The Current State of Knowledge on the Clinical and Methodological Aspects of Extracorporeal Shock Waves Therapy in the Management of Post-Stroke Spasticity—Overview of 20 Years of Experiences
Abstract
1. Introduction
1.1. Development of Post-Stroke Spasticity
1.2. Physical Therapy for Post-Stroke Spasticity
1.3. Shock Waves for Post-Stroke Spasticity
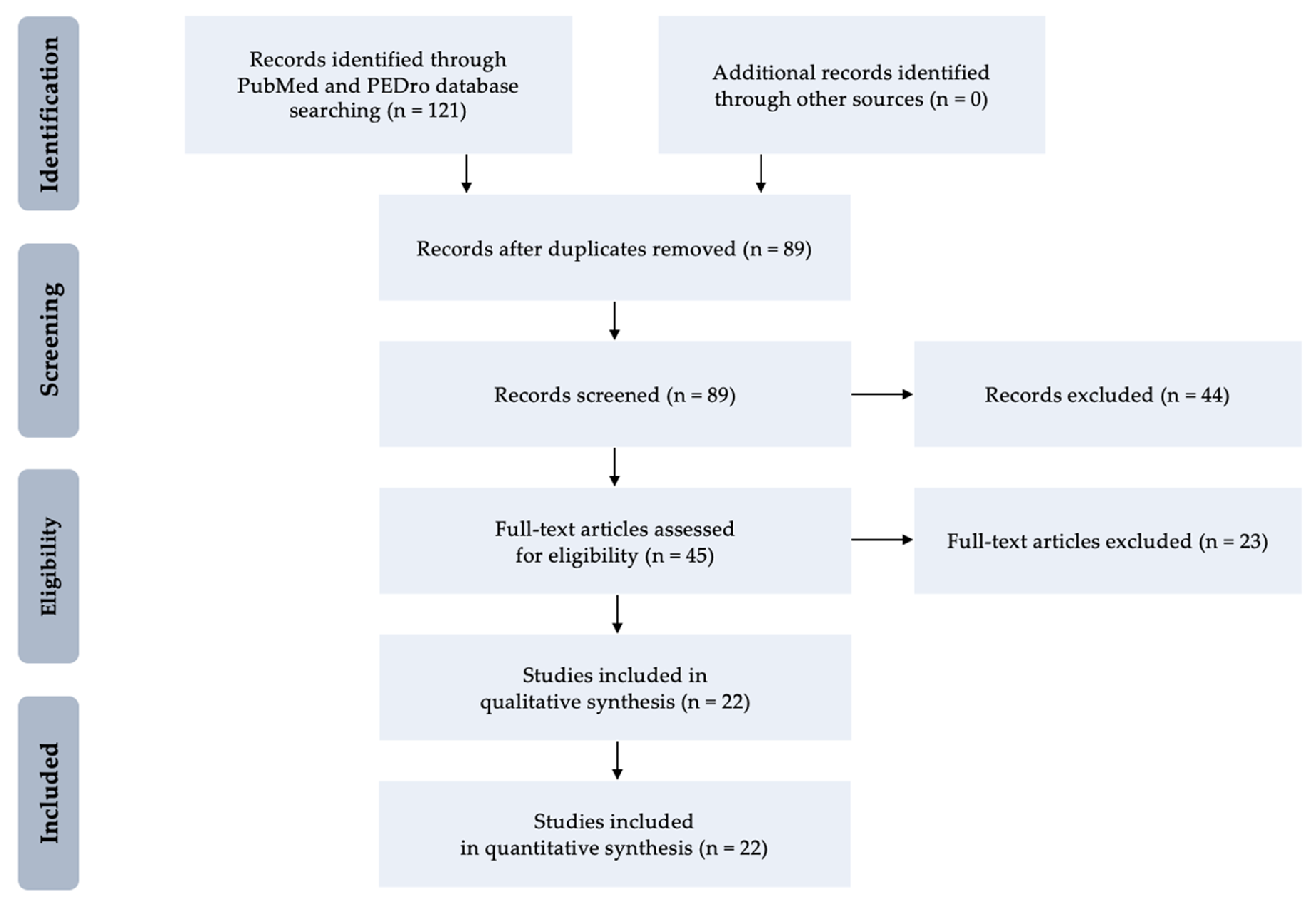
2. Methods
3. Results
3.1. Shock Waves for Upper Limb Spasticity
3.2. Shock Waves for Lower Limb Spasticity
3.3. Recent Reviews and Meta-Analyses
4. Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | Activities of daily living |
| aROM | active range of motion |
| BI | Barthel Index |
| BTX-A | botulinum toxin type A |
| CI | confidence interval |
| CIMT | constraint-induced movement therapy |
| CNS | central nervous system |
| EFD | energy flux density |
| EMG-BF | electromyographic biofeedback training |
| EMG-NMES | electromyography neuromuscular electrical stimulation |
| ES | electrical stimulation |
| ESWT | extracorporeal shock wave therapy |
| FES | functional electrical stimulation |
| FGF | fibroblast growth factors |
| fSWT | focused shock wave therapy |
| IRT | infrared thermography |
| LBP | low back pain |
| LL | lower limb |
| MAS | Modified Ashworth Scale |
| MBI | Modified Barthel Index |
| MMAS | Modified Modified Ashworth scale |
| MRS | Modified Rankin Scale |
| MS | multiple sclerosis |
| MTS | Modified Tardieu Scale |
| MVFT | mirror visual feedback training |
| NCS | neural stem cell |
| NDT | neurodevelopmental treatment by Bobath |
| NICE | National Institute for Health and Clinical Excellence |
| NMES | neuromuscular electrical stimulation |
| NO | nitric oxide |
| NTH-3 | neurotrophin-3 |
| PEDro | Physiotherapy Evidence Database |
| PNF | proprioceptive neuromuscular facilitation |
| pPFT | plantar flexor torque |
| pSWT | planar shock wave therapy |
| RAT | robot-assisted training |
| rSWT | radial shock wave therapy |
| rTMS | transcranial magnetic stimulation |
| SCI | spinal cord injuries |
| sEMG | surface electromyography |
| SMD | standardized mean difference |
| SMT | sensimotor movement training |
| SP | substance P |
| SS | swelling scale |
| tDCS | transcranial direct current stimulation |
| TENS | transcutaneous electrical nerve stimulation |
| TGF | transforming growth factor |
| TRT | task-related training |
| TU | therapeutic ultrasound |
| UL | upper-limb |
| UL-FMA | Upper Limb—Fugl–Meyer Assessment |
| UMN | upper motor neuron |
| VAS | Visual-Analogue Scale |
| VEGF | vascular endothelial growth factor |
| VRBT | virtual reality-based training. |
| WBVT | whole body vibration training |
Appendix A
| PEDro Score | |
|---|---|
| Criteria | Explanation |
| 1. * | Eligibility criteria were specified. |
| 2. | Subjects were randomly allocated to groups. |
| 3. | Allocation was concealed. |
| 4. | The groups were similar at baseline regarding the most important prognostic indicators. |
| 5. | There was blinding of all subjects. |
| 6. | There was blinding of all therapists who administered the therapy. |
| 7. | There was blinding of all assessors who measured at least one key outcome. |
| 8. | Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups. |
| 9. | All subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome was analysed by “intention to treat”. |
| 10. | The results of between-group statistical comparisons are reported for at least one key outcome. |
| 11. | The study provides both point measures and measures of variability for at least one key outcome. |
| NICE Guidelines | |
|---|---|
| Level of Evidence | Type of Evidence |
| 1++ | High-quality meta-analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias. |
| 1+ | Well-conducted meta-analyses, systematic reviews of RCTs, or RCTs with a low risk of bias. |
| 1− | * Meta-analyses, systematic reviews of RCTs, or RCTs with a high risk of bias. * |
| 2++ | High-quality systematic reviews of case-control or cohort studies. High-quality case-control or cohort studies with a very low risk of confounding, bias or chance, and a high probability that the relationship is causal. |
| 2+ | Well-conducted case-control or cohort studies with a low risk of confounding, bias or chance, and a moderate probability that the relationship is causal. |
| 2− | Case control or cohort studies with a high risk of confounding, bias or chance, and asignificant risk that the relationship is not causal. * |
