When Does the Cytokine Storm Begin in COVID-19 Patients? A Quick Score to Recognize It
Abstract
1. Introduction
2. Materials and Methods
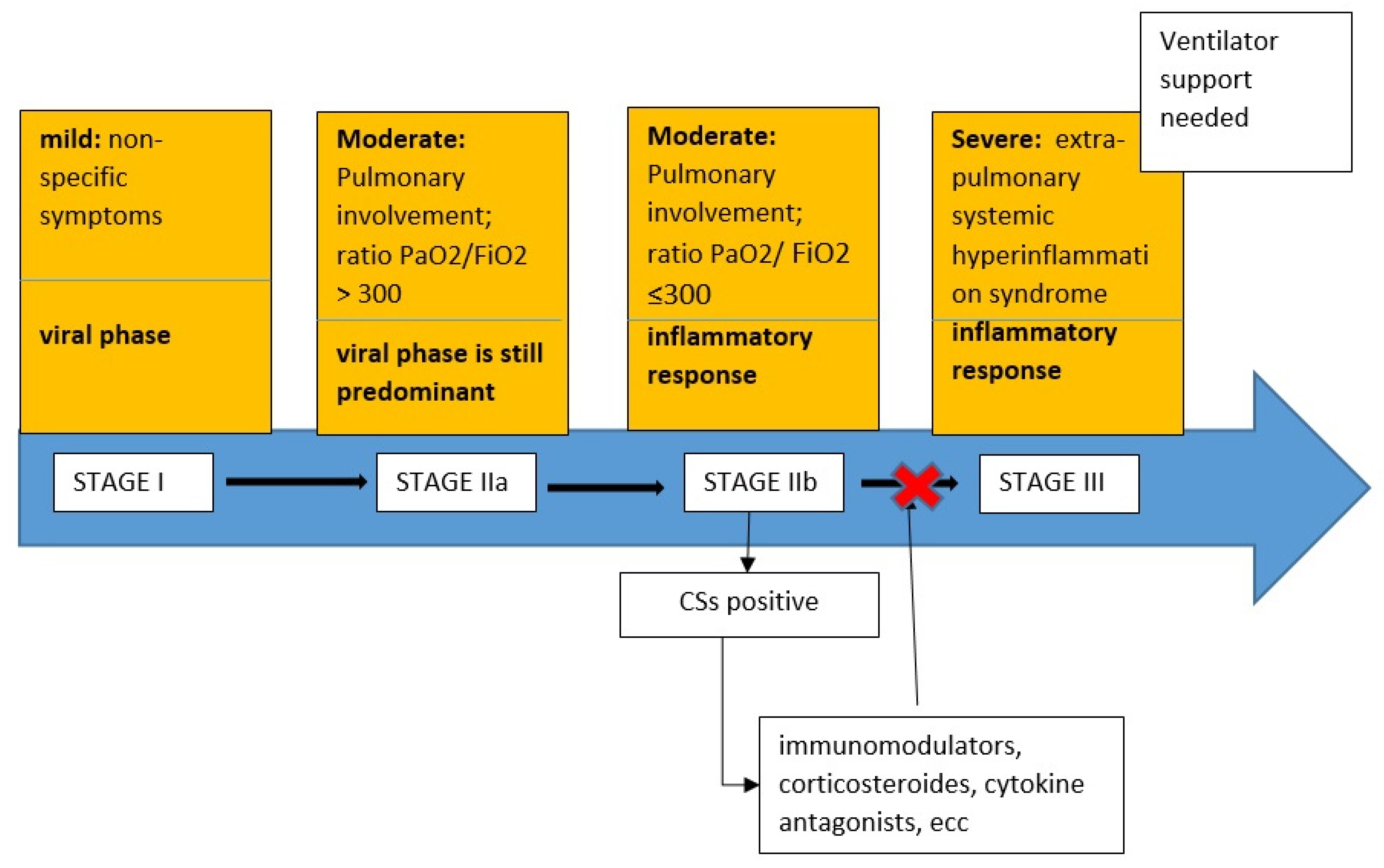
- Stage I (mild): early infection where the viral response phase is predominant, characterized by non-specific symptoms, such as malaise, fever, or cough.
