Impact of Different Positive End-Expiratory Pressures on Lung Mechanics in the Setting of Moderately Elevated Intra-Abdominal Pressure and Acute Lung Injury in a Porcine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation and Instrumentation
2.2. Measurements and Calculations
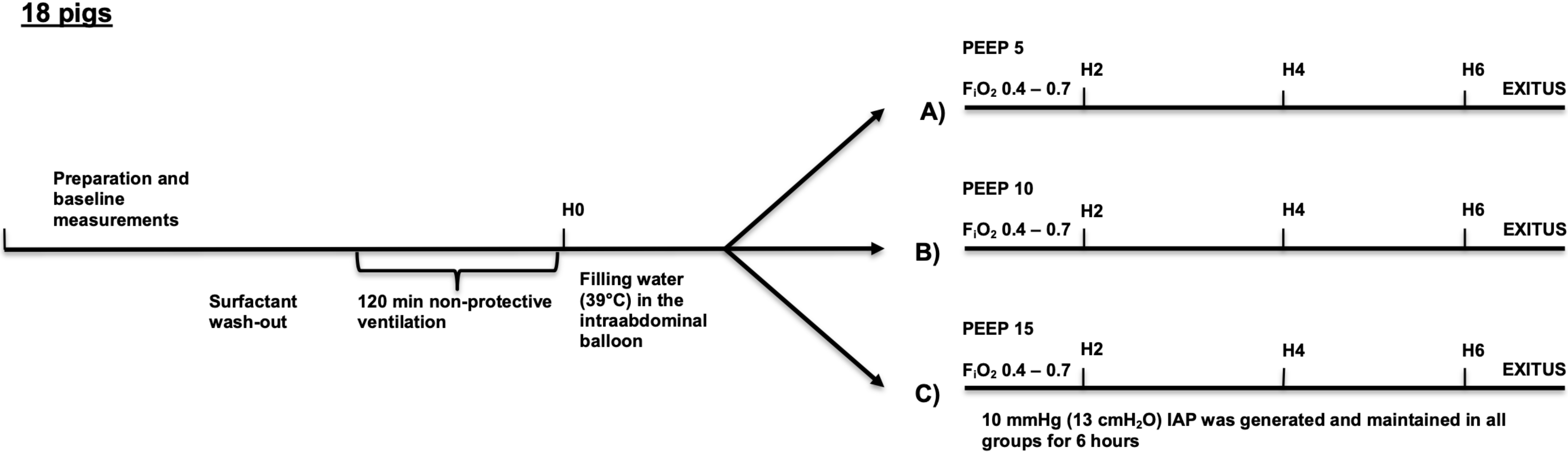
2.3. Experimental Protocol
2.4. Histology
2.5. Wet-To-Dry Ratio
2.6. Statistical Analysis
3. Results
Lung Injury Score and Wet-Dry Weight Ratio (W/D)
4. Discussion
4.1. Main Findings
4.2. Lung Injury Model and Elevated Intra-Abdominal Pressure
4.3. Alterations in Lung Mechanics
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALI | Acute lung injury |
| ARDS | Acute Respiratory Distress Syndrome |
| BAL | Broncho-alveolar-lavage |
| CI | Cardiac index |
| Cstat | Static lung compliance |
| EELV | End-expiratory lung volume |
| EL | Elastance of the lung |
| ERS | Elastance of the respiratory system |
| HR | Heart rate |
| IAH | Intra-abdominal hypertension |
| IAP | Intra-abdominal pressure |
| IQR | Interquartile range |
| MAP | Mean arterial pressure |
| ΔP | Driving pressure |
| ΔPL | Transpulmonary pressure |
| PEs | Esophageal pressure |
| PEEP | Positive end-expiratory pressure |
| PEsInsp | Inspiratory esophageal pressure |
| PEsExp | End-expiratory esophageal pressure |
| P/F ratio | PaO2/FiO2 ratio |
| RR | Respiratory rate |
| TPPExp | Transpulmonary expiratory pressure |
| TPPInsp | Transpulmonary inspiratory pressure |
| VILI | Ventilator-induced lung injury |
| W/D | Wet-to-dry ratio |
References
- Malbrain, M.L.; Chiumello, D.; Pelosi, P.; Bihari, D.; Innes, R.; Ranieri, V.M.; Del Turco, M.; Wilmer, A.; Brienza, N.; Malcangi, V.; et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: A multiple-center epidemiological study. Crit. Care Med. 2005, 33, 315–322. [Google Scholar] [CrossRef]
- Pelosi, P.; Quintel, M.; Malbrain, M.L. Effect of intra-abdominal pressure on respiratory mechanics. Acta Clin. Belg. 2007, 62, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Regli, A.; Pelosi, P.; Malbrain, M. Ventilation in patients with intra-abdominal hypertension: What every critical care physician needs to know. Ann. Intensive Care 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.C.; Xu, Q.P.; Pan, K.H.; Mao, C.; Jin, C.W. Effect of increased intra-abdominal pressure and decompressive laparotomy on aerated lung volume distribution. J. Zhejiang Univ. Sci. B 2010, 11, 378–385. [Google Scholar] [CrossRef]