| 3 | Nonanalytic studies (e.g., case reports and case series). |
| 4 | Expert opinion, formal consensus. |
| NICE Guidelines | |
|---|---|
| Grade | Explanation |
| A | At least one meta-analysis, systematic review, or randomized controlled trial rated as 1++ and directly applicable to the target population or a systematic review of randomized controlled trials or a body of evidence consisting principally of studies rated as 1+ directly applicable to the target population and demonstrating overall consistency of results. |
| B | A body of evidence including studies rated as 2++ directly applicable to the target population and demonstrating overall consistency of results or extrapolated evidence from studies rated as 1++ or 1+. |
| C | A body of evidence including studies rated as * 1− or 2+ directly applicable to the target population and demonstrating overall consistency of results or extrapolated evidence from studies rated as 2++. |
| D | Evidence level * 2−, 3 or 4 or extrapolated evidence from studies rated as 2+. |
References
- Bensmail, D.; Wissel, J.; Laffont, I.; Simon, O.; Scheschonka, A.; Flatau-Baqué, B.; Dressler, D.; Simpson, D.M. Efficacy of IncobotulinumtoxinA for the Treatment of Adult Lower-Limb Post-Stroke Spasticity, Including Pes Equinovarus. Ann. Phys. Rehabil. Med. 2020. [Google Scholar] [CrossRef]
- Schinwelski, M.; Sławek, J. Prevalence of Spasticity Following Stroke and Its Impact on Quality of Life with Emphasis on Disability in Activities of Daily Living. Systematic Review. Neurol. Neurochir. Pol. 2010, 44, 404–411. [Google Scholar] [CrossRef][Green Version]
- Urban, P.P.; Wolf, T.; Uebele, M.; Marx, J.J.; Vogt, T.; Stoeter, P.; Bauermann, T.; Weibrich, C.; Vucurevic, G.D.; Schneider, A.; et al. Occurence and Clinical Predictors of Spasticity after Ischemic Stroke. Stroke 2010, 41, 2016–2020. [Google Scholar] [CrossRef]
- Wissel, J.; Manack, A.; Brainin, M. Toward an Epidemiology of Poststroke Spasticity. Neurology 2013, 80, S13–S19. [Google Scholar] [CrossRef]
- Zorowitz, R.D.; Gillard, P.J.; Brainin, M. Poststroke Spasticity: Sequelae and Burden on Stroke Survivors and Caregivers. Neurology 2013, 80, S45–S52. [Google Scholar] [CrossRef]
- Yelnik, A.P.; Simon, O.; Parratte, B.; Gracies, J.M. How to Clinically Assess and Treat Muscle Overactivity in Spastic Paresis. J. Rehabil. Med. 2010, 42, 801–807. [Google Scholar] [CrossRef]
- Pandyan, A.D.; Gregoric, M.; Barnes, M.P.; Wood, D.; Van Wijck, F.; Burridge, J.; Hermens, H.; Johnson, G.R. Spasticity: Clinical Perceptions, Neurological Realities and Meaningful Measurement. Disabil. Rehabil. 2005, 27, 2–6. [Google Scholar] [CrossRef]
- Li, S. Spasticity, Motor Recovery, and Neural Plasticity after Stroke. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef]
- Kong, K.H.; Lee, J.; Chua, K.S. Occurrence and Temporal Evolution of Upper Limb Spasticity in Stroke Patients Admitted to a Rehabilitation Unit. Arch. Phys. Med. Rehabil. 2012, 93, 143–148. [Google Scholar] [CrossRef]
- Lundström, E.; Terént, A.; Borg, J. Prevalence of Disabling Spasticity 1 Year after First-Ever Stroke. Eur. J. Neurol. 2008, 15, 533–539. [Google Scholar] [CrossRef]
- Opheim, A.; Danielsson, A.; Alt Murphy, M.; Persson, H.C.; Sunnerhagen, K.S. Upper-Limb Spasticity during the First Year after Stroke: Stroke Arm Longitudinal Study at the University of Gothenburg. Am. J. Phys. Med. Rehabil. 2014, 93, 884–896. [Google Scholar] [CrossRef]
- Sommerfeld, D.K.; Eek, E.U.-B.; Svensson, A.-K.; Holmqvist, L.W.; von Arbin, M.H. Spasticity after Stroke: Its Occurrence and Association with Motor Impairments and Activity Limitations. Stroke 2004, 35, 134–139. [Google Scholar] [CrossRef]
- Wissel, J.; Verrier, M.; Simpson, D.M.; Charles, D.; Guinto, P.; Papapetropoulos, S.; Sunnerhagen, K.S. Post-Stroke Spasticity: Predictors of Early Development and Considerations for Therapeutic Intervention. PM R 2015, 7, 60–67. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Bensmail, D.; Yelnik, A. Non-Pharmacological Interventions for Spasticity in Adults: An Overview of Systematic Reviews. Ann. Phys. Rehabil. Med. 2019, 62, 265–273. [Google Scholar] [CrossRef]
- Dymarek, R.; Ptaszkowski, K.; Słupska, L.; Paprocka-Borowicz, M.; Taradaj, J.; Halski, T.; Rosińczuk, J. [Post-stroke spasticity management including a chosen physiotherapeutic methods and improvements in motor control—Review of the current scientific evidence]. Wiad. Lek. 2017, 70, 357–365. [Google Scholar]
