Interobserver Agreement and Plane-Dependent Intraobserver Variability of Shear Wave Sonoelastography in the Differential Diagnosis of Ectopic Thymus Tissue
Abstract
1. Introduction
2. Experimental Section
2.1. Patient Selection and Diagnostic Procedures
2.2. Statistical Analysis
3. Results
3.1. Interobserver Variability
3.2. Intraobserver Variability Related to the Imaging Plane
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| D | difference |
| EET | extrathyroidal ectopic thymic tissue; |
| ET | ectopic thymus; |
| FNAB | fine-needle aspiration biopsy |
| IET | intrathyroidal ectopic thymus; |
| PD | power Doppler; |
| PTC | papillary thyroid carcinoma; |
| R1 | researcher 1 |
| R2 | researcher 2 |
| SD | standard deviation |
| SE | strain elastography |
| SWE | shear wave elastography |
| SWS | shear wave stiffness |
| SWR | shear wave ratio |
| US | ultrasound |
References
- Dobruch-Sobczak, K.; Adamczewski, Z.; Szczepanek-Parulska, E.; Migda, B.; Woliński, K.; Krauze, A.; Prostko, P.; Ruchała, M.; Lewiński, A.; Jakubowski, W.; et al. Histopathological Verification of the Diagnostic Performance of the EU-TIRADS Classification of Thyroid Nodules-Results of a Multicenter Study Performed in a Previously Iodine-Deficient Region. J. Clin. Med. 2019, 8, 1781. [Google Scholar] [CrossRef] [PubMed]
- Adamczewski, Z.; Lewinski, A. Proposed algorithm for management of patients with thyroid nodules/focal lesions, based on ultrasound (US) and fine-needle aspiration biopsy (FNAB); Our own experience. Thyroid Res. 2013, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Kim, S.H.; Jung, S.L.; Kang, B.J.; Kim, J.Y.; Choi, J.J.; Sung, M.S.; Yim, H.W.; Jeong, S.H. Observer variability in the sonographic evaluation of thyroid nodules. J. Clin. Ultrasound 2010, 38, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, Y.X.; Liu, Y.C.; Zhan, J.; Diao, X.H.; Chen, Y.; Zhan, W.W. The values of shear wave elastography in avoiding repeat fine-needle aspiration for thyroid nodules with nondiagnostic and undetermined cytology. Clin. Endocrinol. 