- Stage II (moderate): pulmonary involvement without hypoxia (IIa) or with hypoxia (IIb), as defined by shortness of breath or a PaO2/FiO2 ratio ≤ 300. In this stage there were abnormalities on chest imaging. In Stage IIa, the viral response phase is predominant while the inflammation phase is beginning. In Stage IIb, the viral response phase decreases and the host inflammatory response increases.
- Stage III (systemic hyperinflammation): the most severe stage of illness manifests as an extra-pulmonary systemic hyperinflammation syndrome characterized by ARDS, shock, and cardiac failure.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zh ang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Coronavirus Disease 2019 (COVID-19). Available online: https://covid19.who.int/ (accessed on 13 January 2021).
- Ministero Della Salute. Available online: www.salute.gov (accessed on 13 January 2021).
- WHO. Coronavirus. Available online: https://www.who.int/health-topics/coronavirus#tab=tab_3 (accessed on 14 April 2020).
- Drosten, C.; Meyer, B.; Müller, M. Supplement to: Transmission of MERS-coronavirus in household contacts. N. Engl. J. Med. 2014, 371, 1–10. [Google Scholar] [CrossRef]
- Ahmed, S.; Zimba, O.; Gasparyan, A.Y. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow’s triad. Clin. Rheumatol. 2020, 39, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.K.; Lang, J.; Nauffal, V.; Morrow, D.A.; Bohula, E.A. COVID-19 for the Cardiologist: A Current Review of the Virology, Clinical Epidemiology, Cardiac and Other Clinical Manifestations and Potential Therapeutic Strategies. JACC Basic Transl. Sci. 2020, 5, 518–536. [Google Scholar]
- Gasparyan, A.Y.; Misra, D.P.; Yessirkepov, M.; Zimba, O. Perspectives of Immune Therapy in Coronavirus Disease 2019. J. Korean Med. Sci. 2020, 35, e176. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Sessa, F.; Piscopo, A.; Montana, A.; Torrisi, M.; Patanè, F.G.; Murabito, P.; Volti, G.L.; Pomara, C. No Autopsies on COVID-19 Deaths: A Missed Opportunity and the Lockdown of Science. J. Clin. Med. 2020, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. The pathology of influenza virus infections. Ann. Rev. Pathol. 2008, 3, 499–522. [Google Scholar] [CrossRef]
- Gu, J.; Gong, E.; Zhang, B. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424. [Google Scholar] [CrossRef]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jinag, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensiv. Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z.; Gao, G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020, 95, 332–339. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. Mild versus severe COVID-19: Laboratory markers. Int. J. Infect. Dis. 2020, 95, 304–307. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, M.; Tong, S.; Liu, L.; Tang, L. Predictive factors for disease progression in hospitalized patients with coronavirus disease 2019 in Wuhan, China. J. Clin. Virol. 2020, 127, 104392. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef]
- Arachchillage, D.R.J.; Laffan, M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1233–1234. [Google Scholar] [CrossRef]
- Ibañez, C.; Perdomo, J.; Calvo, A.; Ferrando, C.; Reverter, J.C.; Tassies, D.; Blasi, A. High D dimers and low global fibrinolysis coexist in COVID19 patients: What is going on in there? J. Thromb. Thrombolysis 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Khinda, J.; Janjua, N.Z.; Cheng, S.; van den Heuvel, E.R.; Bhatti, P.; Darvishian, M. Association between markers of immune response at hospital admission and COVID-19 disease severity and mortality: A meta-analysis and meta-regression [published online ahead of print, 2020 Aug 10]. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Aggarwal, S.; Garcia-Telles, N.; Aggarwal, G.; Lavie, C.; Lippi, G.; Henry, B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis 2020, 7, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kappert, K.; Jahić, A.; Tauber, R. Assessment of serum ferritin as a biomarker in COVID-19: Bystander or participant? Insights by comparison with other infectious and non-infectious diseases. Biomarkers 2020, 25, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Yu, J.; Ma, T. Retrospective analysis of laboratory testing in 54 patients with severe- or critical-type 2019 novel coronavirus pneumonia. Lab. Investig. 2020, 100, 794–800. [Google Scholar] [CrossRef]
- Sun, L.; Shen, L.; Fan, J.; Gu, F.; Hu, M.; An, Y.; Zhou, Q.; Fan, H.; Bi, J. Clinical features of patients with coronavirus disease 2019 (COVID-19) from a designated hospital in Beijing, China. J. Med. Virol. 2020, 92, 2055–2066. [Google Scholar] [CrossRef]