- Pollock, A.; Baer, G.; Campbell, P.; Choo, P.L.; Forster, A.; Morris, J.; Pomeroy, V.M.; Langhorne, P. Physical Rehabilitation Approaches for the Recovery of Function and Mobility Following Stroke. Cochrane Database Syst. Rev. 2014, 2014. [Google Scholar] [CrossRef]
- Wang, J.-S.; Lee, S.-B.; Moon, S.-H. The Immediate Effect of PNF Pattern on Muscle Tone and Muscle Stiffness in Chronic Stroke Patient. J. Phys. Ther. Sci. 2016, 28, 967–970. [Google Scholar] [CrossRef]
- Monaghan, K.; Horgan, F.; Blake, C.; Cornall, C.; Hickey, P.P.; Lyons, B.E.; Langhorne, P. Physical Treatment Interventions for Managing Spasticity after Stroke. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Klomjai, W.; Lackmy-Vallée, A.; Roche, N.; Pradat-Diehl, P.; Marchand-Pauvert, V.; Katz, R. Repetitive Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation in Motor Rehabilitation after Stroke: An Update. Ann. Phys. Rehabil. Med. 2015, 58, 220–224. [Google Scholar] [CrossRef]
- McIntyre, A.; Mirkowski, M.; Thompson, S.; Burhan, A.M.; Miller, T.; Teasell, R. A Systematic Review and Meta-Analysis on the Use of Repetitive Transcranial Magnetic Stimulation for Spasticity Poststroke. PM R 2018, 10, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Kautz, S.A.; Schlaug, G.; Meinzer, C.; George, M.S.; Chhatbar, P.Y. Transcranial Direct Current Stimulation for Post-Stroke Motor Recovery: Challenges and Opportunities. PM R 2018, 10, S157–S164. [Google Scholar] [CrossRef] [PubMed]
- Dymarek, R.; Ptaszkowski, K.; Słupska, L.; Halski, T.; Taradaj, J.; Rosińczuk, J. Effects of Extracorporeal Shock Wave on Upper and Lower Limb Spasticity in Post-Stroke Patients: A Narrative Review. Top. Stroke Rehabil. 2016, 23, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Demetrios, M.; Khan, F.; Turner-Stokes, L.; Brand, C.; McSweeney, S. Multidisciplinary Rehabilitation Following Botulinum Toxin and Other Focal Intramuscular Treatment for Post-Stroke Spasticity. Cochrane Database Syst. Rev. 2013, CD009689. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shi, Q.; Wang, X.; Yang, K.; Yang, R. Prediction of Outcome of Extracorporeal Shock Wave Lithotripsy in the Management of Ureteric Calculi. Urol. Res. 2011, 39, 51–57. [Google Scholar] [CrossRef]
- Walewicz, K.; Taradaj, J.; Rajfur, K.; Ptaszkowski, K.; Kuszewski, M.T.; Sopel, M.; Dymarek, R. The Effectiveness Of Radial Extracorporeal Shock Wave Therapy In Patients With Chronic Low Back Pain: A Prospective, Randomized, Single-Blinded Pilot Study. Clin. Interv. Aging 2019, 14, 1859–1869. [Google Scholar] [CrossRef]
- Walewicz, K.; Taradaj, J.; Dobrzyński, M.; Sopel, M.; Kowal, M.; Ptaszkowski, K.; Dymarek, R. Effect of Radial Extracorporeal Shock Wave Therapy on Pain Intensity, Functional Efficiency, and Postural Control Parameters in Patients with Chronic Low Back Pain: A Randomized Clinical Trial. J. Clin. Med. 2020, 9, 568. [Google Scholar] [CrossRef]
- Aschermann, I.; Noor, S.; Venturelli, S.; Sinnberg, T.; Mnich, C.D.; Busch, C. Extracorporal Shock Waves Activate Migration, Proliferation and Inflammatory Pathways in Fibroblasts and Keratinocytes, and Improve Wound Healing in an Open-Label, Single-Arm Study in Patients with Therapy-Refractory Chronic Leg Ulcers. Cell. Physiol. Biochem. 2017, 41, 890–906. [Google Scholar] [CrossRef]
- Liu, T.; Shindel, A.W.; Lin, G.; Lue, T.F. Cellular signaling pathways modulated by low-intensity extracorporeal shock wave therapy. Int. J. Impot. Res. 2019, 31, 170–176. [Google Scholar] [CrossRef]
- Jia, G.; Ma, J.; Wang, S.; Wu, D.; Tan, B.; Yin, Y.; Jia, L.; Cheng, L. Long-Term Effects of Extracorporeal Shock Wave Therapy on Poststroke Spasticity: A Meta-Analysis of Randomized Controlled Trials. J. Stroke Cerebrovasc. Dis. 2020, 29, 104591. [Google Scholar] [CrossRef]
- Rosińczuk, J.; Taradaj, J.; Dymarek, R.; Sopel, M. Mechanoregulation of Wound Healing and Skin Homeostasis. Biomed. Res. Int. 2016, 2016, 3943481. [Google Scholar] [CrossRef] [PubMed]
- d’Agostino, M.C.; Craig, K.; Tibalt, E.; Respizzi, S. Shock Wave as Biological Therapeutic Tool: From Mechanical Stimulation to Recovery and Healing, through Mechanotransduction. Int. J. Surg. 2015, 24, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence: Clinical Guidelines; National Institute for Health and Care Excellence: London, UK, 2003.