- Gattinoni, L.; Pelosi, P.; Suter, P.M.; Pedoto, A.; Vercesi, P.; Lissoni, A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am. J. Respir. Crit. Care Med. 1998, 158, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Regli, A.; Hockings, L.E.; Musk, G.C.; Roberts, B.; Noffsinger, B.; Singh, B.; van Heerden, V.P. Commonly applied positive end-expiratory pressures do not prevent functional residual capacity decline in the setting of intra-abdominal hypertension: A pig model. Crit. Care 2010, 14, R128. [Google Scholar] [CrossRef]
- Regli, A.; Chakera, J.; De Keulenaer, B.L.; Roberts, B.; Noffsinger, B.; Singh, B.; van Heerden, V.P. Matching positive end-expiratory pressure to intra-abdominal pressure prevents end-expiratory lung volume decline in a pig model of intra-abdominal hypertension. Crit. Care Med. 2012, 40, 1879–1886. [Google Scholar] [CrossRef]
- Vidal, M.G.; Ruiz Weisser, J.; Gonzalez, F.; Toro, M.A.; Loudet, C.; Balasini, C.; Canales, H.; Reina, R.; Estenssoro, E. Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Crit. Care Med. 2008, 36, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Reintam Blaser, A.; Parm, P.; Kitus, R.; Starkopf, J. Risk factors for intra-abdominal hypertension in mechanically ventilated patients. Acta Anaesthesiol. Scand. 2011, 55, 607–614. [Google Scholar] [CrossRef]
- Lima, R.; Silva, P.L.; Capelozzi, V.L.; Oliveira, M.G.; Santana, M.C.E.; Cruz, F.F.; Pelosi, P.; Schanaider, A.; Malbrain, M.; Rocco, P.; et al. Early impact of abdominal compartment syndrome on liver, kidney and lung damage in a rodent model. Anaesthesiol. Intensive Ther. 2017, 49, 130–138. [Google Scholar] [CrossRef]
- Pelosi, P.; Vargas, M. Mechanical ventilation and intra-abdominal hypertension: ‘Beyond Good and Evil’. Crit. Care 2012, 16, 187. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Sklar, M.C.; Patel, B.K.; Beitler, J.R.; Piraino, T.; Goligher, E.C. Optimal Ventilator Strategies in Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Baydur, A.; Behrakis, P.K.; Zin, W.A.; Jaeger, M.; Milic-Emili, J. A simple method for assessing the validity of the esophageal balloon technique. Am. Rev. Respir. Dis. 1982, 126, 788–791. [Google Scholar]
- Lachmann, B.; Robertson, B.; Vogel, J. In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesthesiol. Scand. 1980, 24, 231–236. [Google Scholar] [CrossRef]
