Evaluation of Transthoracic Echocardiography in the Assessment of Atherosclerosis of the Left Main Coronary Artery: Comparison with Optical Frequency Domain Imaging (a Pilot Study)
Abstract
1. Introduction
2. Experimental Section
2.1. Patients
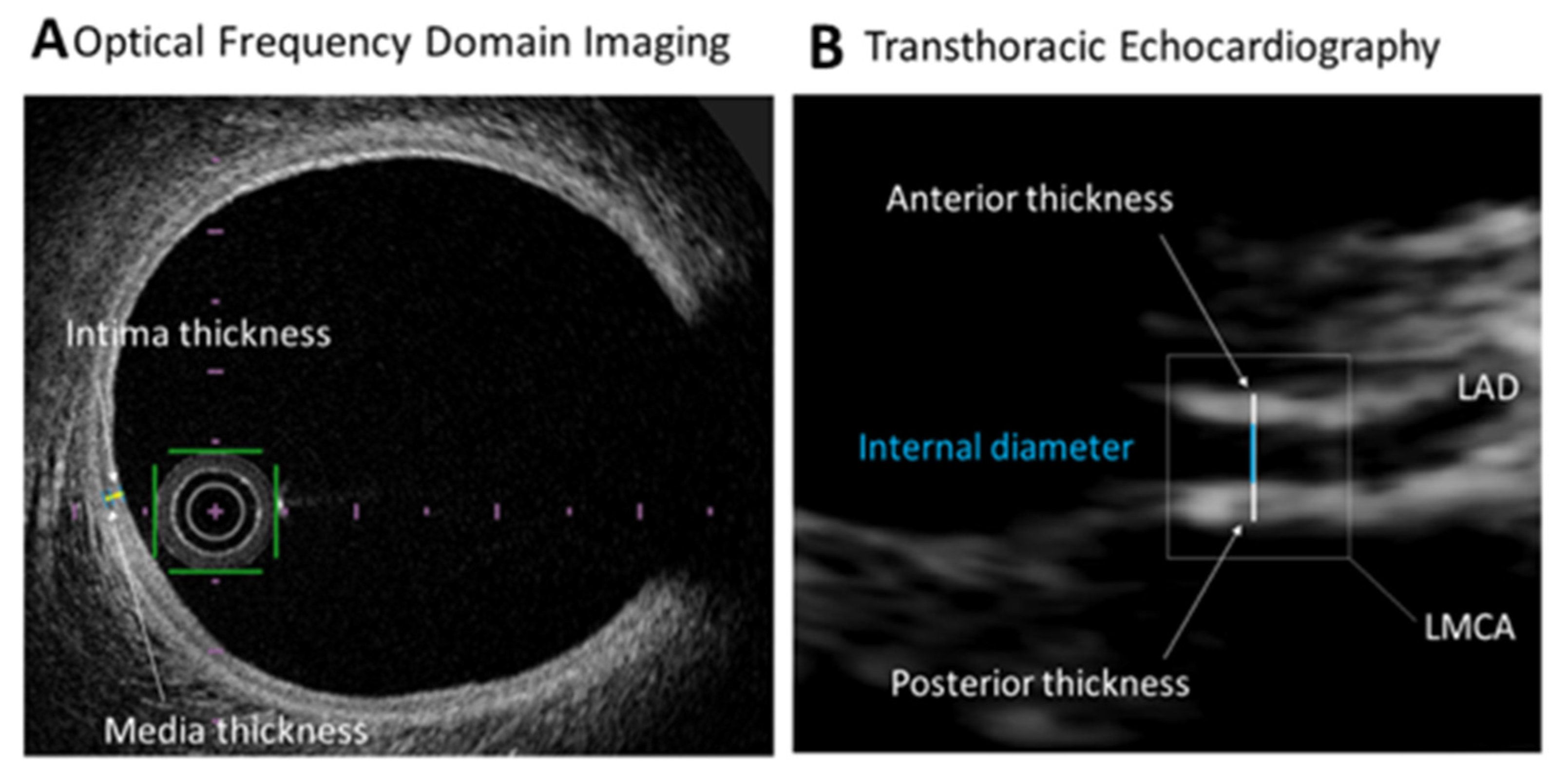
2.2. Optical Frequency Domain Imaging Studies
2.3. Echocardiographic Studies
2.4. Statistical Analysis
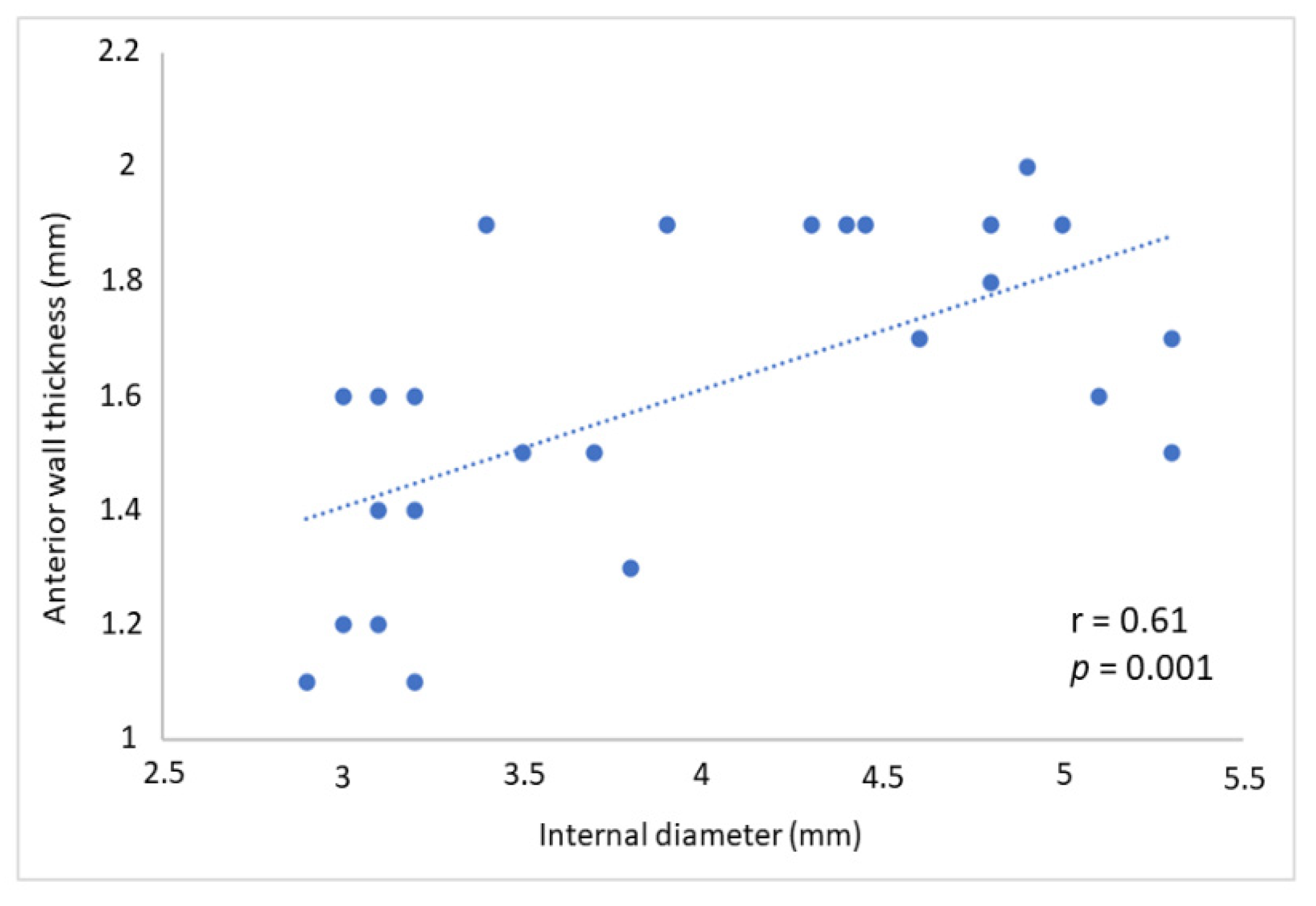
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kubo, T.; Shinke, T.; Okamura, T.; Hibi, K.; Nakazawa, G.; Morino, Y.; Shite, J.; Fusazaki, T.; Otake, H.; Kozuma, K.; et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): One-year angiographic and clinical results. Eur. Heart J. 2017, 38, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.M.; Matta, J.R.; Elgarf, R.; Hamimi, A.; Muniyappa, R.; Ishaq, H.; Hadigan, C.; McConnell, M.V.; Gharib, A.M.; Abd-Elmoniem, K.Z. Sexual Dimorphism of Coronary Artery Disease in a Low- and Intermediate-Risk Asymptomatic Population: Association with Coronary Vessel Wall Thickness at MRI in Women. Radiol. Cardiothorac. Imaging 2019, 1, e180007. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.D.; Newby, D.E. Non-invasive imaging of the coronary arteries. Eur. Heart J. 2019, 40, 2444–2454. [Google Scholar] [CrossRef]
- Rozanski, A.; Muhlestein, J.B.; Berman, D.S. Primary Prevention of CVD: The Role of Imaging Trials. JACC Cardiovasc. Imaging 2017, 10, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Labombarda, F.; Castelnuovo, S.; Perry, R. The use of echocardiography for the non-invasive evaluation of coronary artery disease. Ann. Med. 2017, 49, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Castelnuovo, S.; Macchi, C.; Gandini, S.; Mombelli, G.; Ferri, N.; Labombarda, F.; Sirtori, C.R. Left main coronary wall thickness correlates with the carotid intima media thickness and may provide a new marker of cardiovascular risk. Eur. J. Prev. Cardiol. 2019, 26, 1001–1004. [Google Scholar] [CrossRef]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef]