- Manganotti, P.; Amelio, E. Long-Term Effect of Shock Wave Therapy on Upper Limb Hypertonia in Patients Affected by Stroke. Stroke 2005, 36, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Santamato, A.; Notarnicola, A.; Panza, F.; Ranieri, M.; Micello, M.F.; Manganotti, P.; Moretti, B.; Fortunato, F.; Filoni, S.; Fiore, P. SBOTE Study: Extracorporeal Shock Wave Therapy versus Electrical Stimulation after Botulinum Toxin Type a Injection for Post-Stroke Spasticity-a Prospective Randomized Trial. Ultrasound Med. Biol. 2013, 39, 283–291. [Google Scholar] [CrossRef]
- Troncati, F.; Paci, M.; Myftari, T.; Lombardi, B. Extracorporeal Shock Wave Therapy Reduces Upper Limb Spasticity and Improves Motricity in Patients with Chronic Hemiplegia: A Case Series. NeuroRehabilitation 2013, 33, 399–405. [Google Scholar] [CrossRef]
- Daliri, S.S.; Forogh, B.; Emami Razavi, S.Z.; Ahadi, T.; Madjlesi, F.; Ansari, N.N. A Single Blind, Clinical Trial to Investigate the Effects of a Single Session Extracorporeal Shock Wave Therapy on Wrist Flexor Spasticity after Stroke. NeuroRehabilitation 2015, 36, 67–72. [Google Scholar] [CrossRef]
- Dymarek, R.; Taradaj, J.; Rosińczuk, J. Extracorporeal Shock Wave Stimulation as Alternative Treatment Modality for Wrist and Fingers Spasticity in Poststroke Patients: A Prospective, Open-Label, Preliminary Clinical Trial. Evid. Based Complement. Alternat. Med. 2016, 2016, 4648101. [Google Scholar] [CrossRef]
- Dymarek, R.; Taradaj, J.; Rosińczuk, J. The Effect of Radial Extracorporeal Shock Wave Stimulation on Upper Limb Spasticity in Chronic Stroke Patients: A Single-Blind, Randomized, Placebo-Controlled Study. Ultrasound Med. Biol. 2016, 42, 1862–1875. [Google Scholar] [CrossRef]
- Li, T.-Y.; Chang, C.-Y.; Chou, Y.-C.; Chen, L.-C.; Chu, H.-Y.; Chiang, S.-L.; Chang, S.-T.; Wu, Y.-T. Effect of Radial Shock Wave Therapy on Spasticity of the Upper Limb in Patients With Chronic Stroke. Medicine (Baltimore) 2016, 95. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Yu, H.-K.; Chen, L.-R.; Chang, C.-N.; Chen, Y.-M.; Hu, G.-C. Extracorporeal Shock Waves Versus Botulinum Toxin Type A in the Treatment of Poststroke Upper Limb Spasticity: A Randomized Noninferiority Trial. Arch. Phys. Med. Rehabil. 2018, 99, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Yang, D.J.; Uhm, Y.H.; Yoon, J.H.; Kim, J.H. Effects of Extracorporeal Shock Wave Therapy on Upper Extremity Muscle Tone in Chronic Stroke Patients. J. Phys. Ther. Sci. 2018, 30, 361–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, G.; Yuan, W.; Liu, G.; Qiao, L.; Zhang, Y.; Wang, Y.; Wang, W.; Zhao, M.; Wang, Y.; Wang, J. Effects of Radial Extracorporeal Shockwave Therapy on Spasticity of Upper-Limb Agonist/Antagonist Muscles in Patients Affected by Stroke: A Randomized, Single-Blind Clinical Trial. Age Ageing 2020, 49, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ha, K.W.; Kim, Y.H.; Seol, P.-H.; Kwak, H.-J.; Park, S.-W.; Ryu, B.-J. Effect of Radial Extracorporeal Shock Wave Therapy on Hemiplegic Shoulder Pain Syndrome. Ann. Rehabil. Med. 2016, 40, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.K.; Cho, K.H.; Kim, Y.-J.; Hwang, S.L. Spasticity and Electrophysiologic Changes after Extracorporeal Shock Wave Therapy on Gastrocnemius. Ann. Rehabil. Med. 2011, 35, 599–604. [Google Scholar] [CrossRef]
- Moon, S.W.; Kim, J.H.; Jung, M.J.; Son, S.; Lee, J.H.; Shin, H.; Lee, E.S.; Yoon, C.H.; Oh, M.-K. The Effect of Extracorporeal Shock Wave Therapy on Lower Limb Spasticity in Subacute Stroke Patients. Ann. Rehabil. Med. 2013, 37, 461–470. [Google Scholar] [CrossRef]
- Santamato, A.; Micello, M.F.; Panza, F.; Fortunato, F.; Logroscino, G.; Picelli, A.; Manganotti, P.; Smania, N.; Fiore, P.; Ranieri, M. Extracorporeal Shock Wave Therapy for the Treatment of Poststroke Plantar-Flexor Muscles Spasticity: A Prospective Open-Label Study. Top. Stroke Rehabil. 2014, 21 (Suppl. 1), S17–S24. [Google Scholar] [CrossRef]
- Kim, T.G.; Bae, S.H.; Kim, G.Y.; Kim, K.Y. The Effects of Extracorporeal Shock Wave Therapy on Stroke Patients with Plantar Fasciitis. J. Phys. Ther. Sci. 2015, 27, 523–526. [Google Scholar] [CrossRef]
- Radinmehr, H.; Nakhostin Ansari, N.; Naghdi, S.; Olyaei, G.; Tabatabaei, A. Effects of One Session Radial Extracorporeal Shockwave Therapy on Post-Stroke Plantarflexor Spasticity: A Single-Blind Clinical Trial. Disabil. Rehabil. 2017, 39, 483–490. [Google Scholar] [CrossRef]
- Sawan, S.; Abd-Allah, F.; Hegazy, M.M.; Farrag, M.A.; El-Den, N.H.S. Effect of Shock Wave Therapy on Ankle Plantar Flexors Spasticity in Stroke Patients. NeuroRehabilitation 2017, 40, 115–118. [Google Scholar] [CrossRef]
