Success Rates of Monitoring for Healthcare Professionals with a Substance Use Disorder: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
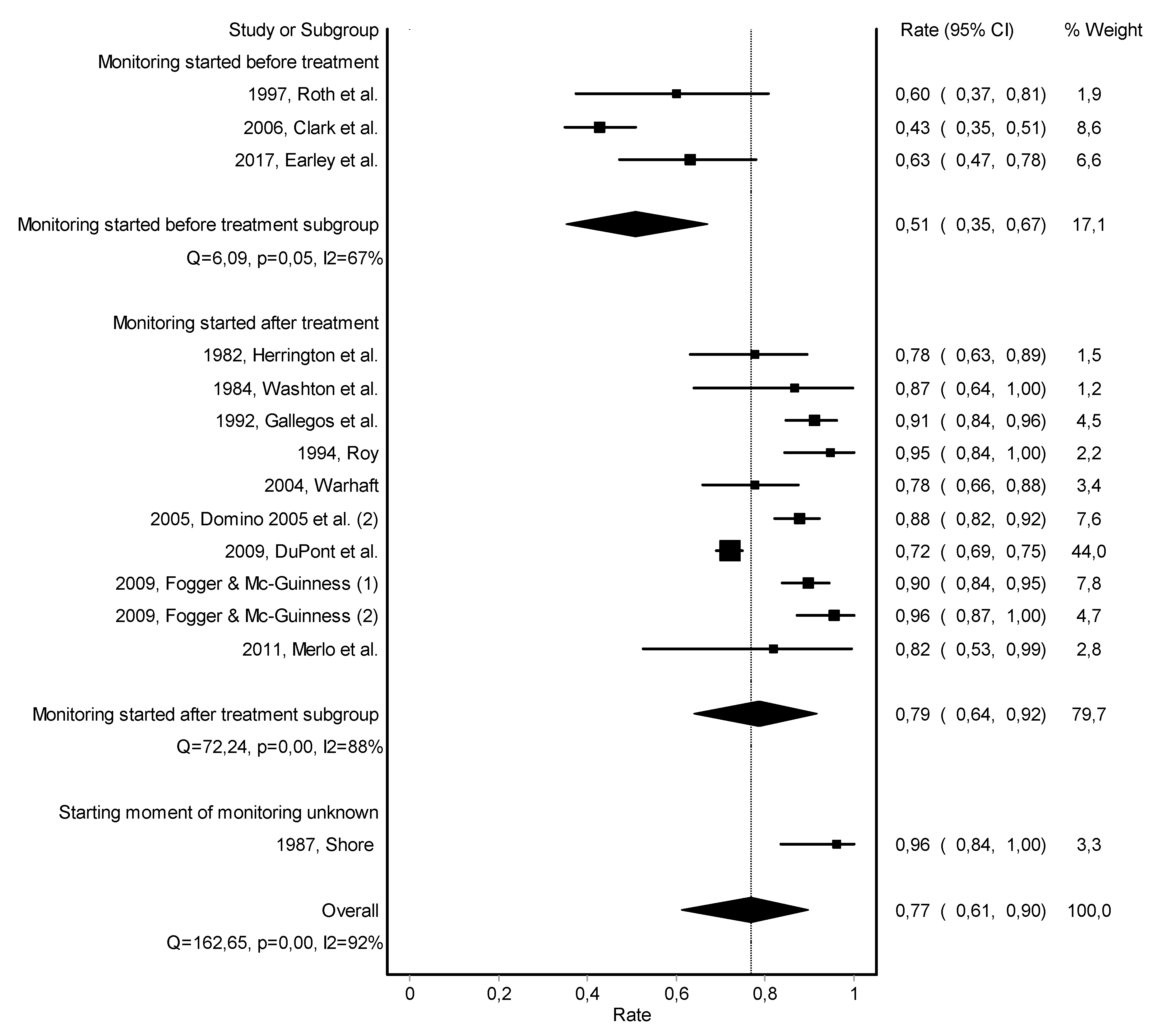
2.1. Search Strategy and Selection Criteria
2.2. Study Selection, Data Extraction, and Quality Assessment
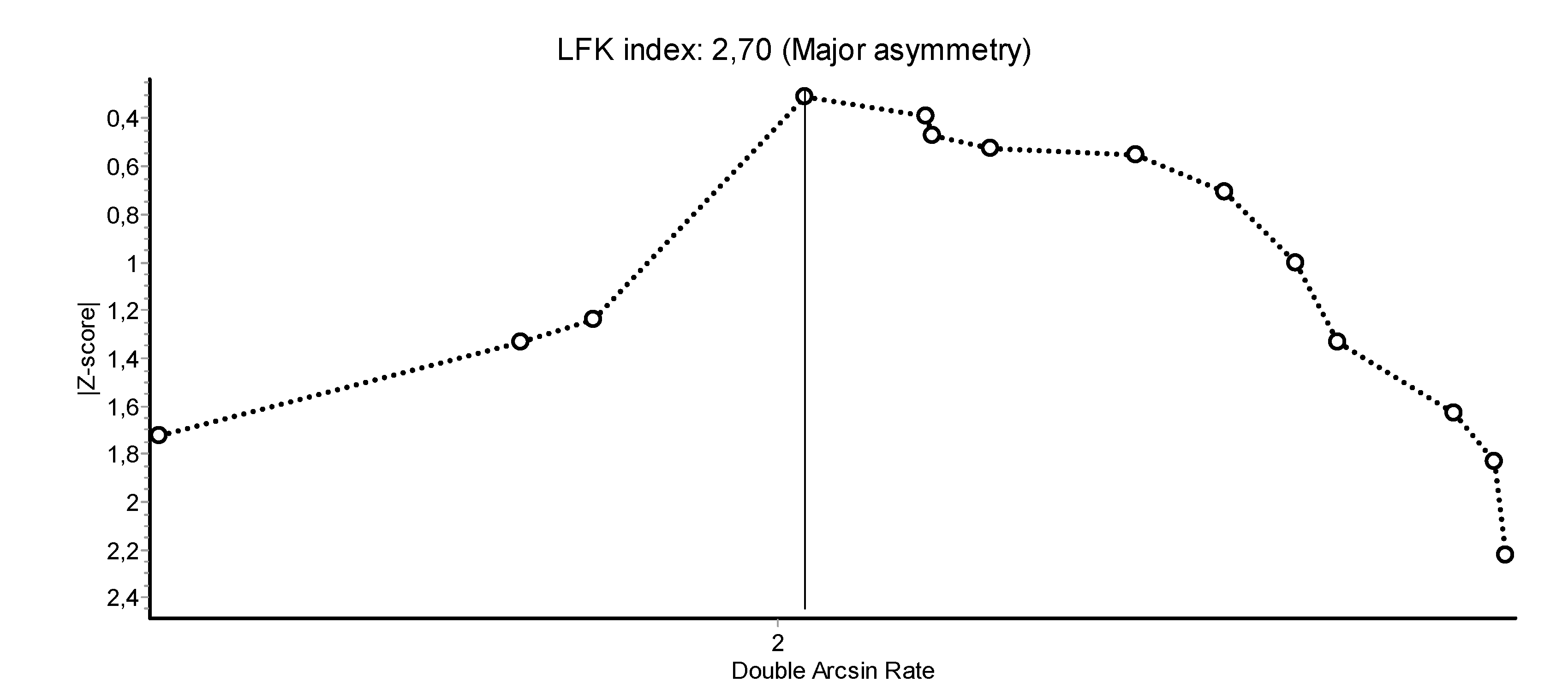
2.3. Data-Analysis
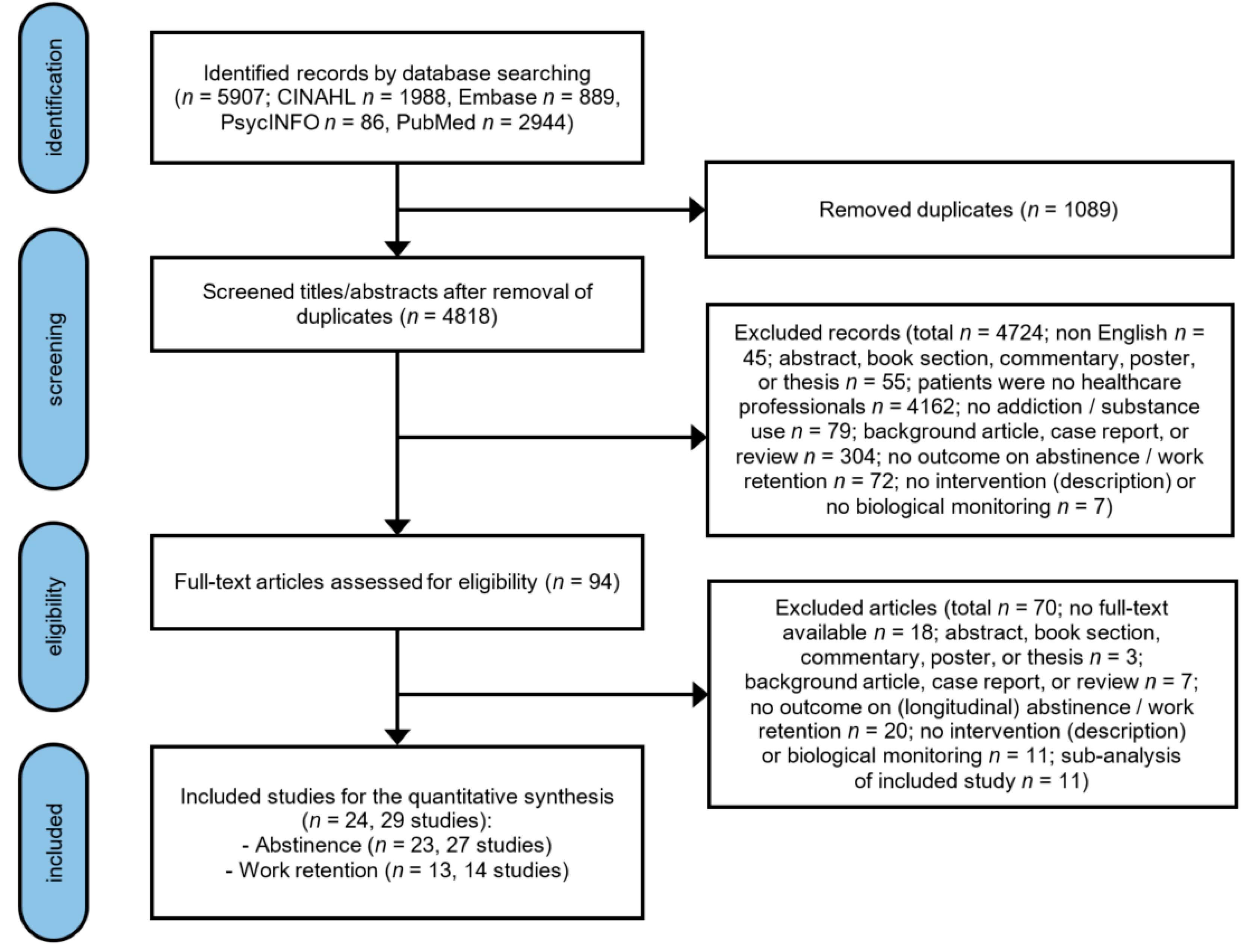
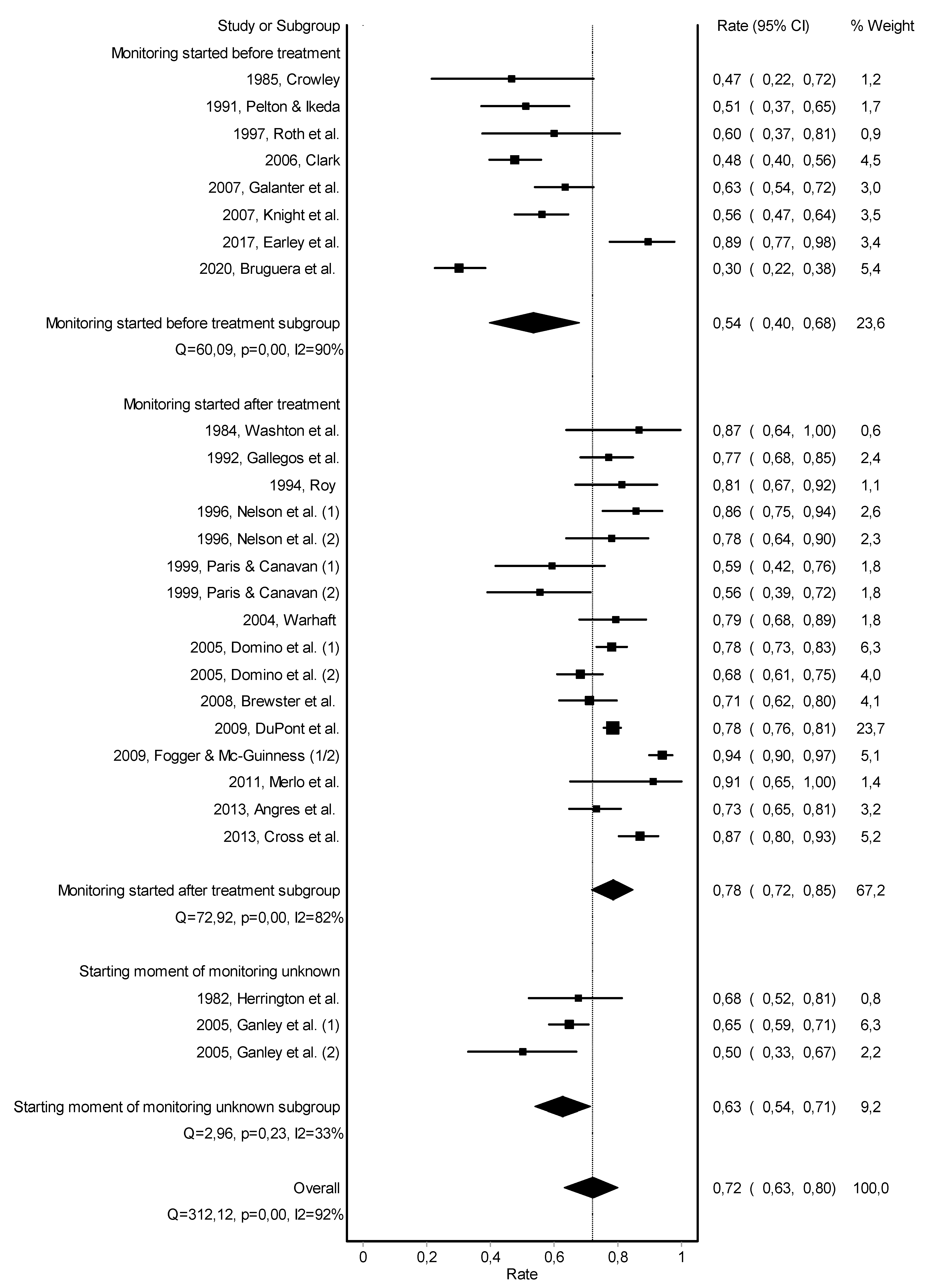
3. Results
3.1. Description of the Included Studies
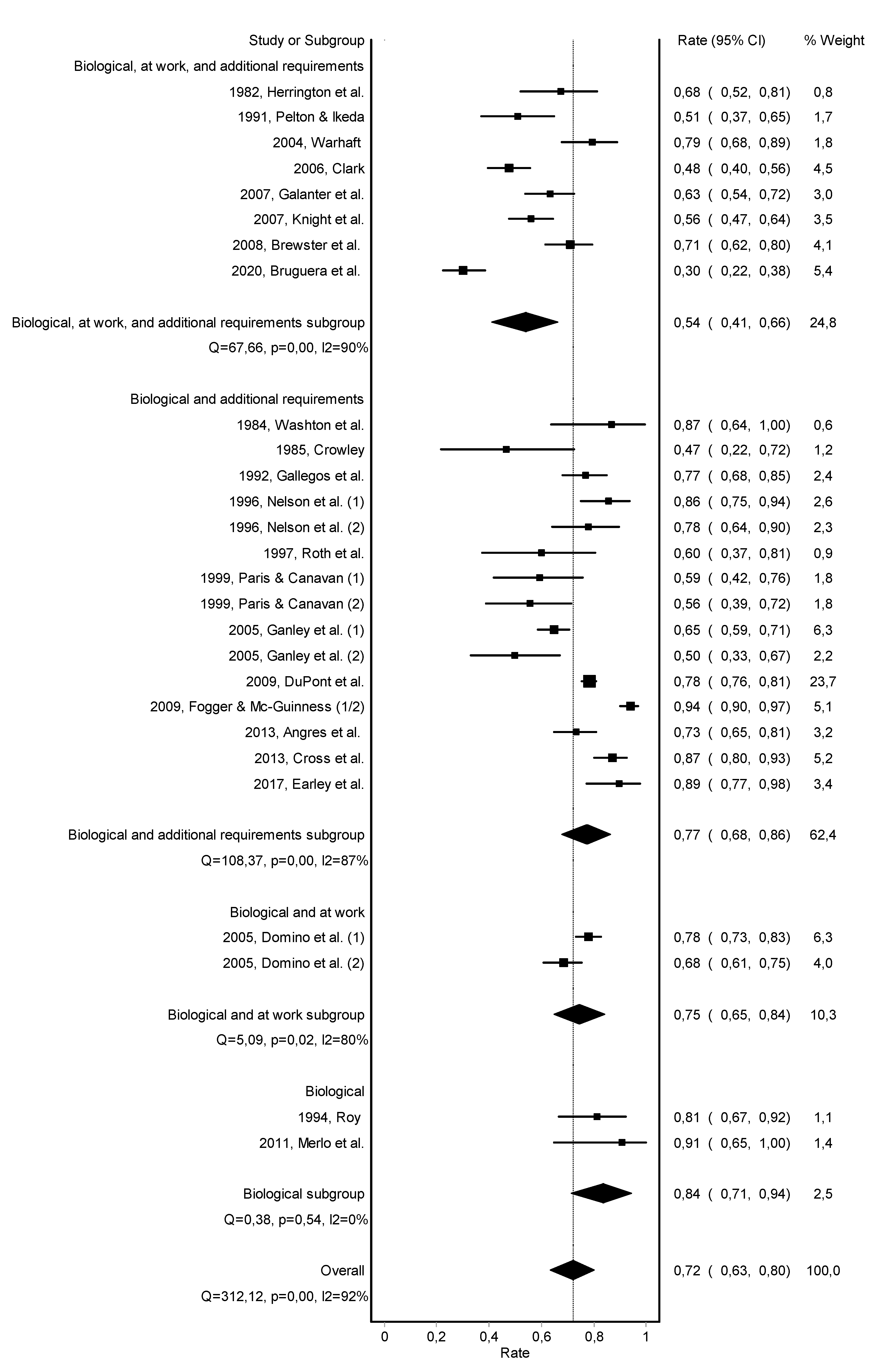
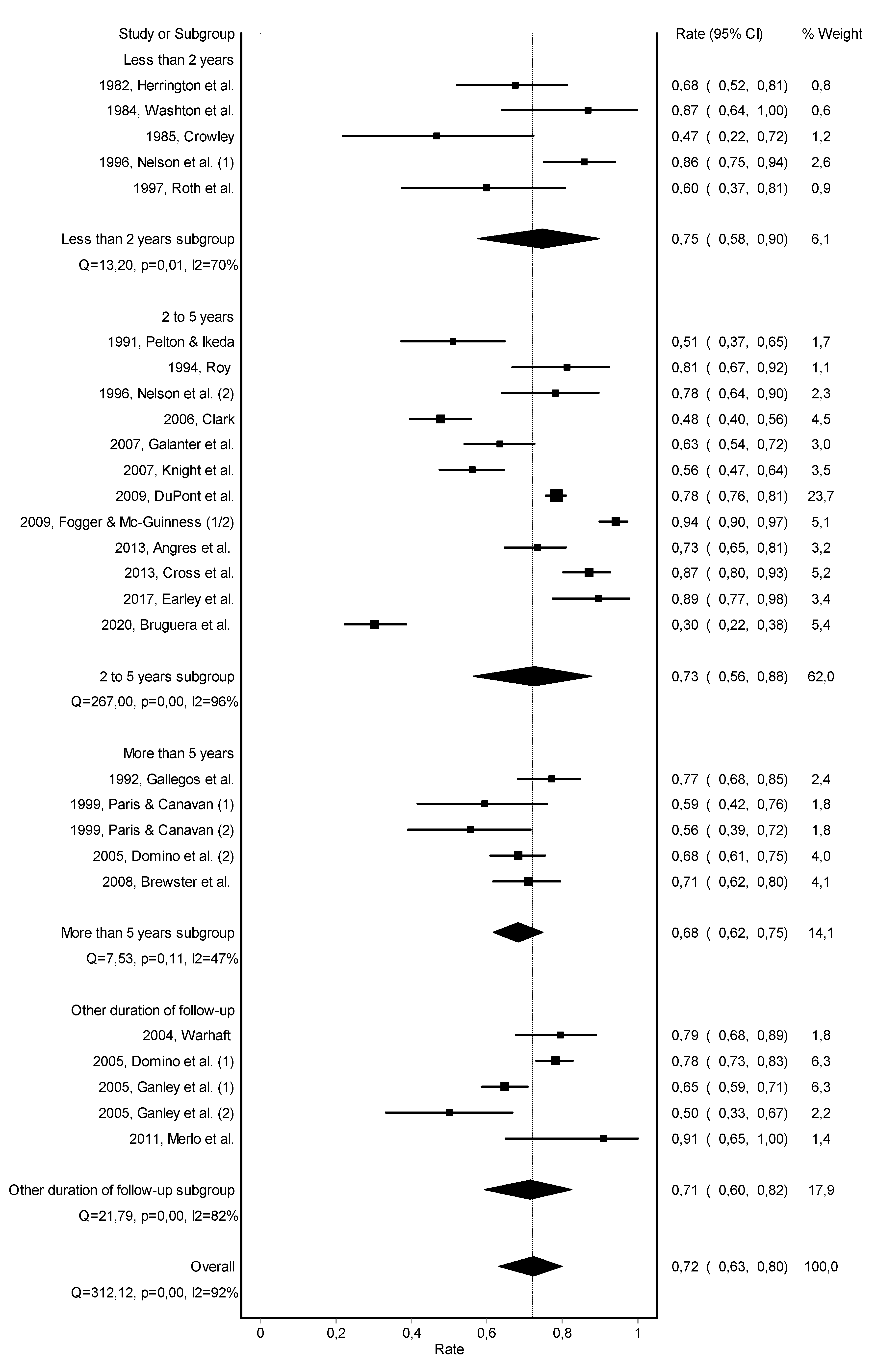
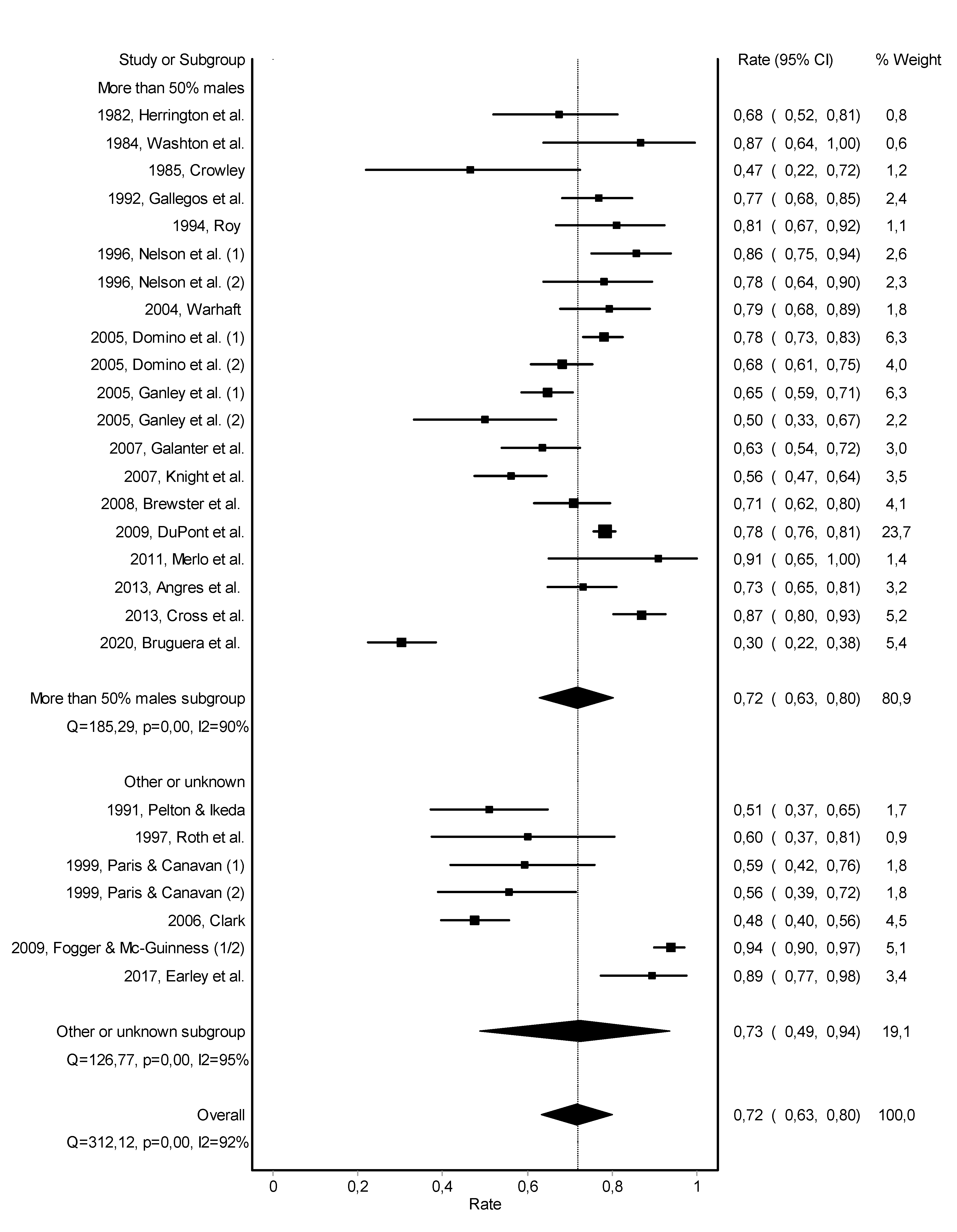
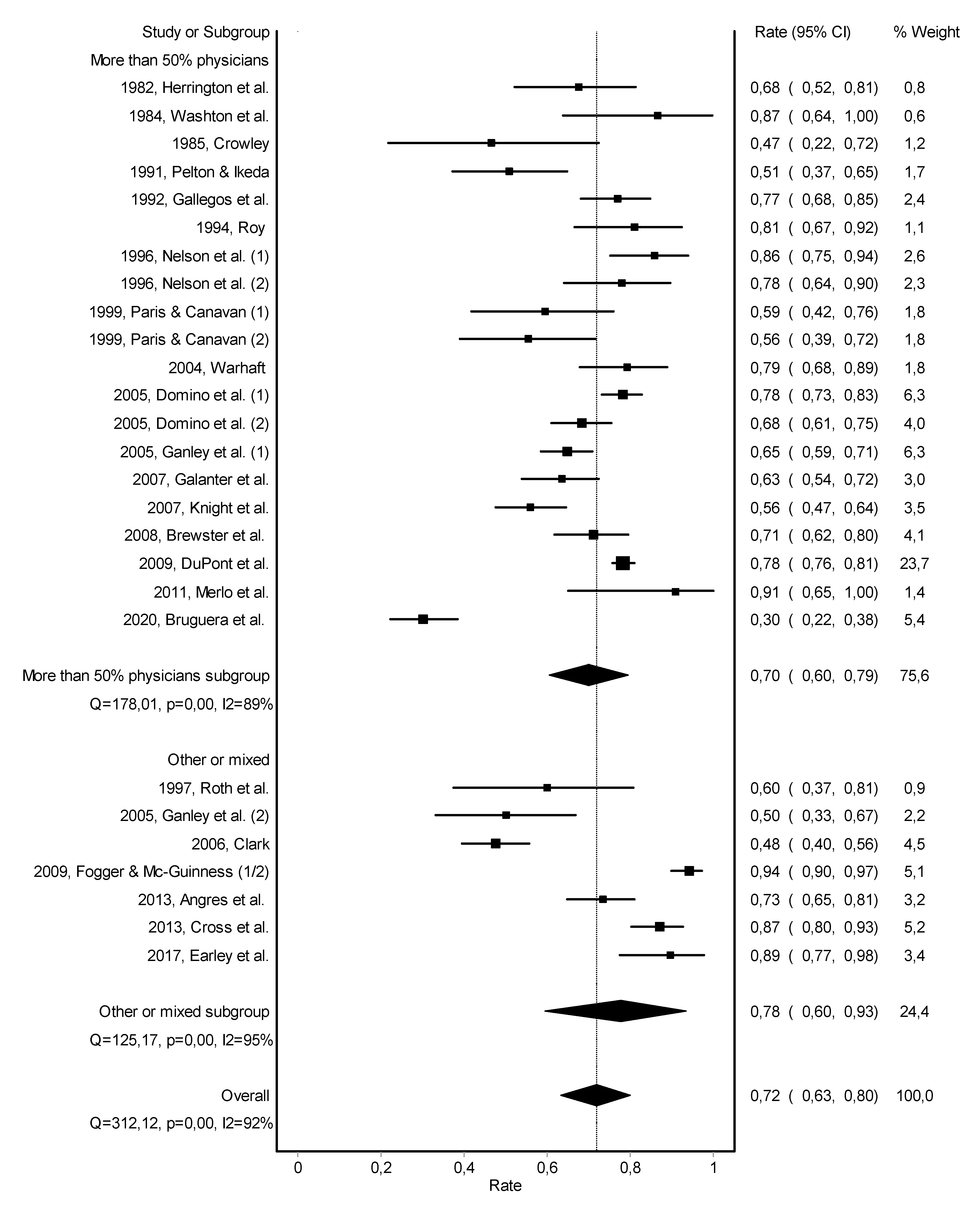
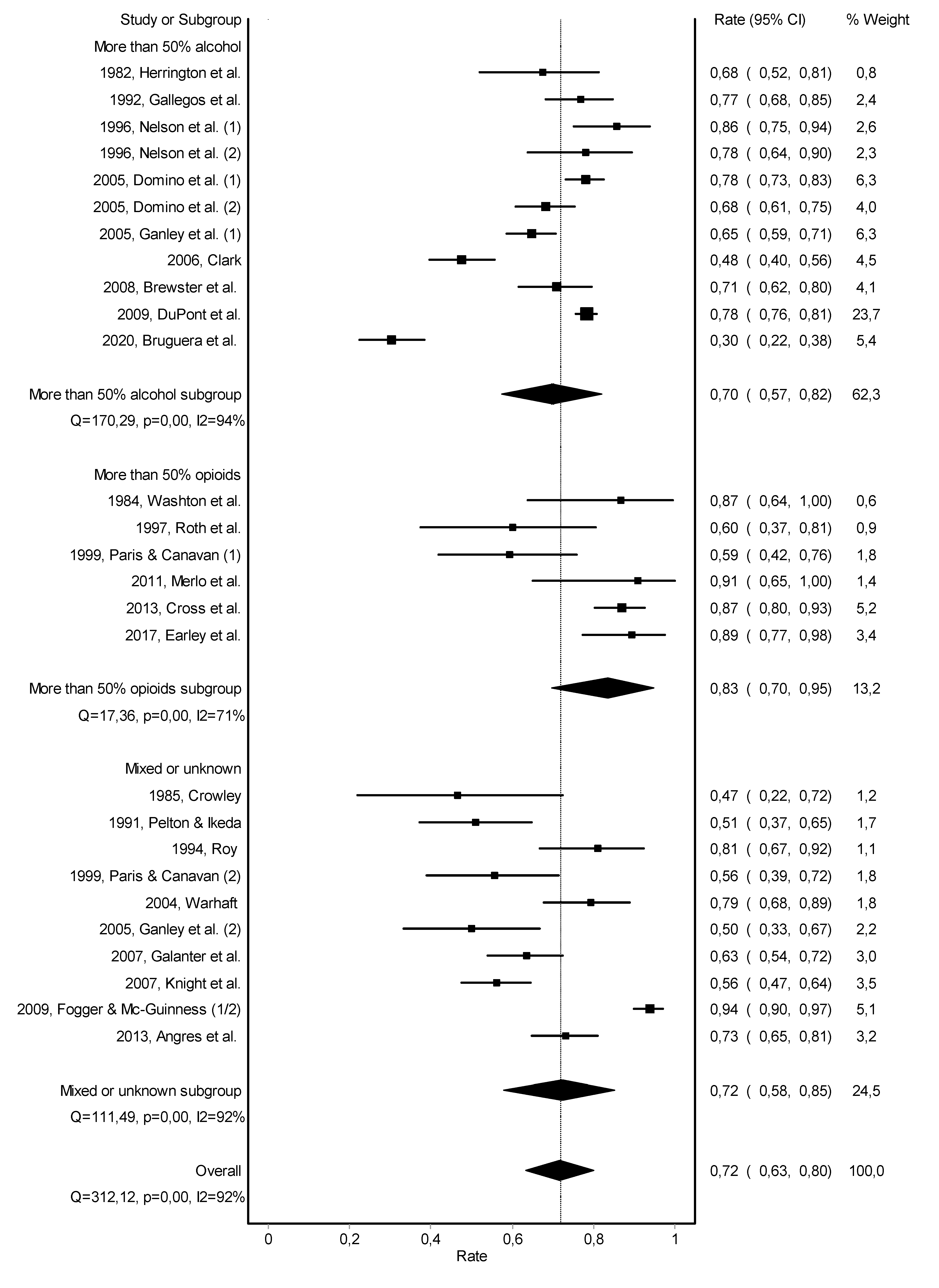
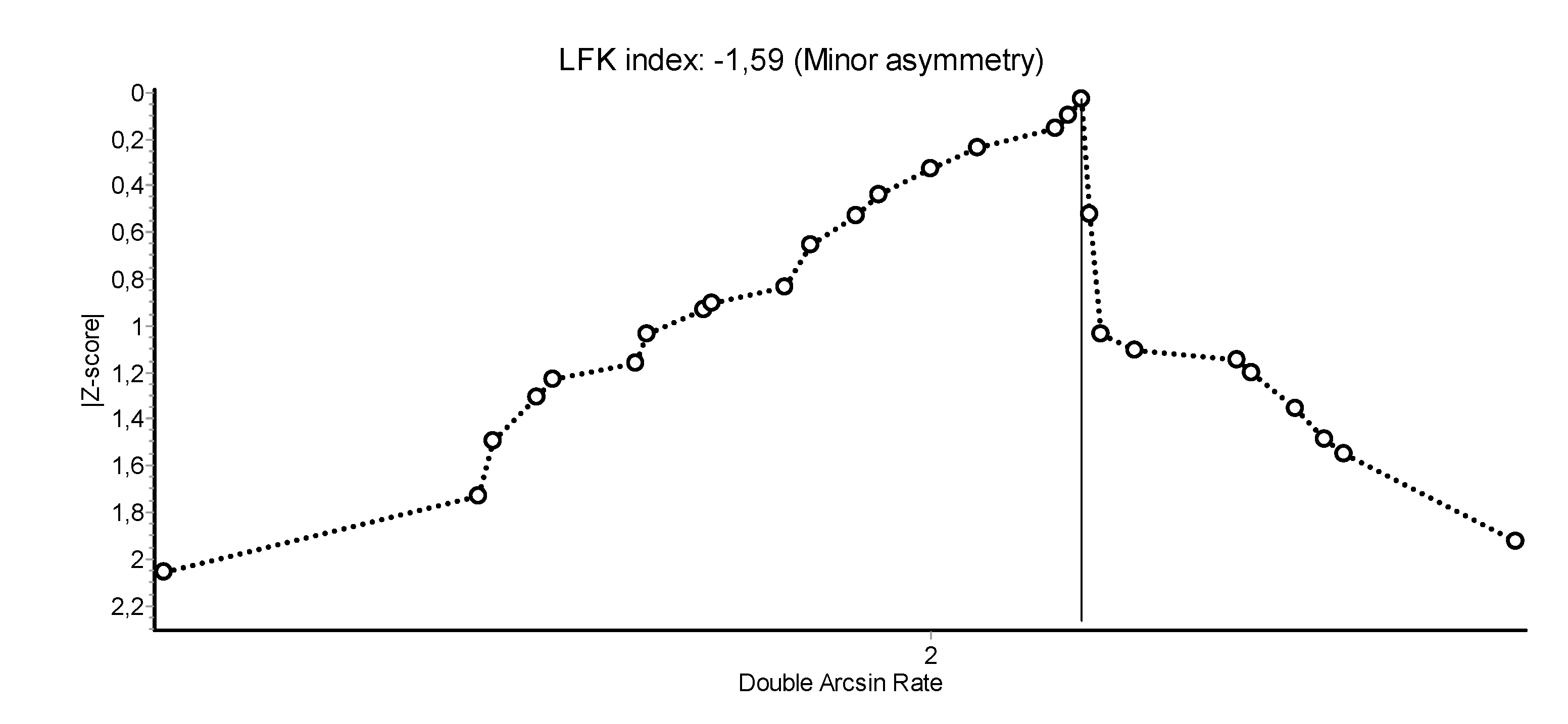
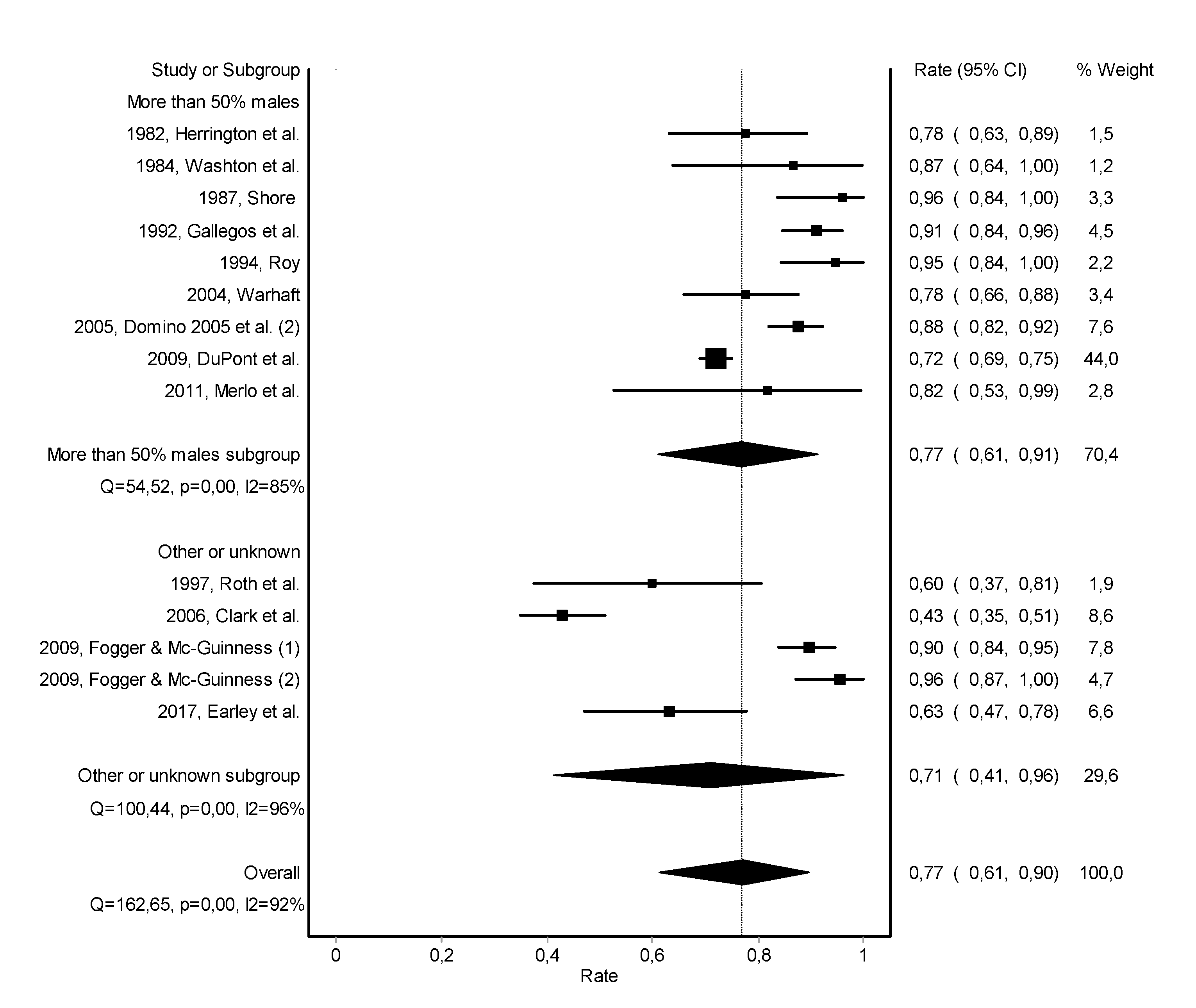
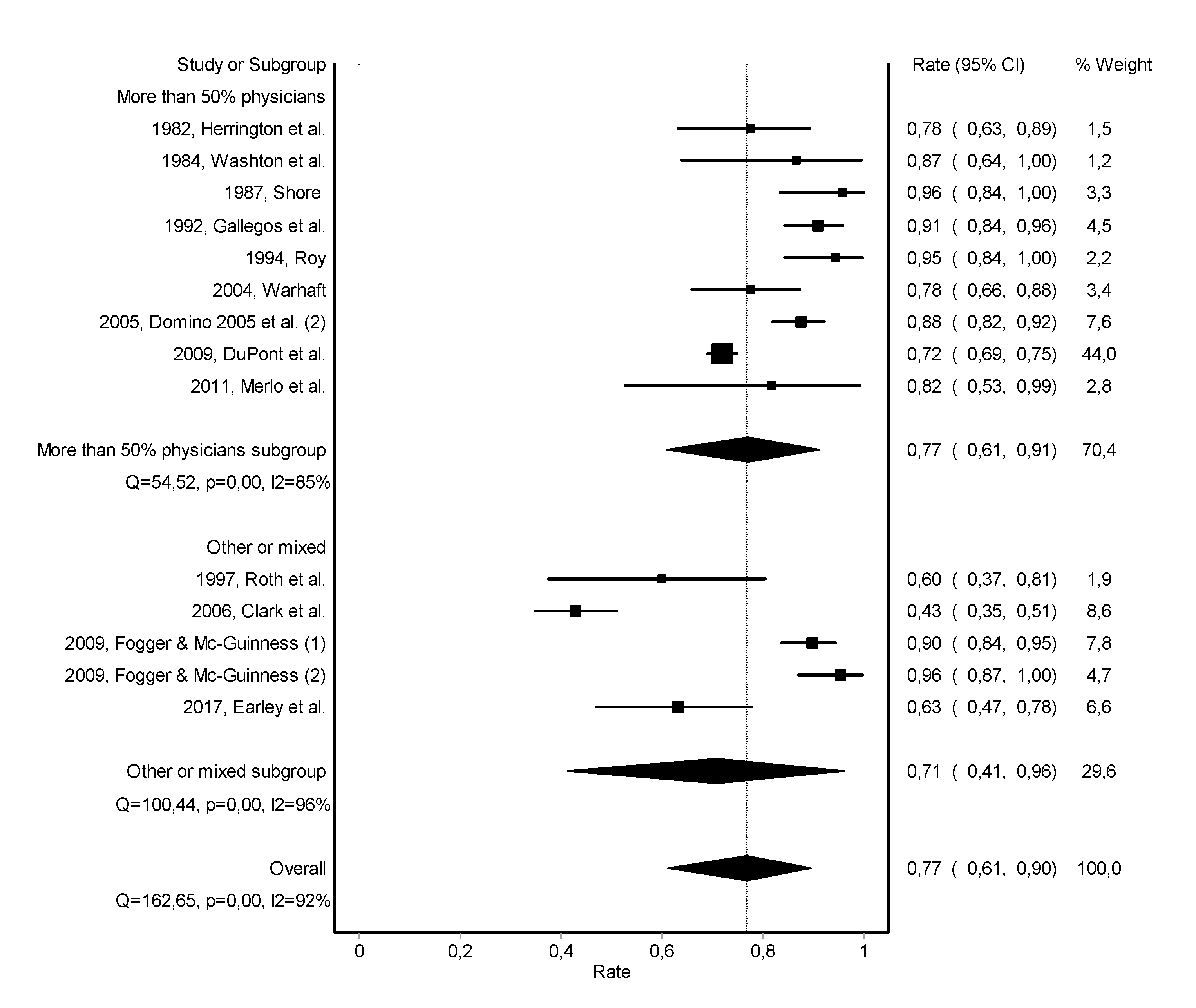
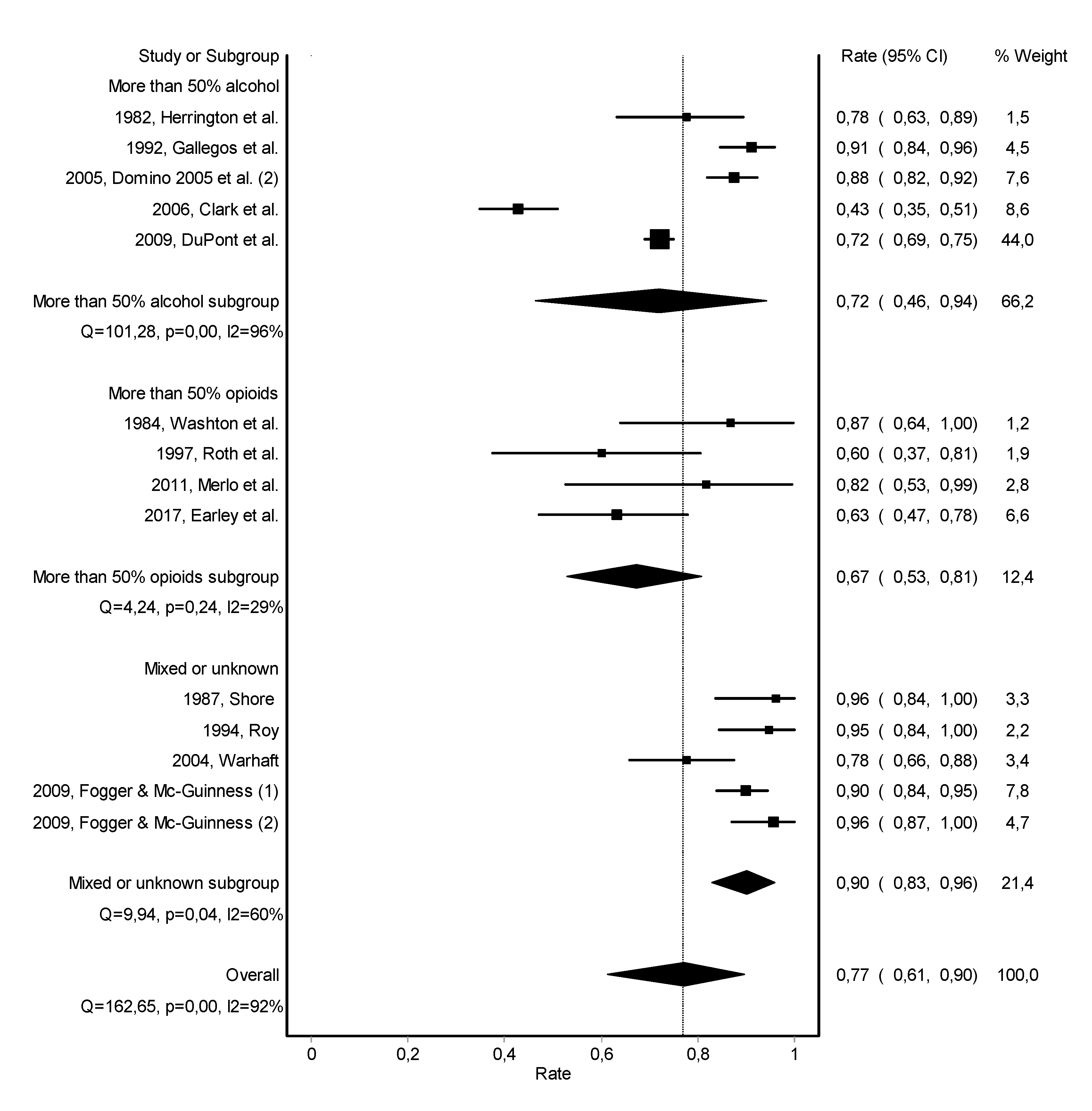
3.2. Abstinence
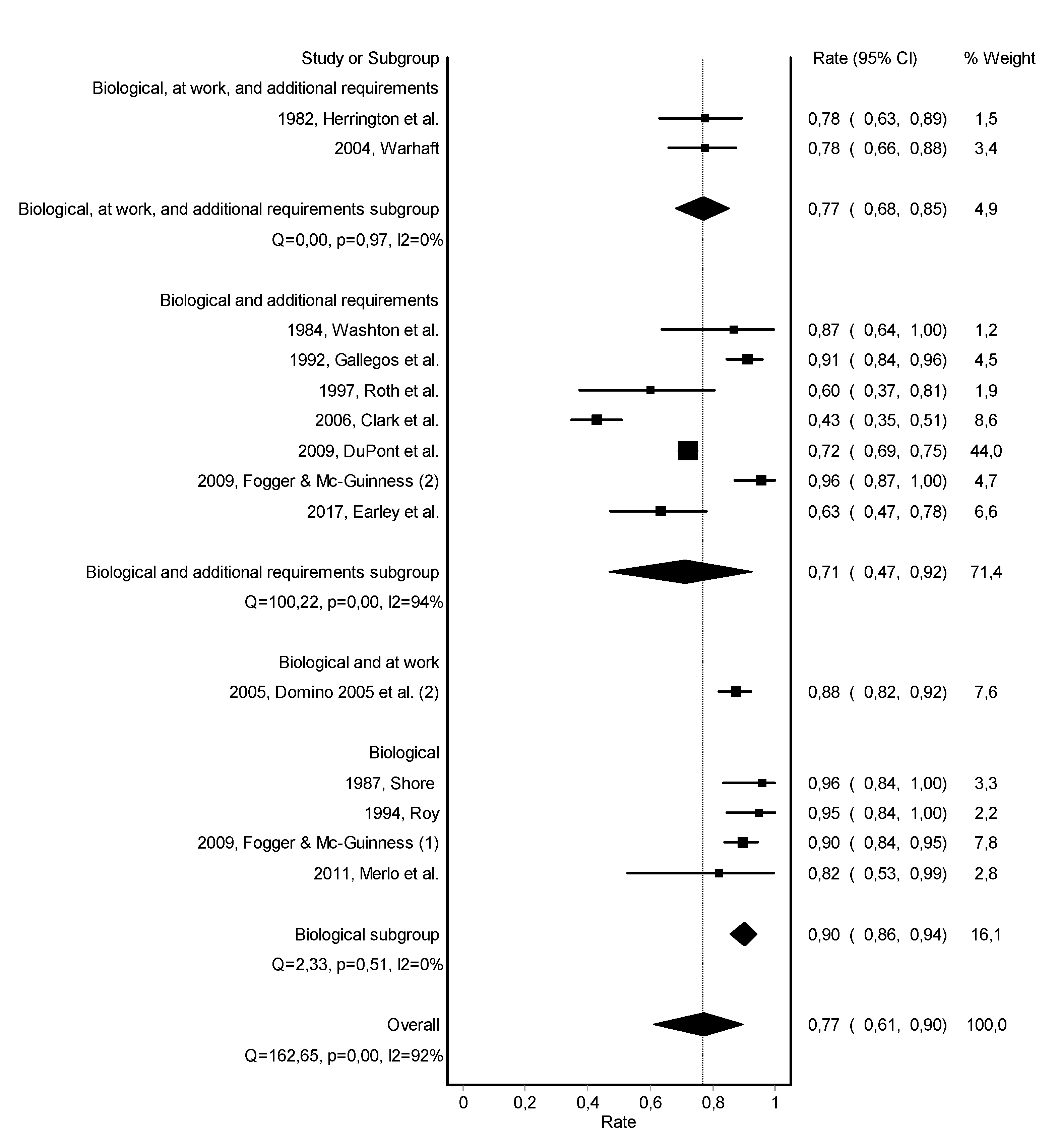
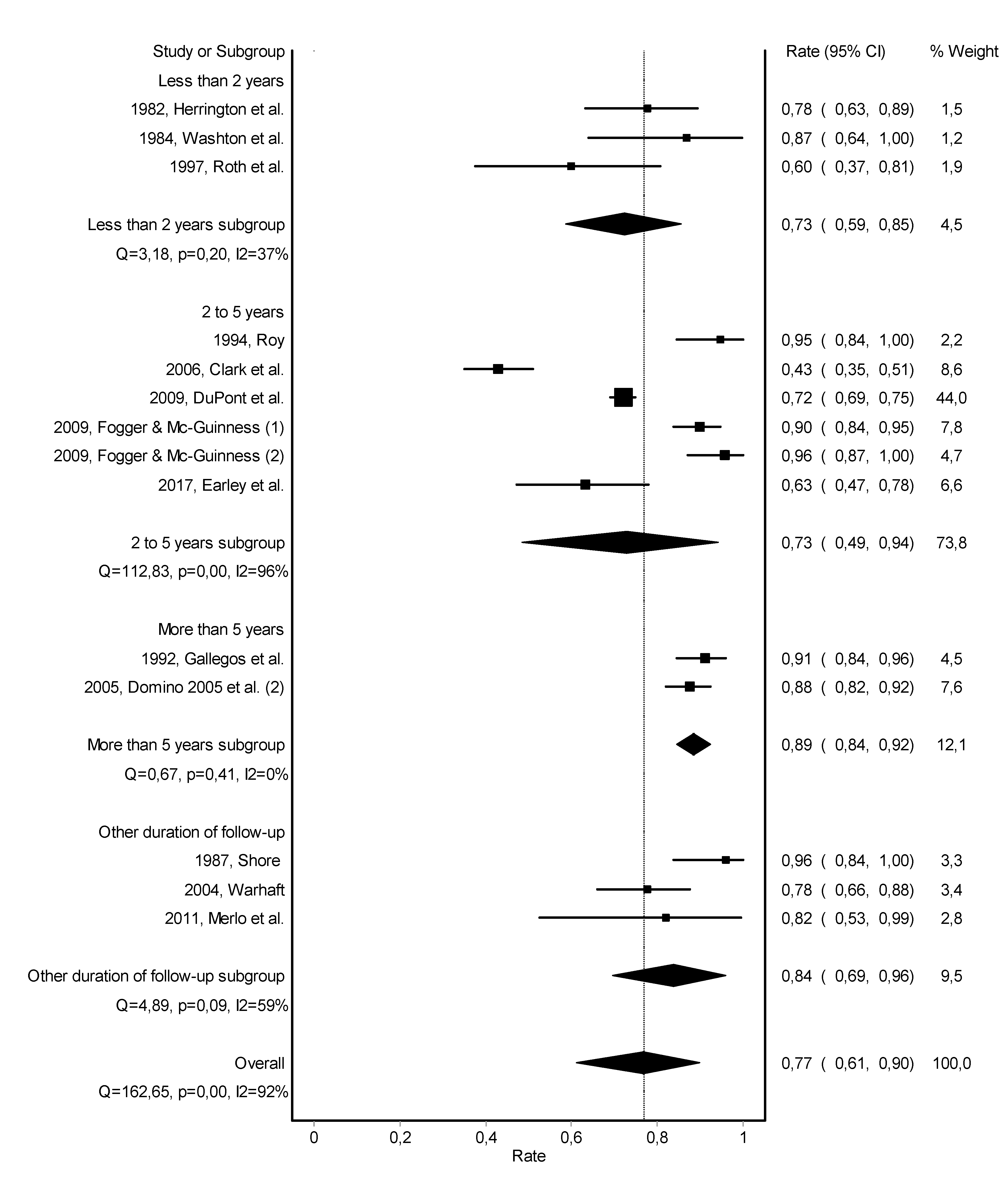
3.3. Work Retention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year, Study | Variables | Total (Max = 10) | Quality Index | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| 1982, Herrington et al. | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0.2 |
| 1984, Washton et al. | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0.2 |
| 1985, Crowley | 0 | 0 | 1 | 1 | 1 | 1 | 4 | 0.4 |
| 1987, Shore | 0 | 1 | 2 | 1 | 1 | 0 | 5 | 0.5 |
| 1991, Pelton & Ikeda | 0 | 0 | 2 | 1 | 1 | 0 | 4 | 0.4 |
| 1992, Gallegos et al. | 1 | 0 | 1 | 1 | 1 | 0 | 4 | 0.4 |
| 1994, Roy | 0 | 0 | 1 | 0 | 1 | 1 | 3 | 0.3 |
| 1996, Nelson et al. (1) | 1 | 0 | 2 | 1 | 1 | 1 | 6 | 0.6 |
| 1996, Nelson et al. (2) | 1 | 0 | 2 | 1 | 1 | 1 | 6 | 0.6 |
| 1997, Roth et al. | 0 | 0 | 1 | 1 | 0 | 1 | 3 | 0.3 |
| 1999, Paris & Canavan (1) | 0 | 0 | 2 | 1 | 1 | 1 | 5 | 0.5 |
| 1999, Paris & Canavan (2) | 0 | 0 | 2 | 1 | 1 | 1 | 5 | 0.5 |
| 2004, Warhaft | 1 | 0 | 2 | 0 | 1 | 0 | 4 | 0.4 |
| 2005, Domino et al. (1) | 1 | 0 | 2 | 1 | 1 | 0 | 5 | 0.5 |
| 2005, Domino et al. (2) | 1 | 0 | 2 | 1 | 1 | 0 | 5 | 0.5 |
| 2005, Ganley et al. (1) | 1 | 1 | 2 | 1 | 1 | 0 | 6 | 0.6 |
| 2005, Ganley et al. (2) | 1 | 1 | 2 | 1 | 1 | 0 | 6 | 0.6 |
| 2006, Clark et al. | 1 | 0 | 2 | 1 | 1 | 1 | 6 | 0.6 |
| 2007, Galanter et al. | 1 | 0 | 1 | 1 | 1 | 1 | 5 | 0.5 |
| 2007, Knight et al. | 1 | 0 | 2 | 1 | 1 | 0 | 5 | 0.5 |
| 2008, Brewster et al. | 1 | 1 | 1 | 1 | 1 | 2 | 7 | 0.7 |
| 2009, DuPont et al. | 1 | 0 | 2 | 1 | 2 | 1 | 7 | 0.7 |