- Wang, F.; Hou, H.; Luo, Y.; Tang, G.; Wu, S.; Huang, M.; Liu, W.; Zhu, Y.; Lin, Q.; Mao, L.; et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Diamanti, A.P.; Rosado, M.M.; Pioli, C.; Sesti, G.; Laganà, B. Cytokine Release Syndrome in COVID-19 Patients, A New Scenario for an Old Concern: The Fragile Balance between Infections and Autoimmunity. Int. J. Mol. Sci. 2020, 21, 3330. [Google Scholar] [CrossRef]
- Ghweil, A.A.; Hassan, M.H.; Mohamed, A.K.; Mohamed, A.O.; Mohammed, H.M.; Abdelazez, A.A.; Osman, H.A.; Bazeed, S.E.S. Characteristics, Outcomes and Indicators of Severity for COVID-19 Among Sample of ESNA Quarantine Hospital’s Patients, Egypt: A Retrospective Study. Infect. Drug Resist. 2020, 13, 2375–2383. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Karakioulaki, M.; Stolz, D. Biomarkers in Pneumonia-Beyond Procalcitonin. Int. J. Mol. Sci. 2019, 20, 2004. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Singanayagam, A.; Hill, A.T. C-Reactive Protein Is an Independent Predictor of Severity in Community-acquired Pneumonia. Am. J. Med. 2008, 121, 219–225. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.-L.; David, S.C.; et al. For the China Medical Treatment Expert Group for Covid-19 Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K. Dysregulated Type I Interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, J.; Zhou, C.; Wu, Z.; Zhong, S.; Liu, J. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 850–857. [Google Scholar] [CrossRef]
- Cameron, M.J.; Bermejo-Martin, J.F.; Danesh, A.; Muller, M.P.; Kelvin, D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res. 2008, 133, 13–19. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Ho, Y.-C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Ménard, T.; Bowling, R.; Mehta, P.; Koneswarakantha, B.; Magruder, E. Leveraging analytics to assure quality during the Covid-19 pandemic—The COVACTA clinical study example. Contemp. Clin. Trials Commun. 2020, 20, 100662. [Google Scholar] [CrossRef]
- Kulanthaivel, S.; Kaliberdenko, V.B.; Balasundaram, K.; Shterenshis, M.; Scarpellini, E.; Abenavoli, L. Tocilizumab in Sars-cov-2 Patients with the Syndrome of Cytokine Storm; a Narrative review. Rev. Recent Clin. Trials 2020, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nasa, P.; Singh, A.; Upadhyay, S.; Bagadia, S.; Polumuru, S.; Shrivastava, P.K.; Sankar, R.; Vijayan, L.; Soliman, M.A.; Ali, A.; et al. Tocilizumab Use in COVID-19 Cytokine-release Syndrome: Retrospective Study of Two Centers. Indian J. Crit. Care Med. 2020, 24, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Toniati, P.; Piva, S.; Cattalini, M.; Garrafa, E.; Regola, F.; Castelli, F.; Franceschini, F.; Airò, P.; Bazzani, C.; Beindorf, E.-A.; et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020, 19, 102568. [Google Scholar] [CrossRef] [PubMed]
- Furlow, B. COVACTA trial raises questions about tocilizumab’s benefit in COVID-19. Lancet Rheumatol. 2020, 2, e592. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Fardet, L.; Galicier, L.; Lambotte, O.; Marzac, C.; Aumont, C.; Chahwan, D.; Coppo, P.; Hejblum, G. Development and Validation of the HScore, a Score for the Diagnosis of Reactive Hemophagocytic Syndrome. Arthr. Rheumatol. 2014, 66, 2613–2620. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G.; Plebani, M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin. Chem. Lab. Med. 2020, 58, 1135–1138. [Google Scholar] [CrossRef]
- Wang, L. C-reactive protein levels in the early stage of COVID-19. Méd. Mal. Infect. 2020, 50, 332–334. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; Wang, Y.; Wang, Z.; Liang, Y.; Huang, T.; Zhang, H.; Sun, W.; Wang, Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020, 92, 577–583. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020, 58, 1131–1134. [Google Scholar] [CrossRef]
- Tan, C.; Huang, Y.; Shi, F.; Tan, K.; Ma, Q.; Chen, Y.; Jiang, X.; Li, X. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J. Med. Virol. 2020, 92, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zuo, P.; Liu, Y.; Zhang, M.; Zhao, X.; Xie, S.; Zhang, H.; Chen, X.; Liu, C. Clinical and Laboratory Predictors of In-hospital Mortality in Patients With Coronavirus Disease-2019: A Cohort Study in Wuhan, China. Clin. Infect. Dis. 2020, 71, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Agenzia Italiana del Farmaco. Studio Randomizzato Multicentrico in Aperto Sull’efficacia Della Somministrazione Precoce del Tocilizumab in Pazienti Affetti da Polmonite da COVID-19. 17 Giugno 2020. Available online: https://www.aifa.gov.it/documents/20142/1123276/studio_RE_Toci_17.06.2020.pdf (accessed on 13 January 2021).