- Grieco, D.L.; Chen, L.; Brochard, L. Transpulmonary pressure: Importance and limits. Ann. Transl. Med. 2017, 5, 285. [Google Scholar] [CrossRef]
- Olegard, C.; Sondergaard, S.; Houltz, E.; Lundin, S.; Stenqvist, O. Estimation of functional residual capacity at the bedside using standard monitoring equipment: A modified nitrogen washout/washin technique requiring a small change of the inspired oxygen fraction. Anesth. Analg. 2005, 101, 206–212. [Google Scholar] [CrossRef]
- Malbrain, M.L.; Deeren, D.H. Effect of bladder volume on measured intravesical pressure: A prospective cohort study. Crit. Care. 2006, 10, R98. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Roberts, D.J.; De Waele, J.; Jaeschke, R.; Malbrain, M.L.; De Keulenaer, B.; Duchesne, J.; Bjorck, M.; Leppaniemi, A.; Ejike, J.C.; et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013, 39, 1190–1206. [Google Scholar] [CrossRef]
- Jakob, S.M.; Knuesel, R.; Tenhunen, J.J.; Pradl, R.; Takala, J. Increasing abdominal pressure with and without PEEP: Effects on intra-peritoneal, intra-organ and intra-vascular pressures. BMC Gastroenterol. 2010, 10, 70. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, M.O.; Deutsch, B.L.; Simeliunas, E.; Diktanaite, D.; Harms, A.; Brune, M.; Uhle, F.; Weigand, M.; Brenner, T.; Kalenka, A. Effect of moderate elevated intra-abdominal pressure on lung mechanics and histological lung injury at different positive end-expiratory pressures. PLoS ONE 2020, 15, e0230830. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.R.; Matute-Bello, G. Experimental models and emerging hypotheses for acute lung injury. Crit. Care Clin. 2011, 27, 735–752. [Google Scholar] [CrossRef]
- Mutoh, T.; Lamm, W.J.; Embree, L.J.; Hildebrandt, J.; Albert, R.K. Volume infusion produces abdominal distension, lung compression, and chest wall stiffening in pigs. J. Appl. Physiol. 1992, 72, 575–582. [Google Scholar] [CrossRef]
- Quintel, M.; Pelosi, P.; Caironi, P.; Meinhardt, J.P.; Luecke, T.; Herrmann, P.; Taccone, P.; Rylander, C.; Valenza, F.; Carlesso, E.; et al. An increase of abdominal pressure increases pulmonary edema in oleic acid-induced lung injury. Am. J. Respir. Crit. Care Med. 2004, 169, 534–541. [Google Scholar] [CrossRef]