| 2009, Fogger & Mc-Guinness (1) | 0 | 0 | 2 | 2 | 1 | 1 | 6 | 0.6 |
| 2009, Fogger & Mc-Guinness (2) | 0 | 0 | 2 | 2 | 1 | 1 | 6 | 0.6 |
| 2011, Merlo et al. | 0 | 0 | 2 | 1 | 1 | 1 | 5 | 0.5 |
| 2013, Cross et al. | 1 | 1 | 2 | 1 | 1 | 2 | 8 | 0.8 |
| 2013, Angres et al. | 0 | 1 | 1 | 1 | 0 | 2 | 5 | 0.5 |
| 2017, Earley et al. | 1 | 1 | 1 | 2 | 2 | 2 | 9 | 0.9 |
| 2020, Bruguera et al. | 1 | 1 | 2 | 1 | 2 | 1 | 8 | 0.8 |
| Variables | 0 | 1 | 2 | |||||
| 1. Clear definition of target population and observation period | No | Yes | ||||||
| 2. Use of diagnostic criteria | Symptom based Not specified | Use of diagnostic system reported | ||||||
| 3. Method of case selection | Not specified | Convenience sampling | Attempts all cases | |||||
| 4. Type of outcome assessment | Not specified | Register Case record | Administered interview | |||||
| 5. Size of study area | Not specified | Small (single site) | Broad (national or multisite) | |||||
| 6. Type of prevalence measure | Range of follow-up duration | Average follow-up duration | Exact follow-up duration | |||||
References
- Kunyk, D. Substance use disorders among registered nurses: Prevalence, risks and perceptions in a disciplinary jurisdiction. J. Nurs. Manag. 2015, 23, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.E.; Lemaire, J.B.; Ghali, W.A. Physician wellness: A missing quality indicator. Lancet 2009, 374, 1714–1721. [Google Scholar] [CrossRef]
- Oreskovich, M.R.; Shanafelt, T.; Dyrbye, L.N.; Tan, L.; Sotile, W.; Satele, D. The prevalence of substance use disorders in American physicians. Am. J. Addict. 2015, 24, 30–38. [Google Scholar] [CrossRef]
- Hughes, P.H.; Storr, C.; Baldwin, D.C.; Williams, K.M.; Conard, S.; Sheehan, D. Patterns of substance use in the medical profession. Med. J. 1992, 41, 311–314. [Google Scholar]
- Trinkoff, A.M.; Eaton, W.W.; Anthony, J.C. The prevalence of substance abuse among registered nurses. Nurs. Res. 1991, 40, 172–175. [Google Scholar] [CrossRef] [PubMed]
- American Medical Association. The sick physician. Impairment by psychiatric disorders, including alcoholism and drug dependence. JAMA 1973, 223, 684–687. [Google Scholar] [CrossRef]
- Braquehais, M.D.; Tresidder, A.; DuPont, R.L. Service provision to physicians with mental health and addiction problems. Curr. Opin. Psychiatry 2015, 28, 324–329. [Google Scholar] [CrossRef]
- Brooks, S.K.; Gerada, C.; Chalder, T. Review of literature on the mental health of doctors: Are specialist services needed? J. Ment. Health 2011, 20, 146–156. [Google Scholar] [CrossRef]