- Taheri, P.; Vahdatpour, B.; Mellat, M.; Ashtari, F.; Akbari, M. Effect of Extracorporeal Shock Wave Therapy on Lower Limb Spasticity in Stroke Patients. Arch. Iran. Med. 2017, 20, 338–343. [Google Scholar] [PubMed]
- Wu, Y.-T.; Chang, C.-N.; Chen, Y.-M.; Hu, G.-C. Comparison of the Effect of Focused and Radial Extracorporeal Shock Waves on Spastic Equinus in Patients with Stroke: A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, S.H.; Yoo, J.-I.; Lee, S.-U. Ultrasonographic Evaluation for the Effect of Extracorporeal Shock Wave Therapy on Gastrocnemius Muscle Spasticity in Patients With Chronic Stroke. PM R 2019, 11, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Radinmehr, H.; Ansari, N.N.; Naghdi, S.; Tabatabaei, A.; Moghimi, E. Comparison of Therapeutic Ultrasound and Radial Shock Wave Therapy in the Treatment of Plantar Flexor Spasticity After Stroke: A Prospective, Single-Blind, Randomized Clinical Trial. J. Stroke Cerebrovasc. Dis. 2019, 28, 1546–1554. [Google Scholar] [CrossRef]
- Yoon, S.H.; Shin, M.K.; Choi, E.J.; Kang, H.J. Effective Site for the Application of Extracorporeal Shock-Wave Therapy on Spasticity in Chronic Stroke: Muscle Belly or Myotendinous Junction. Ann. Rehabil. Med. 2017, 41, 547–555. [Google Scholar] [CrossRef]
- Heckmatt, J.Z.; Pier, N.; Dubowitz, V. Real-Time Ultrasound Imaging of Muscles. Muscle Nerve 1988, 11, 56–65. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, S.-N.; Lee, I.-S.; Jung, H.; Lee, K.-S.; Koh, S.-E. Effects of Extracorporeal Shock Wave Therapy on Spasticity in Patients after Brain Injury: A Meta-Analysis. J. Phys. Ther. Sci. 2014, 26, 1641–1647. [Google Scholar] [CrossRef]
- Guo, P.; Gao, F.; Zhao, T.; Sun, W.; Wang, B.; Li, Z. Positive Effects of Extracorporeal Shock Wave Therapy on Spasticity in Poststroke Patients: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 2470–2476. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, W.; Jiang, W.; Qian, Q. Effects of Extracorporeal Shock Wave Therapy on Spasticity in Post-Stroke Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Rehabil. Med. 2018, 50, 852–859. [Google Scholar] [CrossRef]
- Oh, J.H.; Park, H.D.; Han, S.H.; Shim, G.Y.; Choi, K.Y. Duration of Treatment Effect of Extracorporeal Shock Wave on Spasticity and Subgroup-Analysis According to Number of Shocks and Application Site: A Meta-Analysis. Ann. Rehabil. Med. 2019, 43, 163–177. [Google Scholar] [CrossRef]
- Cabanas-Valdés, R.; Calvo-Sanz, J.; Urrùtia, G.; Serra-Llobet, P.; Pérez-Bellmunt, A.; Germán-Romero, A. The Effectiveness of Extracorporeal Shock Wave Therapy to Reduce Lower Limb Spasticity in Stroke Patients: A Systematic Review and Meta-Analysis. Top. Stroke Rehabil. 2020, 27, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Cabanas-Valdés, R.; Serra-Llobet, P.; Rodriguez-Rubio, P.R.; López-de-Celis, C.; Llauró-Fores, M.; Calvo-Sanz, J. The Effectiveness of Extracorporeal Shock Wave Therapy for Improving Upper Limb Spasticity and Functionality in Stroke Patients: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2020, 34, 1141–1156. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Sun, Q.; Wang, H.; Xie, G. Influence of Transcutaneous Electrical Nerve Stimulation on Spasticity, Balance, and Walking Speed in Stroke Patients: A Systematic Review and Meta-Analysis. J. Rehabil. Med. 2018, 50, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Veluswamy, S.K.; Hombali, A.; Mullick, A.; Manikandan, N.; Solomon, J.M. Effect of Transcutaneous Electrical Nerve Stimulation on Spasticity in Adults With Stroke: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2019, 100, 751–768. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Mehrholz, J.; Wagner, K.; Meissner, D.; Grundmann, K.; Zange, C.; Koch, R.; Pohl, M. Reliability of the Modified Tardieu Scale and the Modified Ashworth Scale in Adult Patients with Severe Brain Injury: A Comparison Study. Clin. Rehabil. 2005, 19, 751–759. [Google Scholar] [CrossRef]
- Dymarek, R.; Ptaszkowski, K.; Ptaszkowska, L.; Kowal, M.; Sopel, M.; Taradaj, J.; Rosińczuk, J. Shock Waves as a Treatment Modality for Spasticity Reduction and Recovery Improvement in Post-Stroke Adults—Current Evidence and Qualitative Systematic Review. Clin. Interv. Aging 2020, 15, 9–28. [Google Scholar] [CrossRef]
- Ansari, N.N.; Naghdi, S.; Hasson, S.; Fakhari, Z.; Mashayekhi, M.; Herasi, M. Assessing the Reliability of the Modified Modified Ashworth Scale between Two Physiotherapists in Adult Patients with Hemiplegia. NeuroRehabilitation 2009, 25, 235–240. [Google Scholar] [CrossRef]