- Hochhausen, N.; Biener, I.; Rossaint, R.; Follmann, A.; Bleilevens, C.; Braunschweig, T.; Leonhardt, S.; Czaplik, M. Optimizing PEEP by Electrical Impedance Tomography in a Porcine Animal Model of ARDS. Respir. Care 2017, 62, 340–349. [Google Scholar] [CrossRef]
- Andrews, P.L.; Sadowitz, B.; Kollisch-Singule, M.; Satalin, J.; Roy, S.; Snyder, K.; Gatto, L.A.; Nieman, G.F.; Habashi, N.M. Alveolar instability (atelectrauma) is not identified by arterial oxygenation predisposing the development of an occult ventilator-induced lung injury. Intensive Care Med. Exp. 2015, 3, 54. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef]
- Silva, P.L.; Negrini, D.; Rocco, P.R. Mechanisms of ventilator-induced lung injury in healthy lungs. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 301–313. [Google Scholar] [CrossRef]
- Steinberg, J.M.; Schiller, H.J.; Halter, J.M.; Gatto, L.A.; Lee, H.M.; Pavone, L.A.; Nieman, G.F. Alveolar instability causes early ventilator-induced lung injury independent of neutrophils. Am. J. Respir. Crit. Care Med. 2004, 169, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.B.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Torquato, J.A.; Lucato, J.J.; Antunes, T.; Barbas, C.V. Interaction between intra-abdominal pressure and positive-end expiratory pressure. Clinics (Sao Paulo) 2009, 64, 105–112. [Google Scholar] [CrossRef] [PubMed]
- De Keulenaer, B.L.; De Waele, J.J.; Powell, B.; Malbrain, M.L. What is normal intra-abdominal pressure and how is it affected by positioning, body mass and positive end-expiratory pressure? Intensive Care Med. 2009, 35, 969–976. [Google Scholar] [CrossRef]
- Regli, A.; De Keulenaer, B.L.; Singh, B.; Hockings, L.E.; Noffsinger, B.; van Heerden, P.V. The respiratory pressure-abdominal volume curve in a porcine model. Intensive Care Med. Exp. 2017, 5, 11. [Google Scholar] [CrossRef]
- Krebs, J.; Pelosi, P.; Tsagogiorgas, C.; Alb, M.; Luecke, T. Effects of positive end-expiratory pressure on respiratory function and hemodynamics in patients with acute respiratory failure with and without intra-abdominal hypertension: A pilot study. Crit. Care 2009, 13, R160. [Google Scholar] [CrossRef]

| Baseline | H0 | |
|---|---|---|
| IAP | 2.3 ± 0.3 | 3.2 ± 0.3 * |
| EELV | 1269 ± 68 | 665 ± 54 * |
| EELV/kg | 25 ± 1 | 13 ± 1 * |
| ∆P | 12 ± 0 | 26 ± 1 * |
| ∆PL | 6 ± 0 | 19 ± 1 * |
| TPPInsp | 6 ± 1 | 17 ± 1 * |
| TPPExp | 0 ± 0 | −2 ± 1 * |