- DuPont, R.L.; McLellan, A.T.; White, W.L.; Merlo, L.J.; Gold, M.S. Setting the standard for recovery: Physicians’ Health Programs. J. Subst. Abus. Treat. 2009, 36, 159–171. [Google Scholar] [CrossRef]
- Gerada, C. The Wounded Healer: Report on the First 10 Years of Practitioner Health Service; NHS: London, UK, 2018. [Google Scholar]
- Jarvis, M.; Williams, J.; Hurford, M.; Lindsay, D.; Lincoln, P.; Giles, L. Appropriate Use of Drug Testing in Clinical Addiction Medicine. J. Addict. Med. 2017, 11, 163–173. [Google Scholar] [CrossRef]
- Weenink, J.W.; Kool, R.B.; Bartels, R.H.; Westert, G.P. Getting back on track: A systematic review of the outcomes of remediation and rehabilitation programmes for healthcare professionals with performance concerns. BMJ Qual. Saf. 2017, 26, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.R.; Sanchez, L.T.; Sherritt, L.; Bresnahan, L.R.; Fromson, J.A. Outcomes of a monitoring program for physicians with mental and behavioral health problems. J. Psychiatr. Pract. 2007, 13, 25–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Barendregt, J.; Doi, S. MetaXL User Guide; EpiGear: Sunrise Beach, Australia, 2016. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
- Doi, S.A.; Thalib, L. A quality-effects model for meta-analysis. Epidemiology 2008, 19, 94–100. [Google Scholar] [CrossRef]
- Herrington, R.E.; Benzer, D.G.; Jacobson, G.R.; Hawkins, M.K. Treating substance-use disorders among physicians. JAMA 1982, 247, 2253–2257. [Google Scholar] [CrossRef]
- Washton, A.M.; Gold, M.S.; Pottash, A.C. Naltrexone in addicted physicians and business executives. NIDA Res. Monogr. 1984, 55, 185–190. [Google Scholar]
- Crowley, T.J. Doctors’ drug abuse reduced during contingency-contracting treatment. Alcohol Drug Res. 1985, 6, 299–307. [Google Scholar]
- Shore, J.H. The Oregon experience with impaired physicians on probation. An eight-year follow-up. JAMA 1987, 257, 2931–2934. [Google Scholar] [CrossRef] [PubMed]
- Pelton, C.; Ikeda, R.M. The California Physicians Diversion Program’s experience with recovering anesthesiologists. J. Psychoact. Drugs 1991, 23, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, K.V.; Lubin, B.H.; Bowers, C.; Blevins, J.W.; Talbott, G.D.; Wilson, P.O. Relapse and recovery: Five to ten year follow-up study of chemically dependent physicians--the Georgia experience. Md. Med. J. 1992, 41, 315–319. [Google Scholar] [PubMed]