2019, 91, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.E.; Dhyani, M.; Anvari, A.; Prescott, J.; Halpern, E.F.; Faquin, W.C.; Stephen, A. Shear-Wave Elastography for the Preoperative Risk Stratification of Follicular-patterned Lesions of the Thyroid: Diagnostic Accuracy and Optimal Measurement Plane. Radiology 2015, 277, 565–573. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.J.; Kim, E.K.; Kim, M.J.; Son, E.J.; Kwak, J.Y. Interobserver agreement in assessing the sonographic and elastographic features of malignant thyroid nodules. AJR Am. J. Roentgenol. 2009, 193, 416–423. [Google Scholar] [CrossRef]
- Dobruch-Sobczak, K.; Migda, B.; Krauze, A.; Mlosek, K.; Słapa, R.Z.; Wareluk, P.; Bakuła-Zalewska, E.; Adamczewski, Z.; Lewiński, A.; Jakubowski, W.; et al. Prospective analysis of inter-observer and intra-observer variability in multi ultrasound descriptor assessment of thyroid nodules. J. Ultrason. 2019, 19, 198–206. [Google Scholar] [CrossRef]
- Bojunga, J.; Herrmann, E.; Meyer, G.; Weber, S.; Zeuzem, S.; Friedrich-Rust, M. Real-time elastography for the differentiation of benign and malignant thyroid nodules: A meta-analysis. Thyroid 2010, 20, 1145–1150. [Google Scholar] [CrossRef]
- Havre, R.F.; Waage, J.R.; Gilja, O.H.; Odegaard, S.; Nesje, L.B. Real-time elastography: Strain ratio measurements are influenced by the position of the reference area. Ultraschall Med. 2012, 33, 559–568. [Google Scholar] [CrossRef]
- Szczepanek-Parulska, E.; Woliński, K.; Stangierski, A.; Gurgul, E.; Ruchała, M. Biochemical and ultrasonographic parameters influencing thyroid nodules elasticity. Endocrine 2014, 47, 519–527. [Google Scholar] [CrossRef]
- Szczepanek-Parulska, E.; Woliński, K.; Stangierski, A.; Gurgul, E.; Biczysko, M.; Majewski, P.; Rewaj-Łosyk, M.; Ruchała, M. Comparison of diagnostic value of conventional ultrasonography and shear wave elastography in the prediction of thyroid lesions malignancy. PLoS ONE 2013, 8, e81532. [Google Scholar] [CrossRef] [PubMed]