| Cstat | 42 ± 2 | 21 ± 1 * |
| ERS | 30 ± 1 | 65 ± 4 * |
| ECW | 15 ± 1 | 18 ± 1 * |
| EL | 15 ± 1 | 47 ± 4 * |
| HR | 66 ± 3 | 83 ± 5 * |
| MAP | 84 ± 2 | 101 ± 2 * |
| P/F ratio | 456 ± 13 | 110 ± 13 * |
| CI | 4.2 ± 0.2 | 4.7 ± 0.2 |
| Lactate | 1.4 ± 0.2 | 1.4 ± 0.1 |
| pH | 7.45 ± 0.01 | 7.37 ± 0.01 |
| RR | 20 (0) | 24 (4) |
| Group A (n = 6) | Group B (n = 6) | Group C (n = 6) | ||
|---|---|---|---|---|
| Weight (kg) | Baseline | 51 ± 3 | 49 ± 3 | 51 ± 3 |
| PEEP | 5 | 10 | 15 | |
| IAP | H0 | 3 ± 1 | 3 ± 0 | 4 ± 1 |
| IAP | H6 | 10 ± 1 * | 10 ± 0 * | 10 ± 0 * |
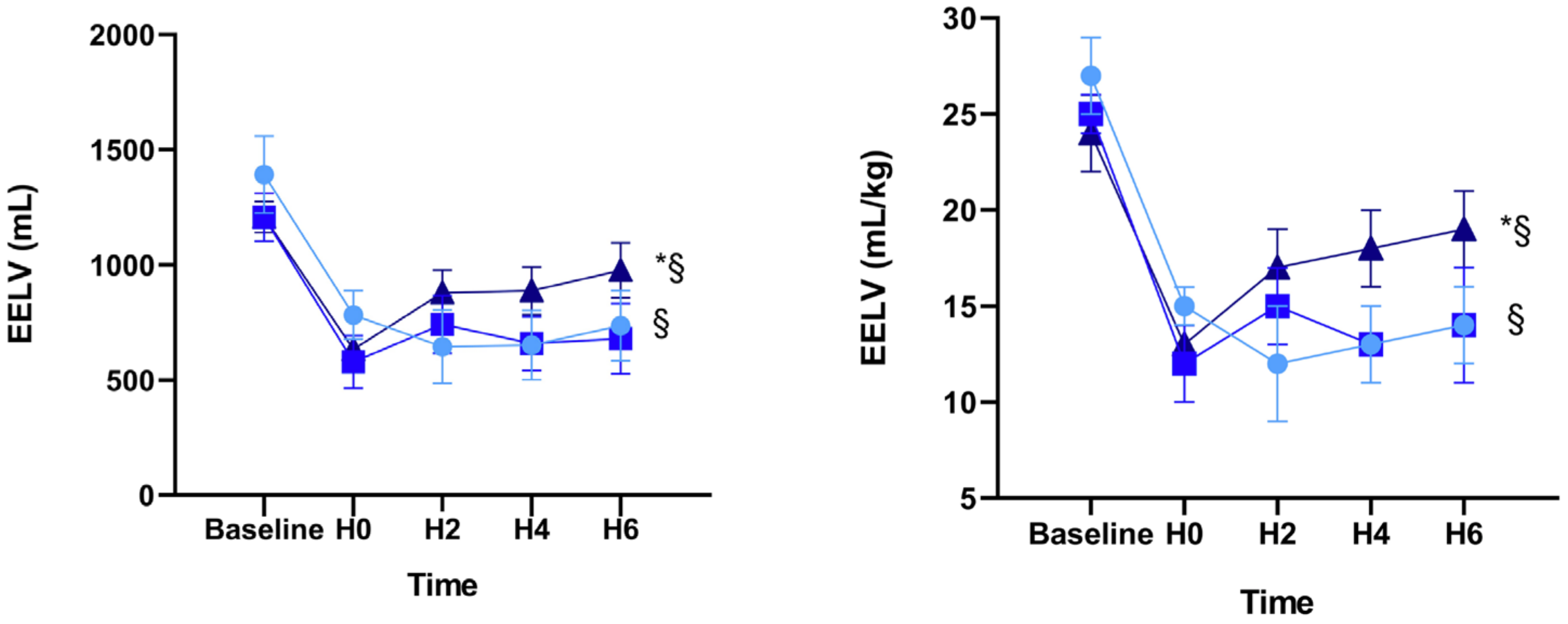
| EELV | H0 | 782 ± 105 | 579 ± 114 | 633 ± 39 |
| EELV | H6 | 736 ± 152 | 680 ± 153 | 976 ± 119 * |
| EELV/kg | H0 | 15 ± 1 | 12 ± 2 | 13 ± 1 |
| EELV/kg | H6 | 14 ± 2 | 13 ± 3 | 19 ± 2 * |
| ∆P | H0 | 22 ± 1 | 26 ± 2 | 29 ± 3 |
| ∆P | H6 | 28 ± 2 *§ | 25 ± 2 | 21 ± 1 |
| ∆PL | H0 | 16 ± 1 | 19 ± 2 | 21 ± 3 |
| ∆PL | H6 | 22 ± 2 *§ | 18 ± 2 | 14 ± 1 |
| TPPInsp | H0 | 15 ± 1 | 17 ± 3 | 20 ± 2 |
| TPPInsp | H6 | 19 ± 2 * | 18 ± 2 | 18 ± 1 |
| TPPExp | H0 | −2 ± 0 | −3 ± 1 | −1 ± 1 |
| TPPExp | H6 | −2 ± 1§ | −1 ± 0$ | 3 ± 1 * |
| Cstat | H0 | 22 ± 1 | 21 ± 3 | 19 ± 2 |
| Cstat | H6 | 18 ± 1 *§ | 21 ± 2 | 24 ± 2 * |
| ERS | H0 | 56 ± 3 | 67 ± 7 | 72 ± 9 |
| ERS | H6 | 69 ± 5 *§ | 66 ± 7 | 53 ± 5 |
| ECW | H0 | 16 ± 2 | 17 ± 2 | 21 ± 2 |
| ECW | H6 | 16 ± 2 | 18 ± 1 | 17 ± 2 * |
| EL | H0 | 40 ± 2 | 50 ± 7 | 52 ± 8 |
| EL | H6 | 54 ± 5 *§ | 48 ± 7 | 35 ± 2 |
| HR | H0 | 70 ± 8 | 84 ± 11 | 93 ± 7 |
| HR | H6 | 88 ± 6 | 84 ± 9 | 75 ± 4 |
| MAP | H0 | 101 ± 4 | 99 ± 2 | 104 ± 6 |
| MAP | H6 | 108 ± 5 | 99 ± 5 | 97 ± 3 |
| P/F ratio | H0 | 130 ± 22 | 120 ± 27 | 81 ± 19 |
| P/F ratio | H6 | 196 ± 39 * | 240 ± 55 * | 320 ± 56 * |
| paCO2 | H0 | 38 ± 2 | 40 ± 2 | 39 ± 2 |
| paCO2 | H6 | 42 ± 1 | 39 ± 1 | 38 ± 1 |
| CI | H0 | 4.5 ± 0.4 | 4.4 ± 0.3 | 5.2 ± 0.4 |
| CI | H6 | 4.2 ± 0.1 § | 3.9 ± 0.2 * | 3.7 ± 0.1 * |
| Lactate | H0 | 1.3 ± 0.3 | 1.3 ± 0.2 | 1.6 ± 0.3 |
| Lactate | H6 | 0.6 ± 0.1 | 0.6 ± 0.1 * | 0.8 ± 0.1 |
| pH | H0 | 7.38 ± 0.02 | 7.36 ± 0.01 | 7.38 ± 0.02 |
| pH | H6 | 7.44 ± 0.04 | 7.44 ± 0.01 | 7.45 ± 0.01 |
| RR | H0 | 23 ± 1 | 23 ± 1 | 25 ± 1 |
| RR | H6 | 25 ± 1 * | 25 ± 1 | 25 ± 1 |
| Crystalloid volume | H6 | 4.9 ± 0.4 § | 5.9 ± 0.6 | 7.1 ± 0.6 |
| Group A | Group B | Group C | |
|---|---|---|---|
| IAP H6 – H0 | 7 ± 1 | 7 ± 0 | 7 ± 1 |