- World Health Organization. Corticosteroids for COVID-19; Living Guidance; 2 September 2020; COVID-19: Clinical Care. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed on 2 September 2020).
- Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management (accessed on 13 January 2021).
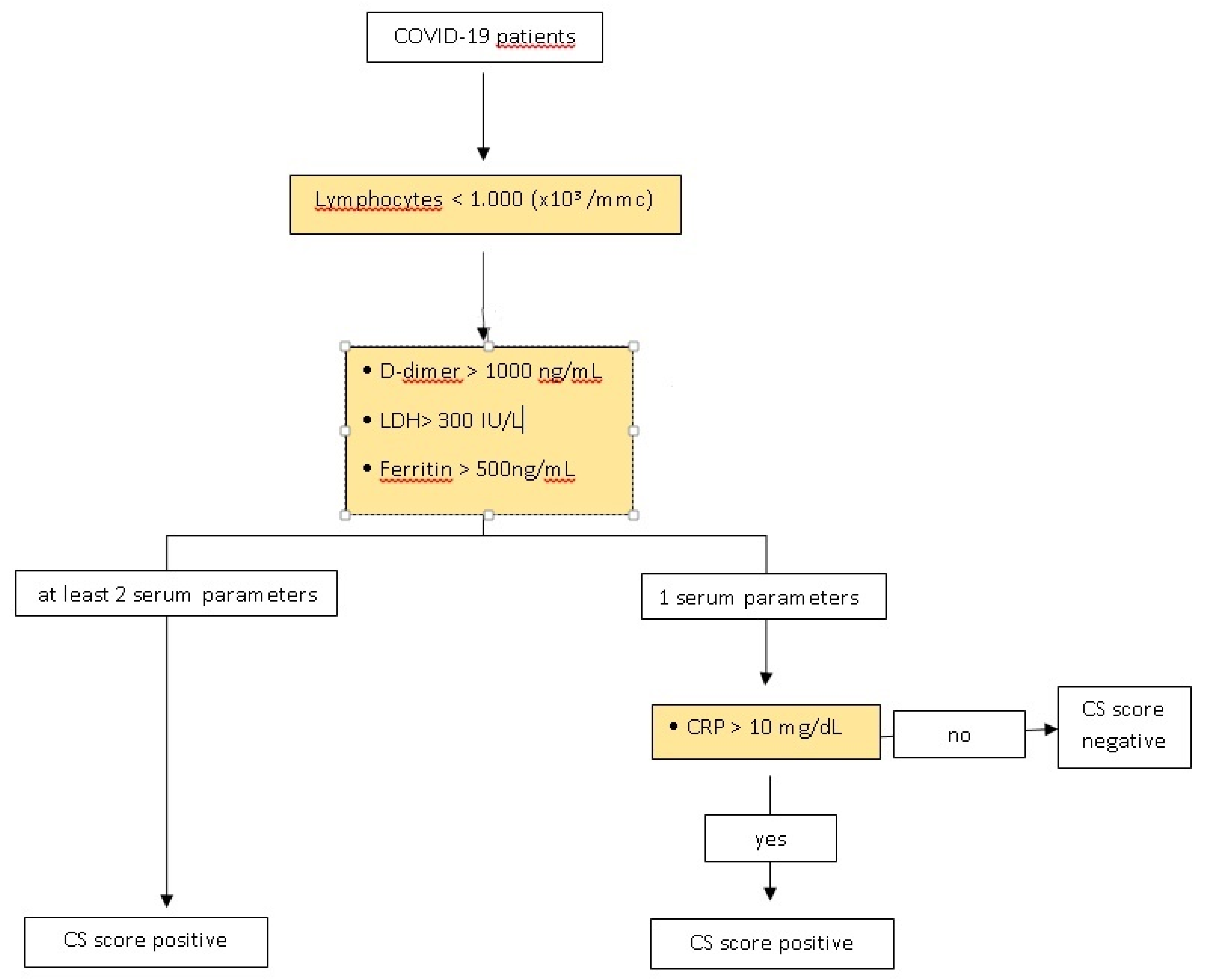
| Cytokine Storm Score (CSs) | |
|---|---|
| At Lymphocytes < 1000 (×103/mmc): | |
| And at least 2 of the following parameters: | positive |
| D-dimer > 1000 ng/mL | |
| LDH> 300 IU/L | |
| Ferritin > 500 ng/mL | |
| OR | |
| At Lymphocytes < 1000 (×103/mmc): | |
| And at least 1 of the following parameters: | positive |
| D-dimer > 1000 ng/mL | |
| LDH> 300 IU/L | |
| Ferritin > 500 ng/mL | |
| AND CRP > 10 mg/dL | |
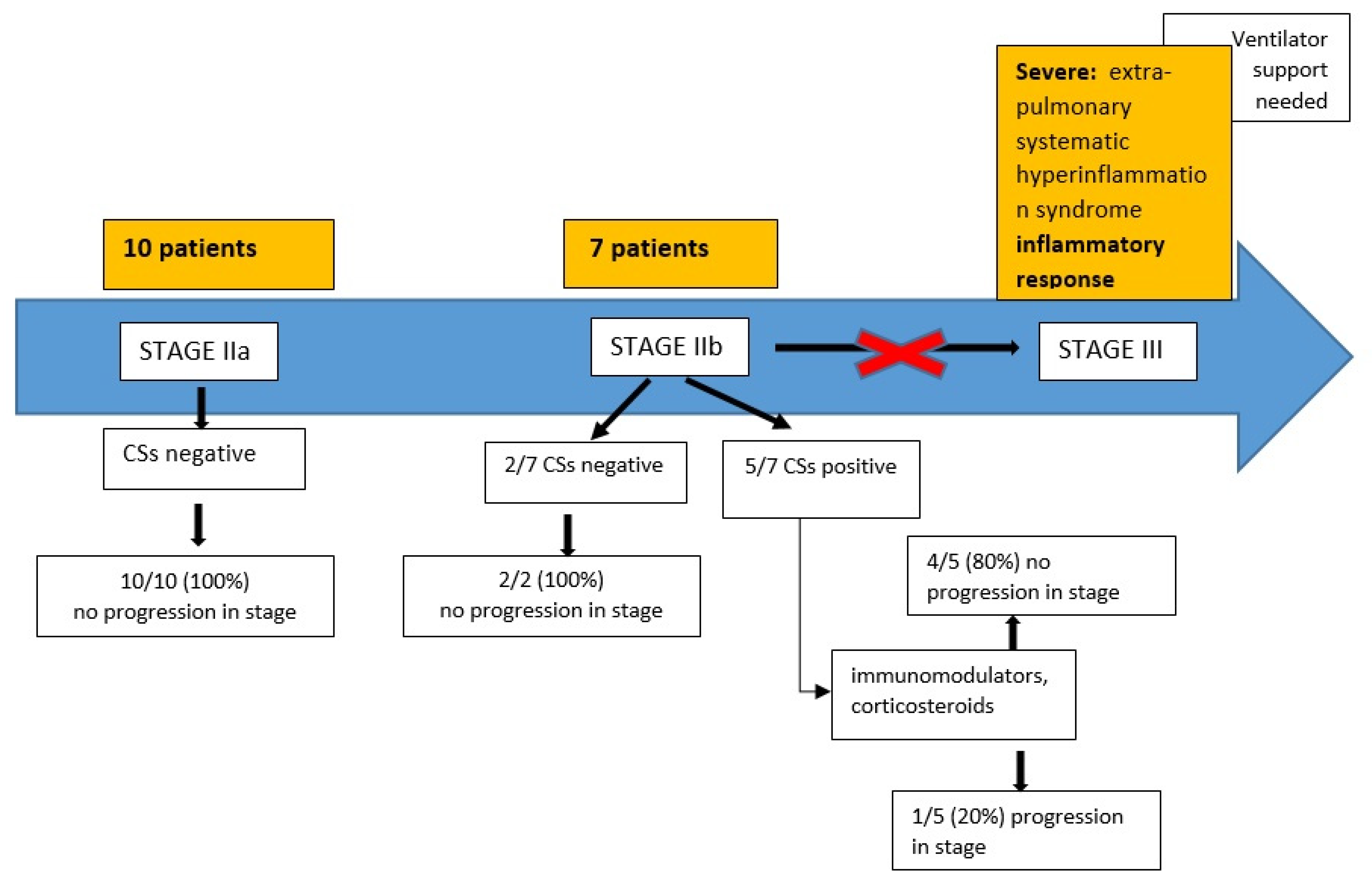
| Total Cases | Average Age | Male/Female | CSs | Progression in Stage | |
|---|---|---|---|---|---|
| COVID-19 patients | 31 | 61.35 | 19/12 | 11/31 (35.4%) positive | 11/31 |