- Roy, A.K., 3rd. Reentry monitoring in the treatment of physicians with substance dependence. South. Med. J. 1994, 87, 881–883. [Google Scholar] [CrossRef]
- Nelson, H.D.; Matthews, A.M.; Girard, D.E.; Bloom, J.D. Substance-impaired physicians probationary and voluntary treatment programs compared. West. J. Med. 1996, 165, 31–36. [Google Scholar]
- Roth, A.; Hogan, I.; Farren, C. Naltrexone plus group therapy for the treatment of opiate-abusing health-care professionals. J. Subst. Abus. Treat. 1997, 14, 19–22. [Google Scholar] [CrossRef]
- Paris, R.T.; Canavan, D.I. Physician substance abuse impairment: Anesthesiologists vs. other specialties. J. Addict. Dis. 1999, 18, 1–7. [Google Scholar] [CrossRef]
- Warhaft, N.J. The Victorian Doctors Health Program: The first 3 years. Med. J. Aust. 2004, 181, 376–379. [Google Scholar] [CrossRef]
- Domino, K.B.; Hornbein, T.F.; Polissar, N.L.; Renner, G.; Johnson, J.; Alberti, S. Risk factors for relapse in health care professionals with substance use disorders. JAMA 2005, 293, 1453–1460. [Google Scholar] [CrossRef]
- Ganley, O.H.; Pendergast, W.J.; Wilkerson, M.W.; Mattingly, D.E. Outcome study of substance impaired physicians and physician assistants under contract with North Carolina Physicians Health Program for the period 1995-2000. J. Addict. Dis. 2005, 24, 1–12. [Google Scholar] [CrossRef]
- Clark, C.; Farnsworth, J. Program for recovering nurses: An evaluation. Medsurg Nurs. Off. J. Acad. Med.-Surg. Nurses 2006, 15, 223–230. [Google Scholar]
- Galanter, M.; Dermatis, H.; Mansky, P.; McIntyre, J.; Perez-Fuentes, G. Substance-abusing physicians: Monitoring and twelve-step-based treatment. Am. J. Addict. 2007, 16, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Brewster, J.M.; Kaufmann, I.M.; Hutchison, S.; MacWilliam, C. Characteristics and outcomes of doctors in a substance dependence monitoring programme in Canada: Prospective descriptive study. BMJ Br. Med. J. 2008, 337, 7679. [Google Scholar] [CrossRef] [PubMed]
- Fogger, S.A.; McGuinness, T. Alabama’s nurse monitoring programs: The nurse’s experience of being monitored. J. Addict. Nurs. 2009, 20, 142–149. [Google Scholar] [CrossRef]
- Merlo, L.J.; Greene, W.M.; Pomm, R. Mandatory naltrexone treatment prevents relapse among opiate-dependent anesthesiologists returning to practice. J. Addict. Med. 2011, 5, 279–283. [Google Scholar] [CrossRef]