- Petzold, G.; Hofer, J.; Ellenrieder, V.; Neesse, A.; Kunsch, S. Liver Stiffness Measured by 2-Dimensional Shear Wave Elastography: Prospective Evaluation of Healthy Volunteers and Patients with Liver Cirrhosis. J. Ultrasound Med. 2019, 38, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Arda, K.; Ciledag, N.; Aktas, E.; Aribas, B.K.; Köse, K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am. J. Roentgenol. 2011, 197, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015, 25, 716–759. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Adamczewski, Z.; Stawerska, R.; Krawczyk, T.; Tomaszewska, M.; Lewiński, A. Sonographic and Elastographic Features of Extra- and Intrathyroidal Ectopic Thymus Mimicking Malignancy: Differential Diagnosis in Children. Front. Endocrinol. 2019, 10, 10:223. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Suzuki, S.; Ohira, T.; Shimura, H.; Midorikawa, S.; Ohtsuru, A.; Sakai, A.; Abe, M.; Yamashita, S.; Suzuki, S. Thyroid examination unit of the radiation medical center for the Fukushima Health Management Survey. Prevalence of ectopic intrathyroidal thymus in Japan: The Fukushima health management survey. Thyroid 2015, 25, 534–537. [Google Scholar] [CrossRef]
- Escobar, F.A.; Pantanowitz, L.; Picarsic, J.L.; Craig, F.E.; Simons, J.P.; Viswanathan, P.A.; Yilmaz, S.; Monaco, S.E. Cytomorphology and sonographic features of ectopic thymic tissue diagnosed in paediatric FNA biopsies. Cytopathology 2018, 29, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Adamczewski, Z.; Stawerska, R.; Stasiak, B.; Lewiński, A. Application of shear wave sonoelastography in differential diagnosis of extra- and intrathyroidal ectopic thymic tissue. J. Clin. Med. 2020, 9, 3816. [Google Scholar] [CrossRef]