| EELVH6 – H0 | −46 ± 115 § | 101 ± 46 | 343 ± 113 |
| EELV/kgH6 – H0 | −1 ± 2 § | 2 ± 1 | 7 ± 2 |
| ∆PH6 – H0 | 5 ± 1 § | 0 ± 1 | −8 ± 4 |
| ∆PL H6 – H0 | 5 ± 1 § | 0 ± 2 | −6 ± 3 |
| TPPInsp H6 – H0 | 5 ± 2 | 1 ± 2 | −2 ± 2 |
| TPPExp H6 – H0 | −1 ± 1 § | 2 ± 1 | 4 ± 1 |
| Cstat H6 – H0 | −4 ± 0 § | 0 ± 1 $ | 6 ± 2 |
| ERS H6 – H0 | 13 ± 3 § | −1 ± 4 | −20 ± 9 |
| ECW H6 – H0 | 0 ± 1 | 1 ± 1 $ | −4 ± 1 |
| EL H6 – H0 | 13 ± 3 § | −2 ± 4 | −16 ± 9 |
| HRH6 – H0 | 17 ± 7 | 0 ± 11 | −19 ± 11 |
| MAPH6 – H0 | 7 ± 7 | 1 ± 5 | −7 ± 8 |
| P/F ratioH6 – H0 | 66 ± 21 § | 120 ± 35 | 239 ± 47 |
| CIH6 – H0 | −0.3 ± 0.4 § | −0.4 ± 0.2 | −1.5 ± 0.4 |
| LactateH6 – H0 | −0.7 ± 0.3 | −0.7 ± 0.1 | −0.8 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedler, M.O.; Simeliunas, E.; Deutsch, B.L.; Diktanaite, D.; Harms, A.; Brune, M.; Dietrich, M.; Uhle, F.; Weigand, M.A.; Kalenka, A. Impact of Different Positive End-Expiratory Pressures on Lung Mechanics in the Setting of Moderately Elevated Intra-Abdominal Pressure and Acute Lung Injury in a Porcine Model. J. Clin. Med. 2021, 10, 306. https://doi.org/10.3390/jcm10020306
Fiedler MO, Simeliunas E, Deutsch BL, Diktanaite D, Harms A, Brune M, Dietrich M, Uhle F, Weigand MA, Kalenka A. Impact of Different Positive End-Expiratory Pressures on Lung Mechanics in the Setting of Moderately Elevated Intra-Abdominal Pressure and Acute Lung Injury in a Porcine Model. Journal of Clinical Medicine. 2021; 10(2):306. https://doi.org/10.3390/jcm10020306
Chicago/Turabian StyleFiedler, Mascha O., Emilis Simeliunas, B. Luise Deutsch, Dovile Diktanaite, Alexander Harms, Maik Brune, Maximilian Dietrich, Florian Uhle, Markus A. Weigand, and Armin Kalenka. 2021. "Impact of Different Positive End-Expiratory Pressures on Lung Mechanics in the Setting of Moderately Elevated Intra-Abdominal Pressure and Acute Lung Injury in a Porcine Model" Journal of Clinical Medicine 10, no. 2: 306. https://doi.org/10.3390/jcm10020306
APA StyleFiedler, M. O., Simeliunas, E., Deutsch, B. L., Diktanaite, D., Harms, A., Brune, M., Dietrich, M., Uhle, F., Weigand, M. A., & Kalenka, A. (2021). Impact of Different Positive End-Expiratory Pressures on Lung Mechanics in the Setting of Moderately Elevated Intra-Abdominal Pressure and Acute Lung Injury in a Porcine Model. Journal of Clinical Medicine, 10(2), 306. https://doi.org/10.3390/jcm10020306