- Agrawal, V.; Sihag, B.K. Extensive xanthomas with severe coronary artery disease in a young patient with familial hypercholesterolemia. Eur. Heart J. 2015, 36, 828. [Google Scholar] [CrossRef]
- Pang, J.; Abraham, A.; Vargas-Garcia, C.; Bates, T.R.; Chan, D.C.; Hooper, A.J.; Bell, D.A.; Burnett, J.R.; Schultz, C.J.; Watts, G.F. An age-matched computed tomography angiographic study of coronary atherosclerotic plaques in patients with familial hypercholesterolaemia. Atherosclerosis 2020, 298, 52–57. [Google Scholar] [CrossRef]
- Lahti, S.J.; Feldman, D.I.; Dardari, Z.; Mirbolouk, M.; Orimoloye, O.A.; Osei, A.D.; Graham, G.; Rumberger, J.; Shaw, L.; Budoff, M.J.; et al. The association between left main coronary artery calcium and cardiovascular-specific and total mortality: The Coronary Artery Calcium Consortium. Atherosclerosis 2019, 286, 172–178. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; Muramatsu, T.; Nakatani, S.; Lee, I.S.; Holm, N.R.; Thuesen, L.; van Geuns, R.J.; van der Ent, M.; Borovicanin, V.; Paunovic, D.; et al. Serial optical frequency domain imaging in STEMI patients: The follow-up report of TROFI study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Shaw, L.J.; Raggi, P.; Morris, D.; Vaccarino, V.; Liu, S.T.; Weinstein, S.R.; Mosler, T.P.; Tseng, P.H.; Flores, F.R.; et al. Prognostic value of number and site of calcified coronary lesions compared with the total score. JACC Cardiovasc. Imaging 2008, 1, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Osei, A.D.; Mirbolouk, M.; Berman, D.; Budoff, M.J.; Miedema, M.D.; Rozanski, A.; Rumberger, J.A.; Shaw, L.; Al Rifai, M.; Dzaye, O.; et al. Prognostic value of coronary artery calcium score, area, and density among individuals on statin therapy vs. non-users: The coronary artery calcium consortium. Atherosclerosis 2020, 316, 79–83. [Google Scholar] [CrossRef]