- Grazhdani, H.; Cantisani, V.; Lodise, P.; Di Rocco, G.; Proietto, M.C.; Fioravanti, E.; Rubini, A.; Redler, A. Prospective evaluation of acoustic radiation force impulse technology in the differentiation of thyroid nodules: Accuracy and interobserver variability assessment. J. Ultrasound 2014, 17, 13–20. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Xu, H.X.; He, Y.; Liu, C.; Guo, L.H.; Liu, L.N.; Xu, J.M. Virtual Touch Tissue Quantification of Acoustic Radiation Force Impulse: A New Ultrasound Elastic Imaging in the Diagnosis of Thyroid Nodules. PLoS ONE 2012, 7, e49094. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.J.; Luo, S.; Kim, M.H.; Ko, S.H.; Kim, Y. Interobserver agreement and intraobserver reproducibility in thyroid ultrasound elastography. AJR Am. J. Roentgenol. 2012, 198, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Veyrieres, J.B.; Albarel, F.; Lombard, J.V.; Berbis, J.; Sebag, F.; Oliver, C.; Petit, P. A threshold value in Shear Wave elastography to rule out malignant thyroid nodules: A reality? Eur. J. Radiol. 2012, 81, 3965–3972. [Google Scholar] [CrossRef] [PubMed]
- Swan, K.Z.; Nielsen, V.E.; Bibby, B.M.; Bonnema, S.J. Is the reproducibility of shear wave elastography of thyroid nodules high enough for clinical use? A methodological study. Clin. Endocrinol. 2017, 86, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, R.; Kikuchi, K.; Koyama, A.; Kershaw, J.; Omatsu, T.; Tachibana, Y.; Suga, M.; Obata, T. Intra- and inter-operator reproducibility of US point shear-wave elastography in various organs: Evaluation in phantoms and healthy volunteers. Eur. Radiol. 2019, 29, 5999–6008. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Lee, J.H.; Shin, J.H.; Kim, S.Y.; Shin, H.J.; Park, J.S.; Choi, Y.J.; Baek, J.H. Shear wave elastography using ultrasound: Effects of anisotropy and stretch stress on a tissue phantom and in vivo reactive lymph nodes in the neck. Ultrasonography 2017, 36, 25–32. [Google Scholar] [CrossRef]
- Gennisson, J.L.; Deffieux, T.; Macé, E.; Montaldo, G.; Fink, M.; Tanter, M. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med. Biol. 2010, 36, 789–801. [Google Scholar] [CrossRef]
- Otsuka, S.; Shan, X.; Kawakami, Y. Dependence of muscle and deep fascia stiffness on the contraction levels of the quadriceps: An in vivo supersonic shear-imaging study. J. Electromyogr. Kinesiol. 2019, 45, 33–40. [Google Scholar] [CrossRef]