- Angres, D.; Bologeorges, S.; Chou, J. A two year longitudinal outcome study of addicted health care professionals: An investigation of the role of personality variables. Subst. Abus. 2013, 7, 49–60. [Google Scholar] [CrossRef]
- Cross, W.; Bologeorges, S.; Angres, D. Issues and data associated with addictive disease in pharmacists. Am. J. Addict. 2012, 21, 383. [Google Scholar]
- Earley, P.H.; Zummo, J.; Memisoglu, A.; Silverman, B.L.; Gastfriend, D.R. Open-label study of injectable extended-release naltrexone (XR-NTX) in healthcare professionals with opioid dependence. J. Addict. Med. 2017, 11, 224–230. [Google Scholar] [CrossRef]
- Bruguera, E.; Heredia, M.; Llavayol, E.; Pujol, T.; Nieva, G.; Valero, S. Integral Treatment Programme for Addicted Physicians: Results from the Galatea Care Programme for Sick Physicians. Eur. Addict. Res. 2020, 26, 122–130. [Google Scholar] [CrossRef]
- Miller, W.R.; Walters, S.T.; Bennett, M.E. How effective is alcoholism treatment in the United States? J. Stud. Alcohol 2001, 62, 211–220. [Google Scholar] [CrossRef]
- van Wamel, A.; Croes, E.; van Vugt, M.; van Rooijen, S. Prevalentie, Zorgaanbod, Effectiviteit en Trends in de Verslavingszorg. Achtergrondstudie in Opdracht van Het College van Zorgverzekeringen; Trimbos Insituut: Utrecht, The Netherlands, 2014. [Google Scholar]
- NIDA. Treatment and Recovery: National Institute on Drug Abuse. 2020. Available online: www.drugabuse.gov/publications/drugs-brains-behavior-science-addiction/treatment-recovery (accessed on 2 November 2020).
- McKay, J.R.; Knepper, C.; Deneke, E.; O’Reilly, C.; DuPont, R.L. Corrigendum to “an initial evaluation of a comprehensive continuing care intervention for clients with substance use disorders: My first year of recovery (MyFYR)”. J. Subst. Abus. Treat. 2017, 75, 97. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.R.; Knepper, C.; Deneke, E.; O’Reilly, C.; DuPont, R.L. An initial evaluation of a comprehensive continuing care intervention for clients with substance use disorders: My First Year of Recovery (MyFYR). J. Subst. Abus. Treat. 2016, 67, 50–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fazzino, T.L.; Bjorlie, K.; Lejuez, C.W. A systematic review of reinforcement-based interventions for substance use: Efficacy, mechanisms of action, and moderators of treatment effects. J. Subst. Abus. Treat. 2019, 104, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Kale, N. Urine Drug Tests: Ordering and Interpreting Results. Am. Fam. Phys. 2019, 99, 33–39. [Google Scholar]