- Labombarda, F.; Coutance, G.; Pellissier, A.; Mery-Alexandre, C.; Roule, V.; Maragnes, P.; Milliez, P.; Saloux, E. Major congenital coronary artery anomalies in a paediatric and adult population: A prospective echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 761–768. [Google Scholar] [CrossRef]
- Gerling, S.; Loose, O.; Zant, R.; Michel, H.; Melter, M.; Gundisch, C.; Krutsch, V.; Krutsch, W. Echocardiographic diagnosis of congenital coronary artery abnormalities in a continuous series of adolescent football players. Eur. J. Prev. Cardiol. 2019, 26, 988–994. [Google Scholar] [CrossRef]
- Meah, M.N.; Dweck, M.R.; Newby, D.E. Cardiovascular imaging to guide primary prevention. Heart 2020, 106, 1267–1275. [Google Scholar] [CrossRef]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100,667 Patients. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef]
- Ali, Z.A.; Maehara, A.; Genereux, P.; Shlofmitz, R.A.; Fabbiocchi, F.; Nazif, T.M.; Guagliumi, G.; Meraj, P.M.; Alfonso, F.; Samady, H.; et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): A randomised controlled trial. Lancet 2016, 388, 2618–2628. [Google Scholar] [CrossRef]
- Kang, D.Y.; Ahn, J.M.; Park, H.; Lee, P.H.; Kang, S.J.; Lee, S.W.; Kim, Y.H.; Park, S.W.; Kim, S.W.; Hur, S.H.; et al. Comparison of optical coherence tomography-guided versus intravascular ultrasound-guided percutaneous coronary intervention: Rationale and design of a randomized, controlled OCTIVUS trial. Am. Heart J. 2020, 228, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Mszar, R.; Nasir, K.; Santos, R.D. Coronary Artery Calcification in Familial Hypercholesterolemia: An Opportunity for Risk Assessment and Shared Decision Making With the Power of Zero? Circulation 2020, 142, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Mszar, R.; Grandhi, G.R.; Valero-Elizondo, J.; Virani, S.S.; Blankstein, R.; Blaha, M.; Mata, P.; Miname, M.H.; Al Rasadi, K.; Krumholz, H.M.; et al. Absence of Coronary Artery Calcification in Middle-Aged