- Aubry, S.; Nueffer, J.P.; Carrié, M. Evaluation of the Effect of an Anisotropic Medium on Shear Wave Velocities of Intra-Muscular Gelatinous Inclusions. Ultrasound Med. Biol. 2017, 43, 301–308. [Google Scholar] [CrossRef]
- Gennisson, J.L.; Grenier, N.; Combe, C.; Tanter, M. Supersonic shear wave elastography of in vivo pig kidney: Influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med. Biol. 2012, 38, 1559–1567. [Google Scholar] [CrossRef]
- Leong, S.S.; Wong, J.H.D.; Md Shah, M.N.; Vijayananthan, A.; Jalalonmuhali, M.; Mohd Sharif, N.H.; Abas, N.K.; Ng, K.H. Stiffness and Anisotropy Effect on Shear Wave Elastography: A Phantom and in Vivo Renal Study. Ultrasound Med. Biol. 2020, 46, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Eby, S.F.; Song, P.; Chen, S.; Chen, Q.; Greenleaf, J.F.; An, K.N. Validation of shear wave elastography in skeletal muscle. J. Biomech. 2013, 46, 2381–2837. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, M.; Murata, Y.; Ishida, R.; Ohashi, I.; Hirata, Y.; Shibuya, H. MR imaging of the thyroid: Correlation between apparent diffusion coefficient and thyroid gland scintigraphy. J. Magn. Reson. Imaging 2003, 17, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Kara, T.; Ateş, F.; Durmaz, M.S.; Akyürek, N.; Durmaz, F.G.; Özbakır, B.; Öztürk, M. Assessment of thyroid gland elasticity with shear-wave elastography in Hashimoto’s thyroiditis patients. J. Ultrasound 2020, 23, 543–551. [Google Scholar] [CrossRef] [PubMed]
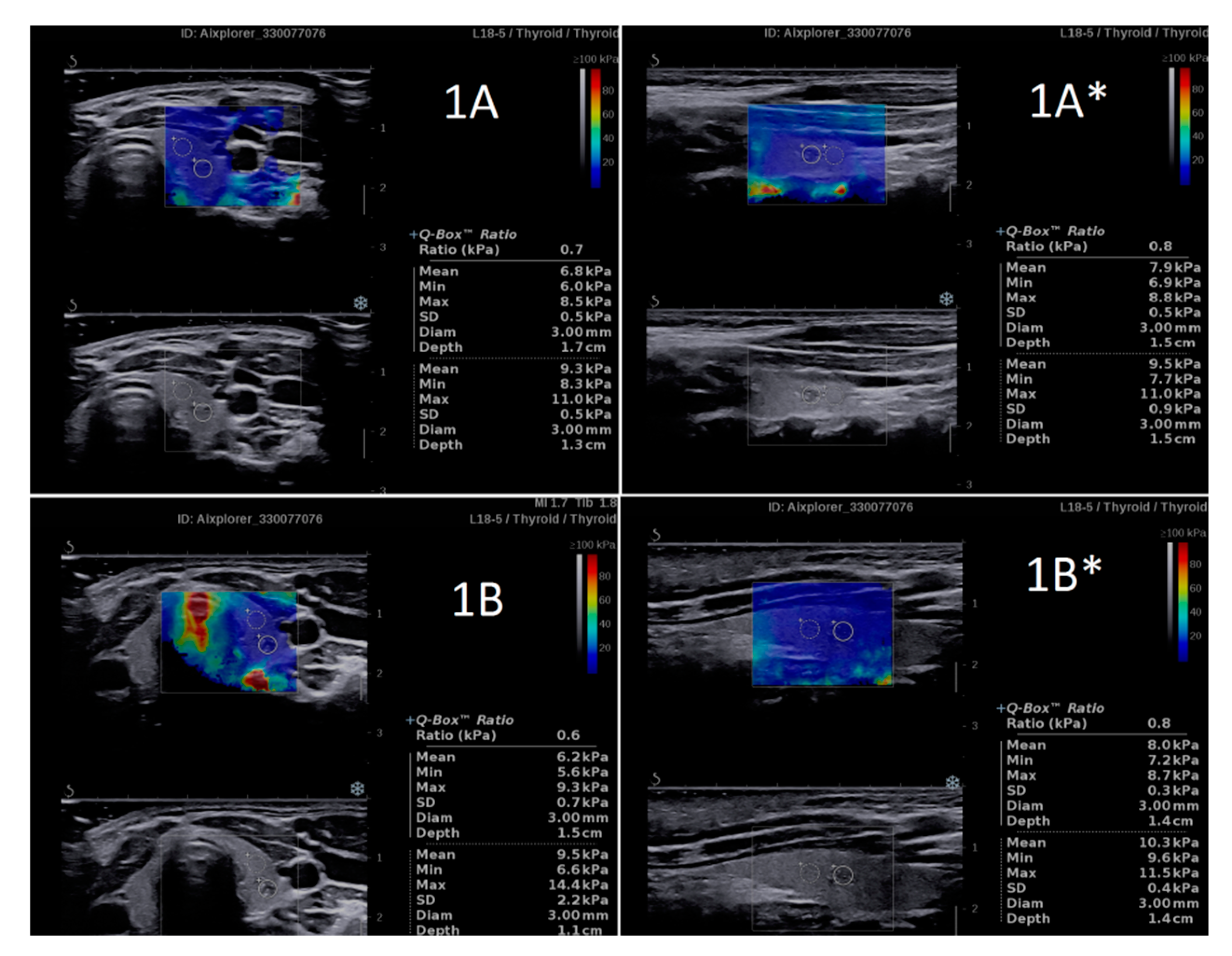
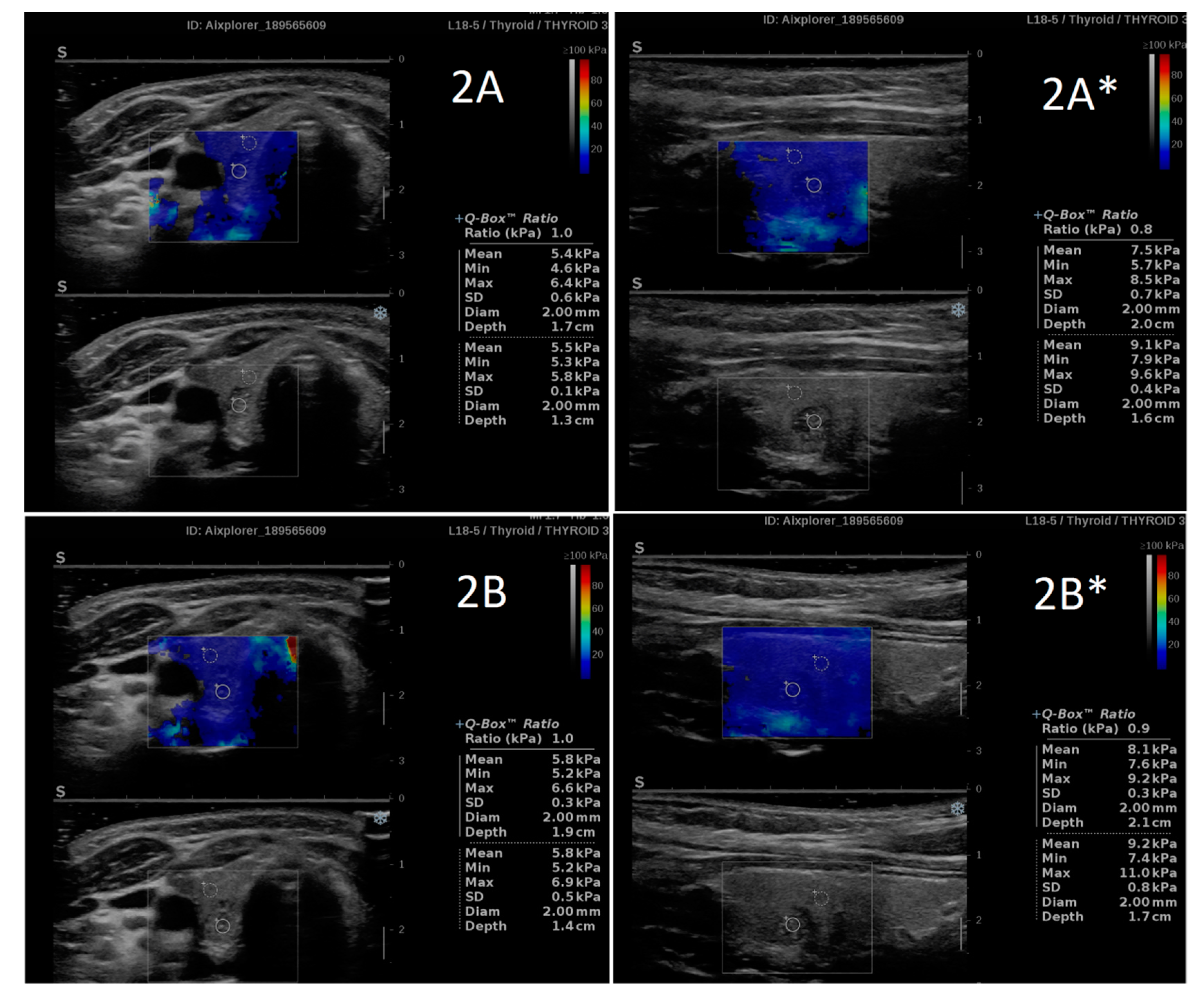
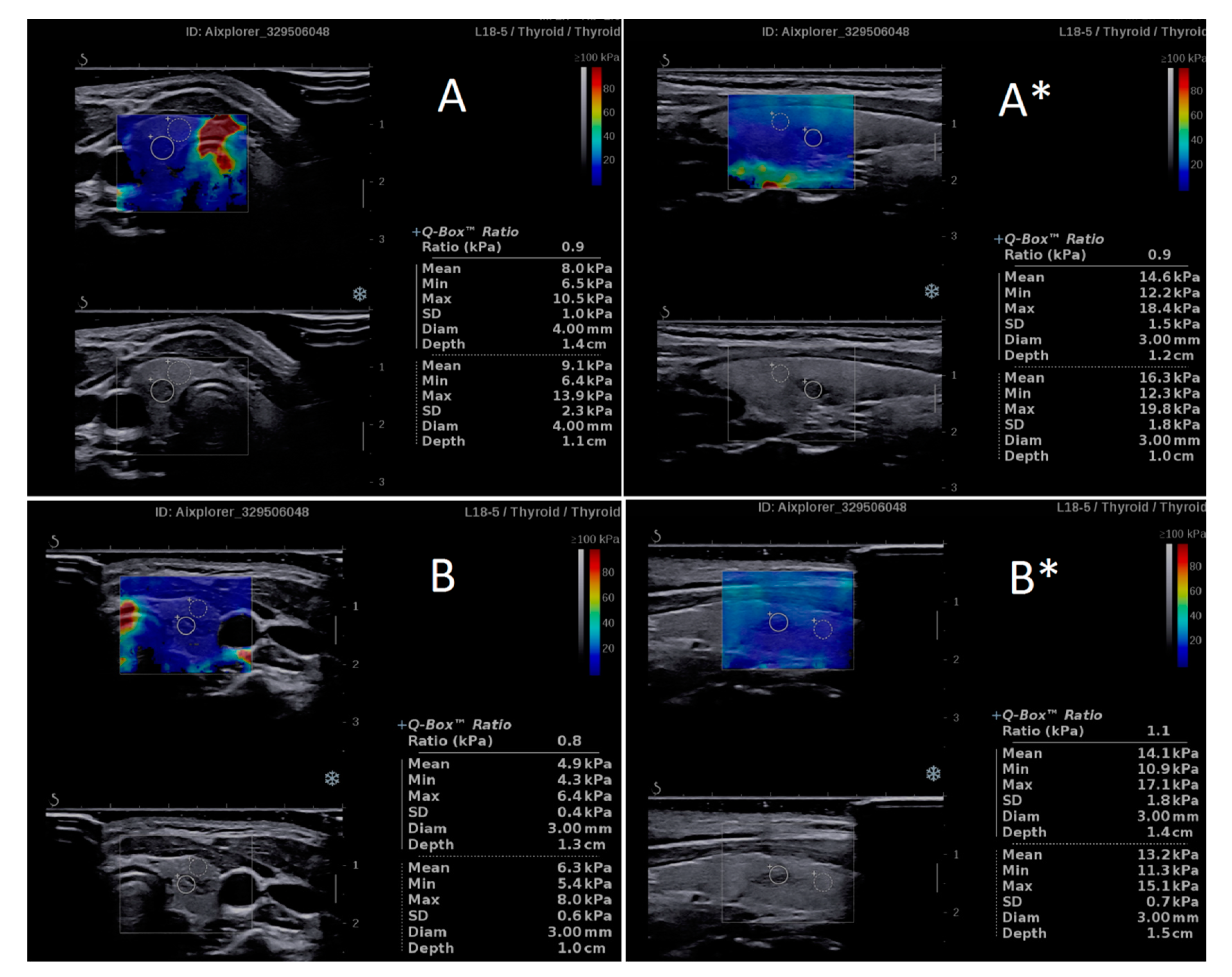
| n | Researcher 1 Mean ± SD | Researcher 2 Mean ± SD | p | D Mean (95%CI) | D% Mean (95%CI) | |
|---|---|---|---|---|---|---|
| Transverse Plane | ||||||
| SWS of ET | 50 | 7.23 ± 1.78 | 7.20 ± 1.54 | 0.84 | −0.04 (−0.37; 0.30) | 10.78 (7.24; 14.32) |
| SWS of EET | 17 | 6.77 ± 1.39 | 7.09 ± 0.99 | 0.18 | 0.32 (−0.26; 0.90) | 11.26 (5.32; 17.21) |