- Ansari, N.N.; Naghdi, S.; Moammeri, H.; Jalaie, S. Ashworth Scales Are Unreliable for the Assessment of Muscle Spasticity. Physiother. Theory Pract. 2006, 22, 119–125. [Google Scholar] [CrossRef]
- Burridge, J.H.; Wood, D.E.; Hermens, H.J.; Voerman, G.E.; Johnson, G.R.; van Wijck, F.; Platz, T.; Gregoric, M.; Hitchcock, R.; Pandyan, A.D. Theoretical and Methodological Considerations in the Measurement of Spasticity. Disabil. Rehabil. 2005, 27, 69–80. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G. Assessing Risk of Bias in Included Studies. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 187–241. ISBN 978-0-470-71218-4. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
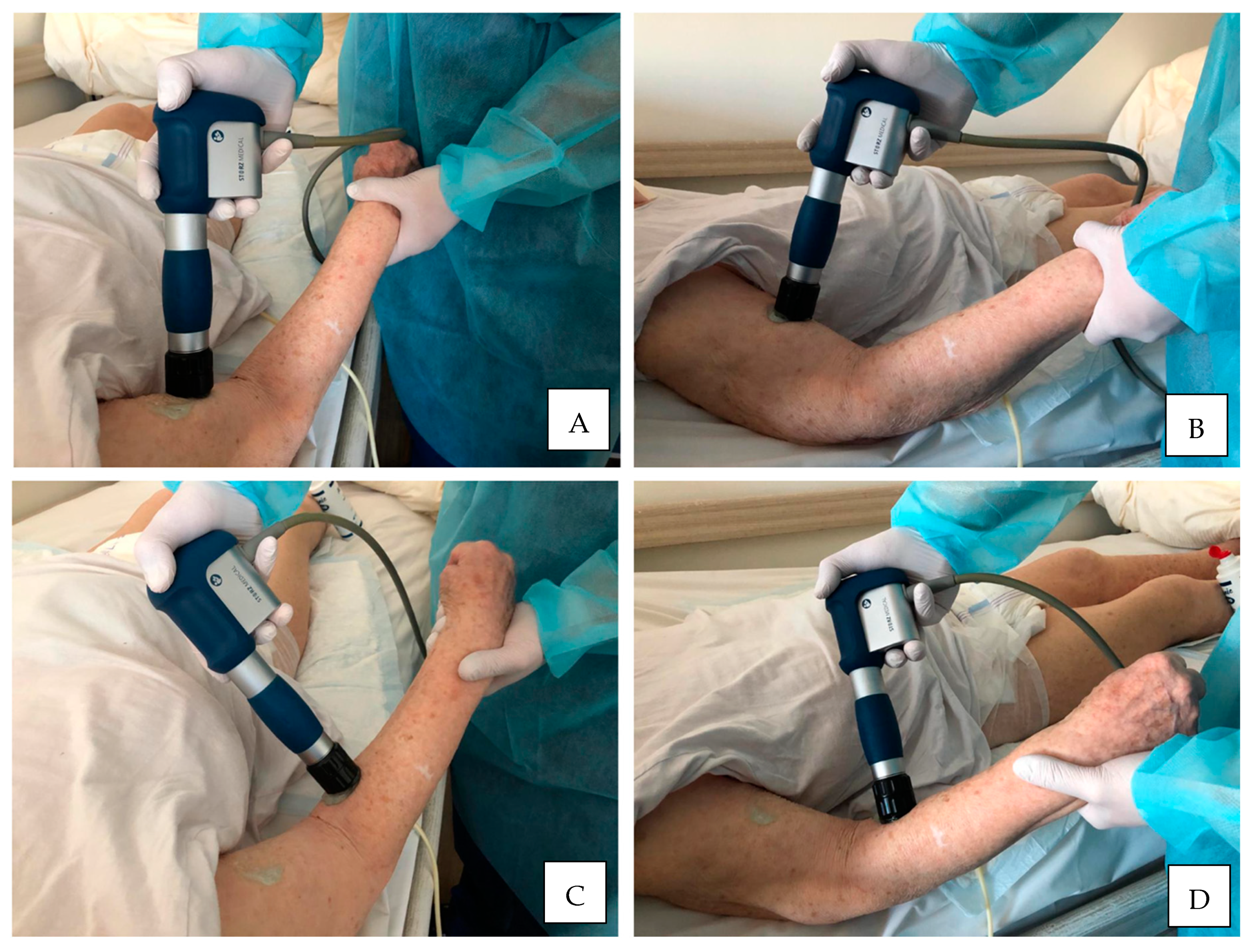
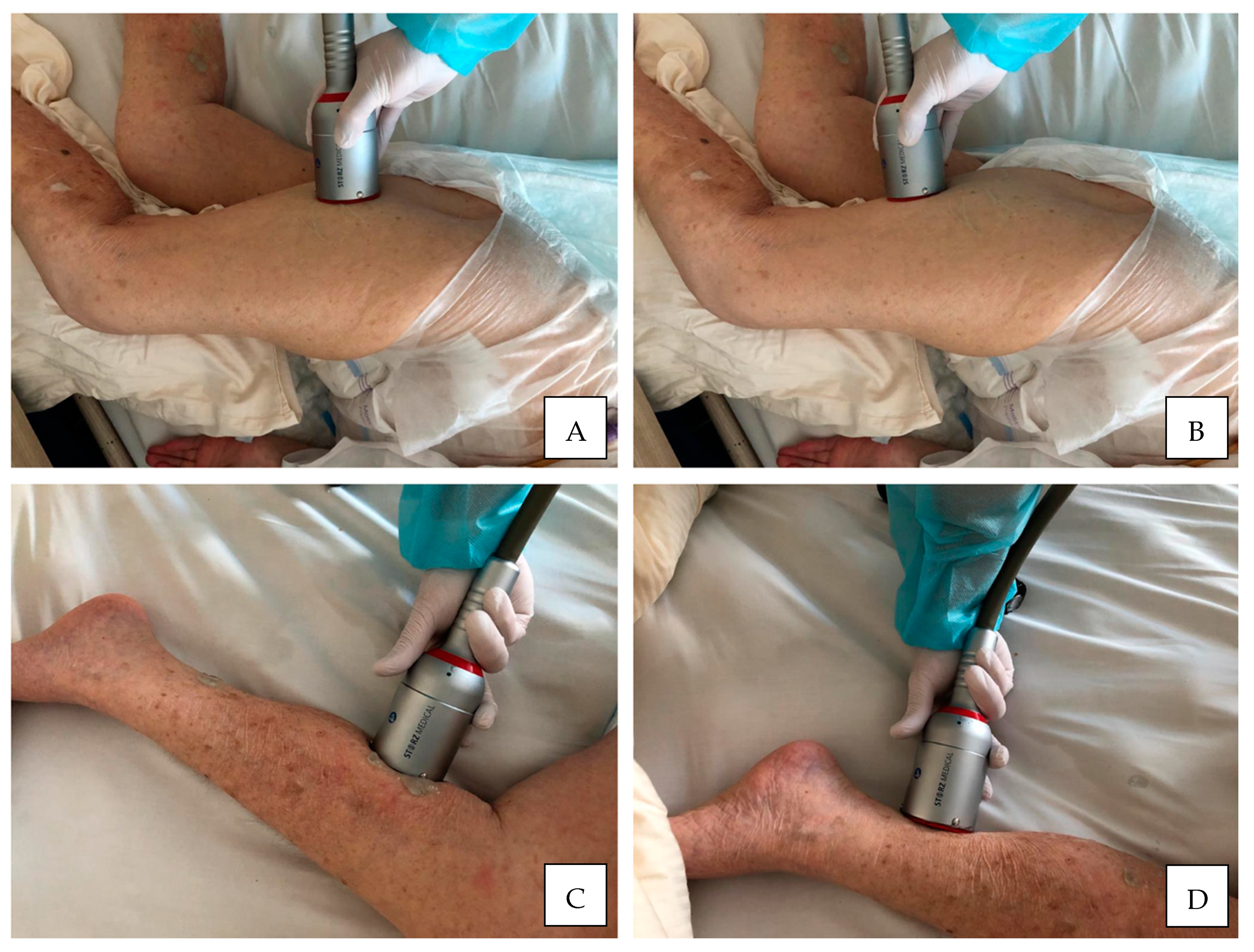
| Authors | Year | Sample (N) | Age (y) | Gender (M/F) | Duration (mo) | Stroke (I/H) | Outcomes | Side Effects |
|---|---|---|---|---|---|---|---|---|
| Manganotti and Amelio [35] | 2005 | 20 | 63.0 | 11/9 | >9 | 15/5 | MAS (+), ROM (+), EMG (−) | not specified |
| Santamato et al. [36] | 2013 | 16 | 64.4 | 9/7 | 10.5 | 8/8 | MAS (+), SFS (+), VAS (+) | none |
| Troncati et al. [37] | 2013 | 12 | 48.0 | 1/11 | not specified | 6/6 | MAS (+), FMA (+), ROM (+) | not specified |
| Daliri et al. [38] | 2015 | 15 | 54.4 | 12/3 | ≥6 | 13/2 | MMAS (+), BRS (−), EMG (+) | not specified |
| Dymarek et al. [39] | 2016 | 30 | 61.4 | 11/19 | 26–77 | 30/0 | MAS (+), sEMG (+), IRT (+) | none |
| Dymarek et al. [40] | 2016 | 20 | 63.1 | 13/7 | 9–120 | 20/0 | MAS (+), sEMG (+), IRT (+) | none |
| Li et al. [41] | 2016 | 20 | 55.4 | 12/8 | 9–144 | 10/10 | MAS (+), FMA (+) | none |
| Kim et al. [44] | 2016 | 17 | 59.9 | 7/10 | 4–60 | 8/9 | VAS (+), CMS (+), ROM (−), FMA (−), MAS (−) | petechiae, bulla |
| Yoon et al. [56] | 2017 | 26 | 58.7 | 26/0 | 2–198 | not specified | MAS (+), MTS (+) | not specified |
| Yoon et al. [56] | 2017 | 28 | 63.1 | 27/1 | 2–198 | not specified | MAS (+), MTS (+) | not specified |
| Wu et al. [42] | 2018 | 21 | 60.0 | 8/13 | >60 | 11/9 | MAS (+), ROM (+), FMA (+) | none |
| Park et al. [43] | 2018 | 15 | 64.2 | 9/6 | 8.1 | 10/5 | FMA (+), STM (+) | not specified |
| Li et al. [44] | 2020 | 27 | 65.0 | 20/7 | >1 | 24/3 | MAS (+), VAS (+), MTS (+), FMA (−) | not specified |
| Authors | Year | Sessions [N] | Pulses [N] | F [Hz] | P [bars] | EFD [mJ/mm2] | Active-SWT | Sham-SWT | Local Anesthesia | Additional Therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| Manganotti and Amelio [35] | 2005 | 1 | 1500/3200 | not specified | 1.5 | 0.03 | fSWT | none | none | not specified |
| Santamato et al. [36] | 2013 | 5 | 2000 | 4 | 1.5 | 0.1 | fSWT | none | none | not specified |
| Troncati et al. [37] | 2013 | 2 | 1600/3200 | not specified | not specified | 0.08 | fSWT | none | not specified | not specified |
| Daliri et al. [38] | 2015 | 1 | 1500 | 4 | 1.5 | 0.03 | rSWT | sound, without energy | not specified | not specified |
| Dymarek et al. [39] | 2016 | 1 | 1500 | 4 | 1.5 | 0.03 | rSWT | plastic cover | none | none |
| Dymarek et al. [40] | 2016 | 1 | 1500 | 4 | 1.5 | 0.03 | rSWT | plastic cover | none | none |
| Li et al. [41] | 2016 | 3 | 2750 | 4 | 3.3 | 0.2 | rSWT | none | none | rehabilitation |
| Kim et al. [44] | 2016 | 3 | 1500 | 12 | 2.0 | 0.1 | rSWT | without trans-mitter | none | not specified |
| Yoon et al. [56] | 2017 | 3 | 1500 | 5 | 1.5 | 0.08 | fSWT | sound, non-contact | not specified | antispastic drugs and physiotherapy |
| Wu et al. [42] | 2018 | 3 | 3000 | 5 | 3.5 | 0.2 | fSWT | none | none | activity training |
| Park et al. [43] | 2018 | 16 | 1500/3200 | not specified | 1.5 | 0.03 | fSWT | sound, without energy | not specified | not specified |
| Li et al. [44] | 2020 | 5 | 6000 | 18 | 1.2–1.4 | 0.06–0.07 | rSWT | none | none | physical therapy |
| Authors | Year | Journal | Protocol | 1. Eligibility Criteria * | 2. Random Allocation | 3. Concealed Allocation | 4. Baseline Comparability | 5. Blind Subjects | 6. Blind Therapists | 7. Blind Assessors | 8. Adequate Follow-Up | 9. Intention-To-Treat Analysis | 10. Between-Group Comparisons | 11. Point and Variability Measures | Total Score in PEDro | Evidence Level in NICE | Level of Recommendation in NICE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Manganotti and Amelio [35] | 2005 | Stroke | CCT | + | − | − | + | − | − | − | + | − | + | + | 4 | 2++ | B |
| Santamato et al. [36] | 2013 | Ultrasound in Medicine and Biology | RCT | + | + | − | + | − | − | + | + | − | + | + | 6 | 1+ | |
| Troncati et al. [37] | 2013 | NeuroRehabilitation | CCS | + | − | − | − | − | − | − | + | − | − | + | 2 | 2− | |