Familial Hypercholesterolemia Patients Without Atherosclerotic Cardiovascular Disease. JACC Cardiovasc. Imaging 2020, 13, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Wunnemann, F.; Sin Lo, K.; Langford-Avelar, A.; Busseuil, D.; Dube, M.P.; Tardif, J.C.; Lettre, G. Validation of Genome-Wide Polygenic Risk Scores for Coronary Artery Disease in French Canadians. Circ. Genom. Precis. Med. 2019, 12, e002481. [Google Scholar] [CrossRef]
- Ajufo, E.; Ayers, C.R.; Vigen, R.; Joshi, P.H.; Rohatgi, A.; de Lemos, J.A.; Khera, A. Value of Coronary Artery Calcium Scanning in Association With the Net Benefit of Aspirin in Primary Prevention of Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2020. [Google Scholar] [CrossRef]
- Pugliese, L.; Spiritigliozzi, L.; Di Tosto, F.; Ricci, F.; Cavallo, A.U.; Di Donna, C.; De Stasio, V.; Presicce, M.; Benelli, L.; D’Errico, F.; et al. Association of plaque calcification pattern and attenuation with instability features and coronary stenosis and calcification grade. Atherosclerosis 2020, 311, 150–157. [Google Scholar] [CrossRef]

| Variables | Population (n = 25) |
|---|---|
| Baseline characteristics | |
| Age (years) | 58 ± 15 |
| Gender (Men %) | 19 (76%) |
| Body mass index (kg/m2) | 25.6 ± 4.2 |
| Body mass index > 30 kg/m2 | 2 (8%) |
| Systemic hypertension | 7 (28%) |
| Hyperlipidemia | 10 (40%) |
| Current smokers | 13 (52%) |
| Diabetes mellitus | 1 (4%) |
| Hyperlipidemia | 10 (39%) |
| Total cholesterol (mmol/L) | 4.9 ± 1.8 |
| Low-density lipoprotein (mmol/L) | 3.2 ± 1.7 |
| High-density lipoprotein (mmol/L) | 1.4 ± 1.1 |
| Triglycerides (mmol/L) | 2.0 ± 1.4 |
| Patients on statin treatment | 19 (76%) |
| Myocardial Infarction | 0 (0%) |
| Coronary artery bypass graft | 0 (0%) |
| Percutaneous coronary intervention | 1 (4%) |
| Estimated glomerular filtration rate < 60 mL/min | 1 (4%) |
| Left ventricular ejection fraction (%) | 54 ± 9 |
| Clinical presentation | |
| Stable angina | 1 (4%) |