| SWS of IET | 33 | 7.47 ± 1.93 | 7.25 ± 1.77 | 0.45 | −0.22 (−0.63; 0.20) | 10.53 (5.89; 15.17) |
| Thyroid SWS | 33 | 8.66 ± 2.42 | 8.54 ± 2.23 | 0.97 | −0.12 (−0.64; 0.39) | 10.49 (6.30; 14.69) |
| SWR of IET | 33 | 0.89 ± 0.21 | 0.88 ± 0.17 | 0.57 | −0.01 (−0.06; 0.04) | 11.3 0(8.01; 14.58) |
| Longitudinal Plane | ||||||
| SWS of ET | 49 | 11.64 ± 3.64 | 12.08 ± 3.64 | 0.10 | 0.43 (−0.12; 0.99) | 11.53 (7.68; 15.38) |
| SWS of EET | 16 | 13.45 ±4.30 | 13.99 ±4.65 | 0.51 | 0.54 (−1.09; 2.16) | 18.54 (9.67; 27.40) |
| SWS of IET | 33 | 10.77 ± 2.96 | 11.15 ± 2.65 | 0.051 | 0.38 (0.00; 0.77) | 8.13 (4.53; 11.73) |
| Thyroid SWS | 50 | 11.84 ± 3.24 | 12.09 ± 2.85 | 0.36 | 0.25 (−0.24; 0.74) | 12.86 (9.24; 16.48) |
| SWS of thyroid adjacent to EET | 17 | 11.08 ± 2.57 | 10.82 ± 2.60 | 0.59 | −0.25 (−1.27; 0.77) | 15.57 (8.29; 22.86) |
| SWS of thyroid adjacent to IET | 33 | 12.23 ± 3.51 | 12.74 ± 2.80 | 0.13 | 0.52 (−0.03; 1.06) | 11.46 (7.23; 15.69) |
| SWR of ET | 49 | 0.86 ± 0.13 | 0.83 ± 0.12 | 0.12 | −0.02 (−0.06; 0.01) | 9.58 (6.32; 12.84) |
| SWR of EET | 16 | 0.84 ± 0.15 | 0.81 ± 0.19 | 0.51 | −0.03 (−0.12; 0.06) | 13.75 (6.08; 21.41) |
| SWR of IET | 33 | 0.87 ±0.12 | 0.85 ± 0.08 | 0.14 | −0.02 (−0.06; 0.01) | 7.57 (4.33; 10.80) |
| Ectopic Thymus | n | Transverse SWS Mean ± SD | Longitudinal SWS Mean ± SD | p | D Mean (95%CI) | D% Mean (95%CI) |
|---|---|---|---|---|---|---|
| Researcher 1 | ||||||
| ETs | 49 | 7.23 ± 1.78 | 11.64 ± 3.64 | <0.0000001 | 4.40 (3.29; 5.52) | 46.47 (38.08; 54.87) |
| EETs | 16 | 6.77 ± 1.39 | 13.45 ±4.30 | <0.001 | 6.68 (4.46; 8.90) | 62.21 (48.29; 76.13) |
| IETs | 33 | 7.47 ± 1.93 | 10.77 ± 2.96 | <0.0001 | 3.30 (2.14; 4.45) | 38.84 (28.95; 48.73) |
| Researcher 2 | ||||||
| ETs | 49 | 7.20 ± 1.54 | 12.08 ± 3.64 | <0.00000001 | 4.88 (3.85; 5.90) | 47.62 (39.87; 55.37) |
| EETs | 16 | 7.09 ± 0.99 | 13.99 ± 4.65 | <0.001 | 6.89 (4.60; 9.17) | 60.65 (47.02; 74.27) |
| IETs | 33 | 7.25 ± 1.77 | 11.15 ± 2.65 | <0.000001 | 3.90 (2.94; 4.86) | 41.31 (32.21; 50.40) |
