0413 | - | - |
| Urgency/24 h baseline | −0.100 ± 0.036 | 0.0049 | -0.192 ± 0.054 | 0.0004 |
| Incontinence/24 h baseline | −0.087 ± 0.053 | 0.0998 | 0.108 ± 0.078 | 0.1659 |
| Micturitions/24 h baseline | −0.069 ± 0.056 | 0.2185 | - | - |
| Nocturia/24 h baseline | −0.160 ± 0.119 | 0.1776 | −0.311 ± 0.155 | 0.0450 |
| Urgency/24 h 4 weeks | −0.067 ± 0.037 | 0.0669 | - | - |
| Incontinence/24 h 4 weeks | −0.126 ± 0.058 | 0.0300 | −0.201 ±00.084 | 0.0165 |
| Micturitions/24 4 weeks | - | - | - | - |
| Nocturia/24 h 4 weeks | - | - | −0.549 ± 0.227 | 0.0153 |
| Parameter | Estimate ± SE | p-Value | Estimate ± SE | p-Value |
|---|---|---|---|---|
| Study I | Study II | |||
| Gender, female | 0.026 ± 0.009 | 0.0056 | 0.011 ± 0.414 | 0.3954 |
| Age, years | 0.026 ± 0.009 | 0.0056 | 0.011 ± 0.012 | 0.3739 |
| Weight, kg | −0.123 ± 0.081 | 0.1592 | 0.019 ± 0.112 | 0.8628 |
| Height, cm | 0.114 ± 0.081 | 0.1592 | −0.028 ± 0.109 | 0.7973 |
| BMI, kg/m2 | 0.340 ± 0.235 | 0.1475 | −0.039 ± 0.327 | 0.9049 |
| OAB duration, months | 0.012 ± 0.002 | <0.0001 | 0.005 ± 0.003 | 0.0677 |
| Urgency/24 h | −0.714 ± 0.025 | <0.0001 | −0.842 ± 0.034 | <0.0001 |
| Incontinence/24 h | −0.003 ± 0.035 | 0.9282 | 0.210 ± 0.043 | <0.0001 |
| Micturitions/24 h | −0.008 ± 0.037 | 0.8364 | 0.029 ± 0.054 | 0.5928 |
| Nocturia/24 h | −0.043 ± 0.008 | 0.6264 | −0.087 ± 0.115 | 0.4483 |
| Dose 30/30 * | 0.109 ± 0.297 | 0.7138 | −0.428 ± 0.328 | 0.1917 |
| Dose 30/45 * | 0.998 ± 0.373 | 0.0076 | 0.343± 0.490 | 0.4483 |
| Parameter | Estimate ± SE | p-Value | Estimate ± SE | p-Value |
|---|---|---|---|---|
| Study I | Study II | |||
| Gender, female | −0.012 ± 0.178 | 0.2662 | −0.331 ± 0.261 | 0.2060 |
| Age, years | 0.012 ± 0.005 | 0.0182 | 0.003 ± 0.008 | 0.7324 |
| Weight, kg | −0.048 ± 0.046 | 0.3025 | −0.051 ± 0.071 | 0.4701 |
| Height, cm | 0.042 ± 0.046 | 0.3741 | 0.043 ± 0.069 | 0.5347 |
| BMI, kg/m2 | 0.130 ± 0.132 | 0.3257 | 0.156 ± 0.206 | 0.4498 |
| OAB duration, months | 0.008 ± 0.001 | <0.0001 | 0.003 ± 0.002 | 0.1493 |
| Urgency/24 h | −0.008 ± 0.014 | 0.5685 | −0.056 ± 0.021 | 0.0086 |
| Incontinence/24 h | −0.765 ± 0.020 | <0.0001 | −0.657 ± 0.028 | <0.0001 |
| Micturitions/24 h | −0.018 ± 0.021 | 0.3851 | 0.011 ± 0.035 | 0.7559 |
| Nocturia/24 h | −0.032 ± 0.050 | 0.3851 | 0.022 ± 0.072 | 0.7655 |
| Dose 30/30 * | −0.103 ± 0.168 | 0.5384 | −0.247 ± 0.208 | 0.2359 |
| Dose 30/45 * | 0.487 ± 0.211 | 0.0211 | −0.128 ± 0.306 | 0.6758 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiri, M.; Schneider, T.; Oelke, M.; Murgas, S.; Michel, M.C. Factors Associated with Decisions for Initial Dosing, Up-Titration of Propiverine and Treatment Outcomes in Overactive Bladder Syndrome Patients in a Non-Interventional Setting. J. Clin. Med. 2021, 10, 311. https://doi.org/10.3390/jcm10020311
Amiri M, Schneider T, Oelke M, Murgas S, Michel MC. Factors Associated with Decisions for Initial Dosing, Up-Titration of Propiverine and Treatment Outcomes in Overactive Bladder Syndrome Patients in a Non-Interventional Setting. Journal of Clinical Medicine. 2021; 10(2):311. https://doi.org/10.3390/jcm10020311
Chicago/Turabian StyleAmiri, Marjan, Tim Schneider, Matthias Oelke, Sandra Murgas, and Martin C. Michel. 2021. "Factors Associated with Decisions for Initial Dosing, Up-Titration of Propiverine and Treatment Outcomes in Overactive Bladder Syndrome Patients in a Non-Interventional Setting" Journal of Clinical Medicine 10, no. 2: 311. https://doi.org/10.3390/jcm10020311
APA StyleAmiri, M., Schneider, T., Oelke, M., Murgas, S., & Michel, M. C. (2021). Factors Associated with Decisions for Initial Dosing, Up-Titration of Propiverine and Treatment Outcomes in Overactive Bladder Syndrome Patients in a Non-Interventional Setting. Journal of Clinical Medicine, 10(2), 311. https://doi.org/10.3390/jcm10020311