| Non ST-elevation myocardial infarction | 4 (16%) |
| ST-elevation myocardial infarction | 20 (80%) |
| Angiographic characteristics | |
| Guiding catheter | |
| Extra Back-Up | 22 (88%) |
| Judkins Left guiding catheter | 3 (12%) |
| Single-vessel disease | 17 (68%) |
| Two-vessel disease | 2 (8%) |
| Three-vessel disease | 6 (24%) |
| LMCA length (mm) | 12 ± 4 |
| LMCA reference diameter (mm) | 4 ± 0.6 |
| Angiographic signs of LMCA atherosclerosis | |
| No lesions | 15 (60%) |
| Lesions ≥ 30% | 10 (40%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labombarda, F.; Roule, V.; Rebouh, I.; Ruscica, M.; Watts, G.F.; Sirtori, C.R. Evaluation of Transthoracic Echocardiography in the Assessment of Atherosclerosis of the Left Main Coronary Artery: Comparison with Optical Frequency Domain Imaging (a Pilot Study). J. Clin. Med. 2021, 10, 256. https://doi.org/10.3390/jcm10020256
Labombarda F, Roule V, Rebouh I, Ruscica M, Watts GF, Sirtori CR. Evaluation of Transthoracic Echocardiography in the Assessment of Atherosclerosis of the Left Main Coronary Artery: Comparison with Optical Frequency Domain Imaging (a Pilot Study). Journal of Clinical Medicine. 2021; 10(2):256. https://doi.org/10.3390/jcm10020256
Chicago/Turabian StyleLabombarda, Fabien, Vincent Roule, Idir Rebouh, Massimiliano Ruscica, Gerald F. Watts, and Cesare R. Sirtori. 2021. "Evaluation of Transthoracic Echocardiography in the Assessment of Atherosclerosis of the Left Main Coronary Artery: Comparison with Optical Frequency Domain Imaging (a Pilot Study)" Journal of Clinical Medicine 10, no. 2: 256. https://doi.org/10.3390/jcm10020256
APA StyleLabombarda, F., Roule, V., Rebouh, I., Ruscica, M., Watts, G. F., & Sirtori, C. R. (2021). Evaluation of Transthoracic Echocardiography in the Assessment of Atherosclerosis of the Left Main Coronary Artery: Comparison with Optical Frequency Domain Imaging (a Pilot Study). Journal of Clinical Medicine, 10(2), 256. https://doi.org/10.3390/jcm10020256