- Brooks, S.K.; Gerada, C.; Chalder, T. Doctors and dentists with mental ill health and addictions: Outcomes of treatment from the Practitioner Health Programme. J. Ment. Health 2013, 22, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.R.; Wolff, K.; Strang, J.; Marshall, E.J. Follow-up of provision of inpatient treatment for UK healthcare professionals with alcohol dependence: Snapshot of a pilot specialist national health service. Subst. Use Misuse 2009, 44, 1916–1925. [Google Scholar] [CrossRef]
- Johnson, R.P.; Connelly, J.C. Addicted physicians. A closer look. JAMA 1981, 245, 253–257. [Google Scholar] [CrossRef]
- Kliner, D.J.; Spicer, J.; Barnett, P. Treatment outcome of alcoholic physicians. J. Stud. Alcohol 1980, 41, 1217–1220. [Google Scholar] [CrossRef]
- Lloyd, G. One hundred alcoholic doctors: A 21-year follow-up. Alcohol Alcohol. 2002, 37, 370–374. [Google Scholar] [CrossRef]
- Morse, R.M.; Martin, M.A.; Swenson, W.M.; Niven, R.G. Prognosis of physicians treated for alcoholism and drug dependence. JAMA 1984, 251, 743–746. [Google Scholar] [CrossRef]
- Murray, R.M. Characteristics and prognosis of alcoholic doctors. Br. Med. J. 1976, 2, 1537–1539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, P.C.; Smith, J.D. Treatment outcomes of impaired physicians in Oklahoma. J. Okla. State Med. Assoc. 1991, 84, 599–603. [Google Scholar] [PubMed]
- Stuyt, E.B.; Gundersen, D.C.; Shore, J.H.; Brooks, E.; Gendel, M.H. Tobacco use by physicians In a physician health program, implications for treatment and monitoring. Am. J. Addict. 2009, 18, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.E.; Kiselanova, N.; Stevens, Q.; Lutz, R.; Mandler, T.; Tran, Z.V. A survey of inhalational anaesthetic abuse in anaesthesia training programmes. Anaesthesia 2008, 63, 616–620. [Google Scholar] [CrossRef]
- Blodgett, J.C.; Maisel, N.C.; Fuh, I.L.; Wilbourne, P.L.; Finney, J.W. How effective is continuing care for substance use disorders? A meta-analytic review. J. Subst. Abus. Treat. 2014, 46, 87–97. [Google Scholar] [CrossRef]
- Lawson, N.; Boyd, J.W. Physician health program outcome data should be viewed with caution. Judges J. 2018, 57, 36. [Google Scholar]
- Mumba, M.N.; Baxley, S.M.; Cipher, D.J.; Snow, D.E. Personal Factors as Correlates and Predictors of Relapse in Nurses With Impaired Practice. J. Addict. Nurs. 2019, 30, 24–31. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019): Cochrane. 2019. Available online: www.training.cochrane.org/handbook (accessed on 2 November 2020).