| Daliri et al. [38] | 2015 | NeuroRehabilitation | RCT | + | − | − | + | + | − | − | − | − | + | + | 4 | 1− | |
| Dymarek et al. [39] | 2016 | Ultrasound in Medicine and Biology | RCT | + | + | − | + | + | − | − | + | − | + | + | 6 | 1+ | |
| Dymarek et al. [40] | 2016 | Evidence-Based Complementary and Alternative Medicine | PCT | + | − | − | − | − | − | − | + | − | − | + | 2 | 2− | |
| Li et al. [41] | 2016 | Medicine (Baltimore) | RCT | + | + | − | + | + | − | + | + | + | + | + | 8 | 1++ | |
| Kim et al. [44] | 2016 | Annals of Rehabilitation Medicine | RCT | + | + | − | + | + | − | + | + | − | + | + | 7 | 1+ | |
| Yoon et al. [56] | 2017 | Annals of Rehabilitation Medicine | RCT | + | + | − | + | − | − | − | + | − | + | + | 5 | 1− | |
| Wu et al. [42] | 2018 | Archives of Physical Medicine and Rehabilitation | RCT | + | + | + | + | − | + | + | + | − | + | + | 8 | 1++ | |
| Park et al. [43] | 2018 | Journal of Physical Therapy Science | RCT | + | + | + | − | + | − | − | − | − | + | + | 6 | 1+ | |
| Li et al. [44] | 2018 | Age and Ageing | RCT | + | + | + | + | − | − | + | − | + | + | + | 7 | 1+ |
| Authors | Year | Sample [N] | Age [y] | Gender [M/F] | Duration [mo] | Stroke [I/H] | Outcomes | Side Effects |
|---|---|---|---|---|---|---|---|---|
| Sohn et al. [46] | 2011 | 10 | 44.9 | 6/4 | 23–77 | 2/8 | MAS (+), EMG (−) | not specified |
| Moon et al. [47] | 2013 | 30 | 52.6 | 17/13 | 80.5 | 16/14 | MAS (+), ROM (−), FMA (−), IDT (+) | none |
| Santamato et al. [48] | 2014 | 23 | 51.6 | 15/8 | 24.9 | 12/11 | MAS (+), ROM (+), EMG (−) | pain, weakeness |
| Kim et al. [49] | 2015 | 10 | 64.1 | 5/5 | 17.6 | 5/5 | STM (+), VAS (+), VGA (+) | not specified |
| Radinmehr et al. [50] | 2017 | 12 | 59.0 | 7/5 | 34.0 | 6 /6 | MMAS (+), ROM (+), IKD (+), TUG (+), EMG (−) | none |
| Sawan et al. [51] | 2017 | 20 | 50.6 | 9/4 | 6–18 | not specified | EMG (+), ROM (+), 10-mWT (+) | not specified |
| Taheri et al. [52] | 2017 | 14 | 44.0 | 9/4 | 12–55 | 11/2 | MAS (+), VAS (+), ROM (+), 3-mWT (+), LEFS (+) | not specified |
| Yoon et al. [56] | 2017 | 13 | 61.0 | 13/0 | 12–184 | not specified | MAS (+), MTS (+) | not specified |
| Yoon et al. [56] | 2017 | 13 | 66.9 | 13/0 | 15–87 | not specified | MAS (+), MTS (+) | not specified |
| Wu et al. [53] | 2018 | 31 | 59.9 | 18 /13 | 50–55 | 20/11 | MAS (+), MTS (+), ROM (+), 10-MWT (+), FPMP (+) | none |
| Lee et al. [54] | 2018 | 9 | 50.0 | 7/2 | >3 | 4/5 | MAS (+), ROM (+), FMA (+), USG (+) | not specified |
| Radinmehr et al. [50] | 2019 | 16 | 60.0 | 9/7 | >1 | not specified | MMAS (+), ROM (+), IKD (+), TUG (+), EMG (−) | none |
| Authors | Year | Sessions [N] | Pulses [N] | F [Hz] | P [bars] | EFD [mJ/mm2] | Active-SWT | Sham-SWT | Local Anesthesia | Additional Therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| Sohn et al. [46] | 2011 | 1 | 1500 | not specified | 2.0 | 0.15 | fSWT | none | none | antispastic drugs and physiotherapy |
| Moon et al. [47] | 2013 | 3 | 1500 | 4 | 2.0 | 0.09 | fSWT | none | none | rehabilitation |
| Santamato et al. [48] | 2014 | 1 | 1500 | not specified | 1.5 | 0.03 | fSWT | none | none | none |
| Kim et al. [49] | 2015 | 3 | 1500 | 4 | 1.5 | 0.089 | not specified | none | none | rehabilitation |
| Radinmehr et al. [50] | 2017 | 1 | 2000 | 5 | 3.0 | 0.3 | rSWT | none | not specified | none |
| Sawan et al. [51] | 2017 | 1 | 1500 | not specified | not specified | not specified | fSWT | none | not specified | physical therapy |
| Taheri et al. [52] | 2017 | 3 | 1500 | 4 | 1.5 | 0.1 | fSWT | sound, without energy | not specified | antispastic drugs and physiotherapy |
| Yoon et al. [56] | 2017 | 3 | 1500 | 5 | 1.5 | 0.08 | fSWT | sound, without contact | not specified | antispastic drugs and physiotherapy |
| Wu et al. [53] | 2018 | 3 | 3000 | 5 | 2.0 | 0.1 | fSWT rSWT | none | not specified | not specified |
| Lee et al. [54] | 2018 | 1 | 2000 | 4 | 1.5 | 0.08 | fSWT | sound, without contact | not specified | physical therapy |
| Radinmehr et al. [50] | 2019 | 1 | 2000 | 5 | 1.5 | 0.08 | fSWT | none | not specified | physical therapy |
| Authors | Year | Journal | Protocol | 1. Eligibility Criteria * | 2. Random Allocation | 3. Concealed Allocation | 4. Baseline Comparability | 5. Blind Subjects | 6. Blind Therapists | 7. Blind Assessors | 8. Adequate Follow-Up | 9. Intention-To-Treat Analysis | 10. Between-Group Comparisons | 11. Point and Variability Measures | Total Score in PEDro | Evidence Level in NICE | Level of Recommendation in NICE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sohn et al. [46] | 2011 | Annals of Rehabilitation Medicine | CCT | + | − | − | − | − | − | − | + | − | − | + | 2 | 2− | C |