| Thyroid | n | Transverse SWS Mean ± SD | Longitudinal SWS Mean ± SD | p | D Mean (95%CI) | D% Mean (95%CI) |
|---|---|---|---|---|---|---|
| R1 | 33 | 8.66 ± 2.42 | 12.23 ± 3.51 | 0.0001 | 3.57 (2.15; 4.98) | 39.69 (29.86; 49.53) |
| R2 | 33 | 8.54 ± 2.23 | 12.74 ± 2.80 | <0.00001 | 4.21 (3.05; 5.36) | 40.79 (31.53; 50.06) |
| n | Transverse SWR Mean ± SD | Longitudinal SWR Mean ± SD | p | D Mean (95%CI) | D% Mean (95%CI) | |
|---|---|---|---|---|---|---|
| R1 | 33 | 0.89 ± 0.21 | 0.87 ± 0.12 | 0.79 | −0.02 (−0.11; 0.07) | 22.01 (15.57; 28.46) |
| R2 | 33 | 0.88 ± 0.17 | 0.85 ± 0.08 | 0.41 | −0.03 (−0.09; 0.02) | 13.99 (9.58; 18.41) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczewski, Z.; Stasiak, M.; Stasiak, B.; Adamczewska, M.; Lewiński, A. Interobserver Agreement and Plane-Dependent Intraobserver Variability of Shear Wave Sonoelastography in the Differential Diagnosis of Ectopic Thymus Tissue. J. Clin. Med. 2021, 10, 214. https://doi.org/10.3390/jcm10020214
Adamczewski Z, Stasiak M, Stasiak B, Adamczewska M, Lewiński A. Interobserver Agreement and Plane-Dependent Intraobserver Variability of Shear Wave Sonoelastography in the Differential Diagnosis of Ectopic Thymus Tissue. Journal of Clinical Medicine. 2021; 10(2):214. https://doi.org/10.3390/jcm10020214
Chicago/Turabian StyleAdamczewski, Zbigniew, Magdalena Stasiak, Bartłomiej Stasiak, Magdalena Adamczewska, and Andrzej Lewiński. 2021. "Interobserver Agreement and Plane-Dependent Intraobserver Variability of Shear Wave Sonoelastography in the Differential Diagnosis of Ectopic Thymus Tissue" Journal of Clinical Medicine 10, no. 2: 214. https://doi.org/10.3390/jcm10020214
APA StyleAdamczewski, Z., Stasiak, M., Stasiak, B., Adamczewska, M., & Lewiński, A. (2021). Interobserver Agreement and Plane-Dependent Intraobserver Variability of Shear Wave Sonoelastography in the Differential Diagnosis of Ectopic Thymus Tissue. Journal of Clinical Medicine, 10(2), 214. https://doi.org/10.3390/jcm10020214