| Population | ((“Health personnel”[MeSH] OR “Medical staff”[MeSH] OR Dentist*[tiab] OR Doctor*[tiab] OR General practitioner*[tiab] OR Health personnel[tiab] OR Healthcare personnel[tiab] OR Healthcare provider*[tiab] OR Healthcare professional*[tiab] OR Medical staff[tiab] OR Nurse*[tiab] OR Nursing staff[tiab] OR Pharmacist*[tiab] OR Physician*[tiab] OR Physician assistant*[tiab]) AND (“Alcohol-related disorders”[MeSH] OR “Alcoholism”[MeSH] OR “Opioid-related disorders”[MeSH] OR “Substance-related disorders”[MeSH] OR Alcohol abuse*[tiab] OR Alcohol addict*[tiab] OR Alcohol dependen*[tiab] OR Alcohol impair*[tiab] OR Alcohol misuse[tiab] OR Alcohol use disorder*[tiab] OR Alcohol-related disorders[tiab] OR Alcoholism[tiab] OR Drug abuse*[tiab] OR Drug addict*[tiab] OR Drug dependen*[tiab] OR Drug impair*[tiab] OR Drug misuse[tiab] OR Drug use disorder*[tiab] OR Opiate abuse*[tiab] OR Opioid abuse*[tiab] OR Opiate addict*[tiab] OR Opioid addict*[tiab] OR Opiate dependen*[tiab] OR Opioid dependen*[tiab] OR Opiate impair*[tiab] OR Opioid impair*[tiab] OR Opiate misuse[tiab] OR Opioid misuse[tiab] OR Opioid-related disorders[tiab] OR Substance abuse*[tiab] OR Substance addict*[tiab] OR Substance dependen*[tiab] OR Substance impair*[tiab] OR Substance misuse[tiab] OR Substance use disorder*[tiab] OR Substance-related disorders[tiab])) OR (“Professional impairment”[MeSH] OR Dentist impair*[tiab] OR Doctor impair*[tiab] OR Nurse impair*[tiab] OR Pharmacist impair*[tiab] OR Physician impair*[tiab] OR Physician assistant impair*[tiab] OR Professional impairment[tiab]) |
| AND | |
| Intervention | (“Health services”[MeSH] OR “Occupational health”[MeSH] OR “Mental disorders”[MeSH] OR “Referral and consultation”[MeSh] OR Employee assistance program*[tiab] OR Employee health service*[tiab] OR Health agenc*[tiab] OR Health program*[tiab] OR Health service*[tiab] OR Occupational health[tiab] OR Occupational health service*[tiab] OR Mental disorders[tiab] OR Referral and consultation[tiab]) OR (“Biological monitoring”[MeSH] OR “Mental health recovery”[MeSH] OR “Psychiatric rehabilitation”[MeSH] OR Biological monitor*[tiab] OR Mental health rehabilitation[tiab] OR Mental health recovery[tiab] OR Physiologic monitor*[tiab] OR Psychiatric rehabilitation[tiab] OR Psychosocial rehabilitation[tiab] OR Recover*[tiab]) OR (“Self-help groups”[MeSH] OR Self-help group*[tiab] OR Support group*[tiab] OR Alcoholics anonym*[tiab] OR Narcotics anonym*[tiab]) |
| AND | |
| Outcome | (“Outcome assessment, health care”[MeSH] OR “Program evaluation”[MeSH] OR “Treatment outcome”[MeSH] OR Outcome assessment*[tiab] OR Outcome measure*[tiab] OR Program effect*[tiab] OR Program evaluation[tiab] OR Treatment effect*[tiab] OR Treatment failure*[tiab] OR Treatment outcome*[tiab] OR Recovery rate*[tiab] OR Rehabilitation outcome*[tiab]) OR (“Alcohol abstinence”[MeSH] OR “Recurrence”[MeSH] OR Abstinence[tiab] OR Alcohol abstinence[tiab] OR Drug abstinence[tiab] OR Opioid abstinence[tiab] OR Substance use abstinence[tiab] OR Recurrence[tiab] OR Relapse*[tiab]) OR (“Return to work”[MeSH] OR “Work performance”[MeSH] OR Job perform*[tiab] OR Job retention[tiab] OR Return to work[tiab] OR Work perform*[tiab] OR Work resum*[tiab] OR Work retention[tiab]) |
| Year, Study Country (State) | Design | Time Frame | Subjects (N) | Males (%) | Type of Healthcare Professional (%) | Type of Substance Use (%) | Source of Referral (%) |
|---|---|---|---|---|---|---|---|
| 1982, Herrington et al. [20] US (Wisconsin) | retrospective review | 1979–1982 | 40 | 95 | general practitioner (28); anesthesiologist (13); psychiatrist (10); internal medicine (8); dentist (8); obstetrics-gynecology (8); surgeon (5); other (20) | alcohol (58); narcotics (38); other (5) | coworker (63); family member (18); legal system (13); self-referral (8) |
| 1984, Washton et al. [21] US (New York; New Jersey) | retrospective review | 1979–1981 | 15 | 100 | physician (100) | opioids (100) | - |
| 1985, Crowley [22] US (Colorado) | prospective descriptive | - | 15 | 100 | physician (60); dentist (33); veterinarian (7) | - | licensing board (40); hospital or coworkers (33); family member (7); treatment provider (7); self-referral (13) |
| 1987, Shore [23] US (Oregon) | retrospective review | 1977–1985 | 25 | - | physician (100) | - | - |
| 1991, Pelton & Ikeda [24] US (California) | retrospective review | 1980–1990 | 51 | - | anesthesiologist (100) | opioids (49) | - |
| 1992, Gallegos et al. [25] US (Georgia) | retrospective review | 1982–1992 | 100 | 92 | family and general practitioner (23); surgeon (22); anesthesiologist (17); psychiatrist (15); internal medicine (12); emergency medicine (4); pediatrician (3); radiologist (1); dermatologist (1); occupational medicine (1); rehabilitation medicine (1) | alcohol (71); cocaine (21); meperidine hydrochloride (19); diazepam (18); marijuana (17); percodan (12); fentanyl citrate (11); codeine sulfate (9); amphetamine (7) | - |
| 1994, Roy [26] US (Louisiana) | retrospective review | >1989 | 37 | 89 | physician (68); dentist (16); pharmacist (5); veterinarian (3); other (8) | prescription drug (43); alcohol (27); polysubstance (16); cocaine (14) | - |
| 1996, Nelson et al. (1) [27] US (Oregon) | retrospective review | 1990–1992 | 56 | 91 | surgery (59); internal medicine (32); family practitioner (21); emergency medicine (7); anesthesiology (6); pathology (4); pediatrician (4); obstetrics-gynecology (3); psychiatry (2); neurology (2); dermatology (1); radiology (1); unknown (1) | alcohol (75); opioids and cocaine (21); amphetamines and sedatives (4) | self-referral (15); immediate contact (39); third party (46) |
| 1996, Nelson et al. (2) [27] US (Oregon) | retrospective review | 1990–1992 | 41 | 90 | alcohol (87); opioids and cocaine (8); amphetamines and sedatives (5) | self-referral (7); immediate contact (15); third party (73); unknown (5) | |
| 1997, Roth et al. [28] US (Connecticut) | retrospective review | - | 20 | 15 | nurse (85); anesthesiology nurse (10); pharmacist (5) | opioids (100); alcohol (85); cocaine (40); benzodiazepines (30) | licensing board (90); self-referral (10) |
| 1999, Paris & Canavan (1) [29] US (New Jersey) | retrospective review | 1982–1994 | 32 | - | anesthesiologist (59); anesthesiology residents (41) | opioids (78) | - |
| 1999, Paris & Canavan (2) [29] US (New Jersey) | retrospective review | 1982–1994 | 36 | - | physician (75); resident (25) | opioids (42) | - |
| 2004, Warhaft [30] Australia | retrospective review | 2001–2004 | 58 | 86 | general practitioner (34); anesthesiologist (10); surgeon (7); pathologist (5); radiologist (5); physician (3); obstetrics-gynecology (2); occupational medicine (2); pediatrician (2); psychiatry (2); other (28) | alcohol (36); pethidine (31); heroin (12); codeine (5); benzodiazepines (5); amphetamines (3); cocaine (3); nitrous oxide (2); ketamine (2) | - |
| 2005, Domino et al. [31] US (Washington) | retrospective cohort | 1991–2001 | 292 | 84 | physician (79); physician assistant (11); veterinarian (5); osteopath (2); dentist/dental surgeon (1); podiatrist/pharmacist (1) | alcohol (56); opioids (32); cocaine (3); benzodiazepines (2); other (7) | - |
| 2005, Ganley et al. (1) [32] US (North Carolina) | retrospective review | 1991–2001 | 233 | 87 | physician (100) | alcohol (50); opioids (25); polysubstance (16); other (8) | licensing board; hospital; coworker; family member; self-referral |
| 2005, Ganley et al. (2) [32] US (North Carolina) | retrospective review | 1991–2001 | 34 | 74 | physician assistant (100) | alcohol (44); opioids (35); polysubstance (6); other (15) | licensing board; hospital; coworker; family member; self-referral |
| 2006, Clark et al. [33] US (Idaho) | retrospective review | 1985–2000 | 147 | 18 | registered nurse (57); licensed practical nurse (38); advanced practice registered nurse (3) | alcohol (72); legal oral opioids (45); inhalants (8); stimulants (23); marijuana (21); legal injected narcotics (31); illegal injected opioids (33); prescription drugs (20) | employer (50); licensing board (14); coworker (6); treatment provider (6); self-referral (14) |
| 2007, Galanter et al. [34] US (New York; Nevada) | retrospective review | 2003–2004 | 104 | 92 | anesthesiologist (21); internal medicine (15); surgeon (14); family practitioner (10); obstetrics-gynecology (9); pediatrician (8); psychiatrist (8); general practitioner (4); emergency medicine (4); radiologist (3); other (5) | alcohol (36); opioids (34); other or mixed (30) | - |
| 2007, Knight et al. [13] US (Massachusetts) | retrospective observations | 1993–2003 | 132 | 82 | internal medicine (31); psychiatrist (12); surgeon (12); anesthesiologist (11); emergency medicine (8); family practitioner (6); obstetrics-gynecology (6); radiologist (4); pediatrician (3); other (6) | - | - |
| 2008, Brewster et al. [35] Canada | prospective descriptive | 1995–2007 | 100 | 90 | general or family practitioner (51); specialist (49) | alcohol (51); opioids (37); other (13) | - |
| 2009, DuPont et al. [9] US (Maryland; Pennsylvania; Indiana; Florida) | retrospective review | 1995–2001 | 904 | 86 | family practitioner (20); internal medicine (13); anesthesiologist (11); emergency medicine (7); psychiatrist (7); other (42) | alcohol (50); opioids (33); stimulants (8); other (9) | licensing board, hospital, malpractice insurance company (55); family member, coworker, employer (45) |
| 2009, Fogger & Mc-Guinness (1) [36] US (Alabama) | cross-sectional survey | - | 127 | - | registered nurse (77); licensed practical nurse (13); advanced practice registered nurse (8) | opioids (36) | - |
| 2009, Fogger & Mc-Guinness (2) [36] US (Alabama) | cross-sectional survey | - | 45 | - | registered nurse (78); licensed practical nurse (18); advanced practice registered nurse (4) | - | |
| 2011, Merlo et al. [37] US (Florida) | retrospective review | ≥ 2005 | 11 | 100 | anesthesiologist (100) | opioids (100) | - |
| 2013, Angres et al. [38] US (Illinois) | prospective cohort | - | 116 | 68 | physician (48); nurse (24); pharmacist (18); dentist (7); optometrist (1); physician assistant (1); other (1) | - | licensing board (100) |