| Moon et al. [47] | 2013 | Annals of Rehabilitation Medicine | CCT | + | − | − | + | − | − | − | + | − | + | + | 4 | 2++ | |
| Santamato et al. [48] | 2014 | Topics in Stroke Rehabilitation | PCT | + | − | − | − | − | − | − | − | − | − | + | 1 | 2− | |
| Kim et al. [48] | 2015 | Journal of Physical Therapy Science | PCT | + | − | − | − | − | − | − | + | − | − | + | 3 | 2+ | |
| Radinmehr et al. [49] | 2017 | Disability and Rehabilitation | RCT | + | + | − | − | + | + | − | − | − | + | + | 5 | 1− | |
| Sawan et al. [50] | 2017 | NeuroRehabilitation | CCT | + | − | − | − | − | − | − | + | − | + | + | 3 | 2+ | |
| Taheri et al. [51] | 2017 | Archives of Iranian Medicine | RCT | + | + | − | + | − | − | − | + | − | + | + | 5 | 1− | |
| Yoon et al. [56] | 2017 | Annals of Rehabilitation Medicine | RCT | + | + | − | + | − | − | − | + | − | + | + | 5 | 1− | |
| Wu et al. [53] | 2018 | European Journal of Physical and Rehabilitation Medicine | RCT | + | + | + | + | + | + | − | + | − | + | + | 8 | 1++ | |
| Lee et al. [54] | 2018 | Physical Medicine and Rehabilitation | RCT | + | + | + | + | + | + | + | + | − | + | + | 9 | 1++ | |
| Radinmehr et al. [50] | 2019 | Journal of Stroke and Cerebrovascular Diseases | RCT | + | + | + | + | − | − | + | − | + | + | + | 7 | 1+ |
| Practical Implications | |
|---|---|
| Practical Component | Explanation and Justification |
| fSWT devices | EM, EH, or PE devices: therapeutic effects on the level of cells (molecular changes) |
| rSWT devises | PN devices: therapeutic effects on the level of tissues (morphological changes) |
| UL muscles | Biceps brachii, flexor carpi radialis, flexor carpi ulnaris, palmar interosseous, flexor digitorum superficialis |
| LL muscles | Triceps surae, biceps femoris |
| P [bar] | Typically, 1.5–2.0 but sometimes higher values even up to 3.0–3.5 |
| EFD [mJ/mm2] | Typically, 0.03–0.1 but sometimes higher values even up to 0.2–0.3 |
| TED [J/mm2] * | Typically, 0.05–0.45 but sometimes higher values even up to 0.9–1.2 |
| F [Hz] | Normally, range of 4–5 but some reports indicate even up to 12–18 |
| Pulses [N] | Normally, 1500–2000 per muscle belly, occasionally to 3000, but also even up to 6000 |
| Sessions [N] | Usually, 1–2 per week and 3–5 totally during treatment period, sometimes even up to 16 |
| ESWT transducer | Applicator head placed perpendiculary and directly over the muscle belly or myotendinous junction |
| USG gel application | Ultrasonic gel as a contact medium applied on the skin within the treatment area to reduce tissue resistance |
| LA administration | Usually not recommended but it depends on individual pain threshold of each patients |
| AE risk management | Should be carefully observed and reported such as local episodes: pain, bruises, petechiae, muscle weakness |
| Future Directions | |
|---|---|
| Research Questions | Methodological Advices and Research Directions |
| 1. How to improve the research methodology? |
|
| 2. Which patients should be enrolled in the study? |
|
| 3. Which study outcomes should be considered? |
|
| 4. How should the shock wave treatment be performed? |
|
| 5. How to perform the sham procedure in placebo group? |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opara, J.; Taradaj, J.; Walewicz, K.; Rosińczuk, J.; Dymarek, R. The Current State of Knowledge on the Clinical and Methodological Aspects of Extracorporeal Shock Waves Therapy in the Management of Post-Stroke Spasticity—Overview of 20 Years of Experiences. J. Clin. Med. 2021, 10, 261. https://doi.org/10.3390/jcm10020261
Opara J, Taradaj J, Walewicz K, Rosińczuk J, Dymarek R. The Current State of Knowledge on the Clinical and Methodological Aspects of Extracorporeal Shock Waves Therapy in the Management of Post-Stroke Spasticity—Overview of 20 Years of Experiences. Journal of Clinical Medicine. 2021; 10(2):261. https://doi.org/10.3390/jcm10020261
Chicago/Turabian StyleOpara, Józef, Jakub Taradaj, Karolina Walewicz, Joanna Rosińczuk, and Robert Dymarek. 2021. "The Current State of Knowledge on the Clinical and Methodological Aspects of Extracorporeal Shock Waves Therapy in the Management of Post-Stroke Spasticity—Overview of 20 Years of Experiences" Journal of Clinical Medicine 10, no. 2: 261. https://doi.org/10.3390/jcm10020261
APA StyleOpara, J., Taradaj, J., Walewicz, K., Rosińczuk, J., & Dymarek, R. (2021). The Current State of Knowledge on the Clinical and Methodological Aspects of Extracorporeal Shock Waves Therapy in the Management of Post-Stroke Spasticity—Overview of 20 Years of Experiences. Journal of Clinical Medicine, 10(2), 261. https://doi.org/10.3390/jcm10020261