| Stage IIa | 10/31 (32%) | 56.4 | 5/5 | 10/10 (100%): negative | 0/10 |
| Stage IIb | 21/31 (68%) | 63.7 | 14/7 | 10/21 (48%): negative | 0/10 |
| Stage III | 11/21 (52%): positive | 11/11 (100%) | |||
| Patients Stage IIb Who Progressed (n = 11) | Time (Hours) from CSs Positive and Intubation |
|---|---|
| 2 | 12 h |
| 4 | 24 h |
| 4 | 48 h |
| 1 | 96 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappanera, S.; Palumbo, M.; Kwan, S.H.; Priante, G.; Martella, L.A.; Saraca, L.M.; Sicari, F.; Vernelli, C.; Di Giuli, C.; Andreani, P.; et al. When Does the Cytokine Storm Begin in COVID-19 Patients? A Quick Score to Recognize It. J. Clin. Med. 2021, 10, 297. https://doi.org/10.3390/jcm10020297
Cappanera S, Palumbo M, Kwan SH, Priante G, Martella LA, Saraca LM, Sicari F, Vernelli C, Di Giuli C, Andreani P, et al. When Does the Cytokine Storm Begin in COVID-19 Patients? A Quick Score to Recognize It. Journal of Clinical Medicine. 2021; 10(2):297. https://doi.org/10.3390/jcm10020297
Chicago/Turabian StyleCappanera, Stefano, Michele Palumbo, Sherman H. Kwan, Giulia Priante, Lucia Assunta Martella, Lavinia Maria Saraca, Francesco Sicari, Carlo Vernelli, Cinzia Di Giuli, Paolo Andreani, and et al. 2021. "When Does the Cytokine Storm Begin in COVID-19 Patients? A Quick Score to Recognize It" Journal of Clinical Medicine 10, no. 2: 297. https://doi.org/10.3390/jcm10020297
APA StyleCappanera, S., Palumbo, M., Kwan, S. H., Priante, G., Martella, L. A., Saraca, L. M., Sicari, F., Vernelli, C., Di Giuli, C., Andreani, P., Mariottini, A., Francucci, M., Sensi, E., Costantini, M., Bruzzone, P., D’Andrea, V., Gioia, S., Cirocchi, R., & Tiri, B. (2021). When Does the Cytokine Storm Begin in COVID-19 Patients? A Quick Score to Recognize It. Journal of Clinical Medicine, 10(2), 297. https://doi.org/10.3390/jcm10020297