| 2013, Cross et al. [39] US (Illinois) | prospective descriptive | 1994–2011 | 116 | 78 | pharmacist (100) | oral opioids (71); alcohol (22); illegal drugs (9); stimulants (8); injected opioids (3) | - |
| 2017, Earley et al. [40] US (Georgia) | single-arm multisite, open label | 2009–2012 | 38 | 18 | nurse (79); physician (11); pharmacist (3); other (8) | opioids (100) | - |
| 2020, Bruguera et al. [41] Spain | prospective descriptive | 2008–2016 | 126 | 60 | family practitioner (17); psychiatrist (9); anesthesiologist (9); pediatrician (6); orthopedic surgeons (6); internal medicine (3); resident (4); other (47) | alcohol (63); sedatives, hypnotics, anxiolytics (15); opioids (7); stimulants (6); cannabis (4); cocaine (2); mixed (3) | self-referral (75); coworker or family member (20); other (6) |
| Year, Study | Name of the Health Program | Recommended Type of Treatment | Monitoring Elements | Monitoring Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Start of Monitoring | Biological Monitoring | Monitoring at Work | Other Agreements | Follow-Up: % Abstinence | Follow-Up: % Work Retention | |||
| 1982, Herrington et al. [20] | Impaired Physician Treatment Program | inpatient (95%) | after treatment completion | urine | yes | participate in Alcoholics or Narcotics Anonymous groups, attendance of local meetings in the community, and attendance of weekly sessions of Milwaukee Doctors in Alcoholics Anonymous | 0 to 3 years: 68% no relapse | 0 to 3 years: 78% working |
| 1984, Washton et al. [21] | Regent or Fair Oaks Hospital | inpatient 4–10 weeks | after treatment completion | urine | pharmacotherapy with naltrexone, group therapy, individual therapy, and family/couples therapy | 0 to 1.5 years: 87% no relapse | 0 to 1.5 years: 87% working | |
| 1985, Crowley [22] | Halsted Clinic | outpatient | with treatment initiation | urine | - | counseling sessions | 2 years (average): 47% no relapse | - |
| 1987, Shore [23] | rehabilitation program | - | - | urine | - | long term supervision by the medical board | - | 0 to 8 years: 75% working |
| 1991, Pelton & Ikeda [24] | California Physicians Diversion Program | - | with treatment initiation | urine | yes | attendance of two diversion group meetings per week, and two or more 12-step meetings per week | 3 to 5 years: 51% no relapse 18% brief relapse | - |
| 1992, Gallegos et al. [25] | Georgia Impaired Physicians Program continuing care program | Georgia Impaired Physicians Treatment Program | after treatment completion | urine | - | primary care physician attends to their medical needs, recovery mentor, five Alcoholics or Narcotics Anonymous meetings per week, and one Caduceus Club meeting per week | more than 5 years: 77% no relapse | more than 5 years: 91% working |
| 1994, Roy [26] | reentry monitoring | - | after treatment completion | urine | - | group therapy | 2 years (average): 81% no relapse8% brief relapse | 2 years (average): 95% working |
| 1996, Nelson et al. (1) [27] | Diversion Program | inpatient and/or outpatient | after treatment completion | urine | - | group therapy | 1.5 years (average): 86% no relapse | - |
| 1996, Nelson et al. (2) [27] | Probationary Program | inpatient and/or outpatient | after treatment completion | urine | - | group therapy | 2.3 years (average): 78% no relapse | - |
| 1997, Roth et al. [28] | special treatment program | inpatient and/or outpatient | with treatment initiation | urine | - | pharmacotherapy with naltrexone for 6 months | 1.8 years (average): 60% no relapse | 1.8 years (average): 60% working |
| 1999, Paris & Canavan (1) [29] | Physician Health Program | - | after treatment completion | urine | - | participate in an aftercare group for 1 year, monthly face-to-face appointment with PHP employee, and attendance of Alcoholics Anonymous meetings | 7.8 years (average): 59% no relapse | - |
| 1999, Paris & Canavan (2) [29] | Physician Health Program | - | after treatment completion | urine | - | participate in an aftercare group for 1 year, monthly face-to-face appointment with PHP employee, and attendance of Alcoholics Anonymous meetings | 7.2 years (average): 56% no relapse | - |
| 2004, Warhaft [30] | Case Management, Aftercare and Monitoring Program | - | after treatment completion | urine and/or breath | yes | attendance at Caduceus group andattendance at mutual help group (Alcoholics or Narcotics Anonymous) | 0 to 3 years: 79% no relapse | 0 to 3 years: 78% working |
| 2005, Domino et al. (1) [31] | Washington Physician Health Program | - | after treatment completion | urine | yes | frequent contact for behavioral assessment and regulatory board reports | 0 to 5 years: 78% no relapse | - |
| 2005, Domino et al. (2) [31] | Washington Physician Health Program | - | after treatment completion | urine | yes | frequent contact for behavioral assessment and regulatory board reports | more than 5 years: 68% no relapse | more than 5 years: 88% working |
| 2005, Ganley et al. (1) [32] | North Carolina Physician Health Program | inpatient and/or outpatient | - | urine hair | meetings with volunteer monitor, participate in Alcoholics Anonymous and other self-help groups, and participate in Caduceus meetings | 1 to 6 years: 65% no relapse 26% brief relapse | - | |
| 2005, Ganley et al. (2) [32] | North Carolina Physician Health Program | inpatient and/or outpatient | - | urine hair | meetings with volunteer monitor, participate in Alcoholics Anonymous and other self-help groups, and participate in Caduceus meetings | 1 to 6 years: 50% no relapse 9% brief relapse | - | |
| 2006, Clark et al. [33] | Program for Recovering Nurses | mainly outpatient (69%) | with treatment initiation | urine | yes | aftercare counseling and attendance at recovery nursing support groups | 3.8 years (average): 48% no relapse | 3.8 years (average): 43% working |
| 2007, Galanter et al. [34] | Committee for Physician Health | inpatient and/or outpatient | with treatment initiation | urine | yes | 12-step/therapy monitor | 3.4 years (average): 63% no relapse | - |
| 2007, Knight et al. [13] | Physician Health Services | individual psychotherapy | with treatment initiation | urine | yes | attendance at Caduceus meetings or Alcoholics Anonymous | 0 to 3 years: 56% no relapse | - |
| 2008, Brewster et al. [35] | Ontario Physician Health Program | usually inpatient abstinence based 4–6 weeks | after treatment completion | urine | yes | visits to addiction medicine doctor, visits to a family doctor for routine health needs, and attendance at mutual support groups in community | 5 years (exact): 71% no relapse and 14% brief relapse | - |
| 2009, Du Pont et al. [9] | 16 American Physician Health Programs | inpatient and/or outpatient | after treatment completion | urine | - | participate in Alcoholics or Narcotics Anonymous groups, participate in aftercare groups, and follow-up from Physician Health Program monitor | 4.5 years (average): 78% no relapse | 4.5 years (average): 72% working |
| 2009, Fogger & McGuinness (1) [36] | Voluntary Discipline Alternative Program | inpatient and/or outpatient | after treatment completion | urine | - | - | 3 years (average): 94% no relapse | 2.5 years (average): 90% working |
| 2009, Fogger & McGuinness (2) [36] | Probationary Program | inpatient and/or outpatient | after treatment completion | urine | - | attendance of 12-steps meetings at least three times per week and attendance aftercare meeting at least one time per week for one year | 4.4 years (average): 96% working | |
| 2011, Merlo et al. [37] | Professional Resource Network | - | after treatment completion | urine | - | pharmacotherapy with naltrexone for at least 2 years | 3.4 years (average): 91% no relapse | 3.4 years (average): 82% working |
| 2013, Angres et al. [38] | After-Care program | abstinence based 6–8 weeks | after treatment completion | urine | - | participate in Caduceus aftercare group weekly | 2 years (exact): 73% no relapse | - |
| 2013, Cross et al. [39] | Chicago treatment program for professionals | inpatient abstinence based 8–10 weeks | after treatment completion | urine | - | participate in Alcoholics or Narcotics Anonymous groups, participate in Caduceus aftercare group, and follow-up from Physician Health Program monitor | 2 years (exact): 87% no relapse | - |
| 2017, Earley et al. [40] | Injectable extended-release naltrexone | intensive outpatient | with treatment initiation | urine | - | attendance at mutual support meetings | 2 years (exact): 89% no relapse | 2 years (exact): 63% working |
| 2020, Bruguera et al. [41] | Galatea Addiction Programme | inpatient (62%) and/or outpatient 7–8 weeks | with treatment initiation | urine hair | yes | participate in psychotherapy group weekly | 2 years (average): 30% no relapse | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geuijen, P.M.; van den Broek, S.J.M.; Dijkstra, B.A.G.; Kuppens, J.M.; de Haan, H.A.; de Jong, C.A.J.; Schene, A.H.; Atsma, F.; Schellekens, A.F.A. Success Rates of Monitoring for Healthcare Professionals with a Substance Use Disorder: A Meta-Analysis. J. Clin. Med. 2021, 10, 264. https://doi.org/10.3390/jcm10020264
Geuijen PM, van den Broek SJM, Dijkstra BAG, Kuppens JM, de Haan HA, de Jong CAJ, Schene AH, Atsma F, Schellekens AFA. Success Rates of Monitoring for Healthcare Professionals with a Substance Use Disorder: A Meta-Analysis. Journal of Clinical Medicine. 2021; 10(2):264. https://doi.org/10.3390/jcm10020264
Chicago/Turabian StyleGeuijen, Pauline M., Sophie J. M. van den Broek, Boukje A. G. Dijkstra, Joanneke M. Kuppens, Hein A. de Haan, Cornelis A. J. de Jong, Aart H. Schene, Femke Atsma, and Arnt F. A. Schellekens. 2021. "Success Rates of Monitoring for Healthcare Professionals with a Substance Use Disorder: A Meta-Analysis" Journal of Clinical Medicine 10, no. 2: 264. https://doi.org/10.3390/jcm10020264
APA StyleGeuijen, P. M., van den Broek, S. J. M., Dijkstra, B. A. G., Kuppens, J. M., de Haan, H. A., de Jong, C. A. J., Schene, A. H., Atsma, F., & Schellekens, A. F. A. (2021). Success Rates of Monitoring for Healthcare Professionals with a Substance Use Disorder: A Meta-Analysis. Journal of Clinical Medicine, 10(2), 264. https://doi.org/10.3390/jcm10020264





