Myocardial Work Does Not Have Additional Diagnostic Value in the Assessment of ATTR Cardiac Amyloidosis
Abstract
:1. Introduction
2. Material and Methods
2.1. Patient Population
2.2. Echocardiographic Examination
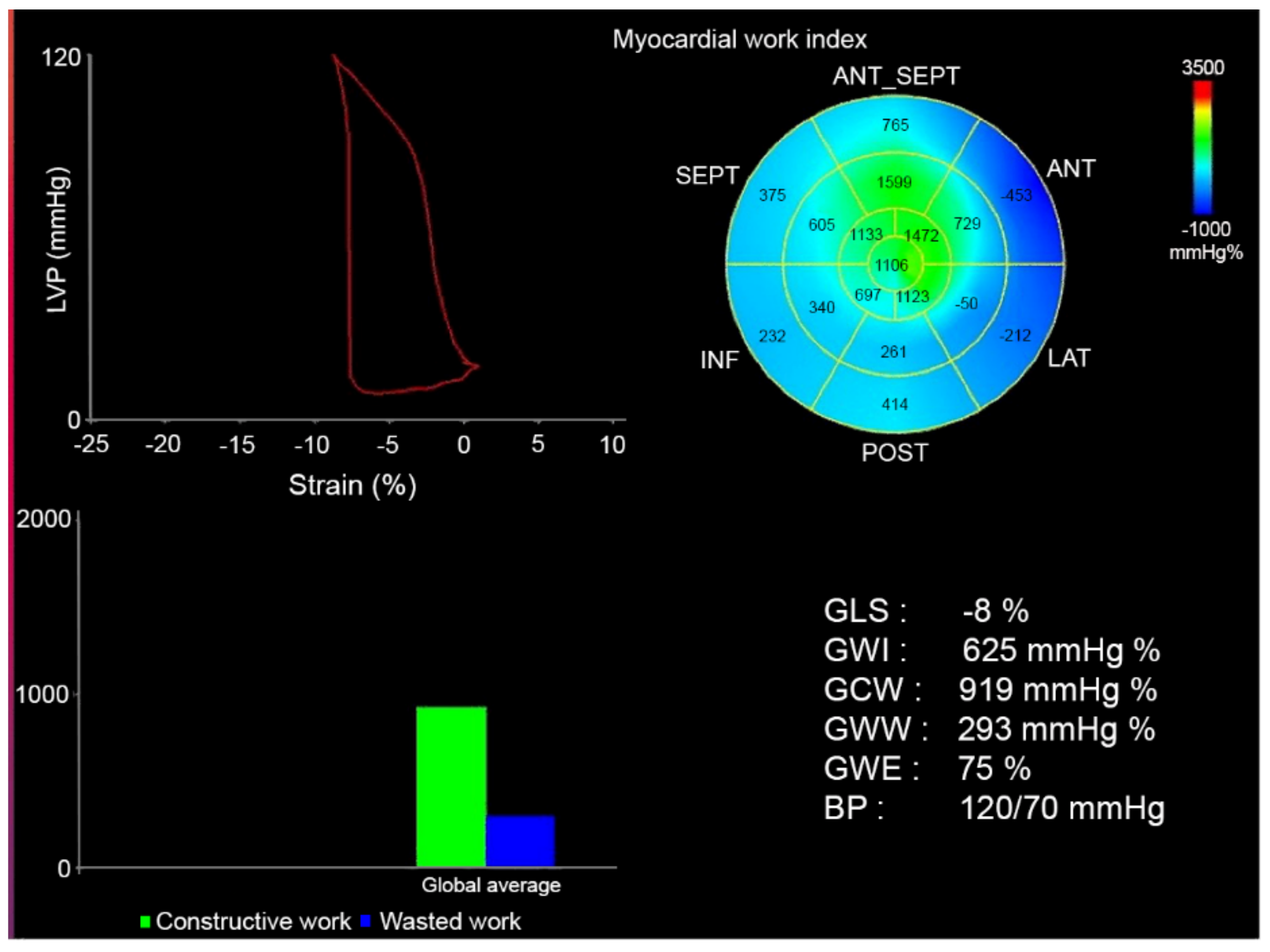
2.3. Assessment of Myocardial Work
- Global Work Index (GWI): total work within the area of the LV GLS = area within the pressure-strain curve (mmHg/%)
- Global Constructive Work (GCW): work performed by the LV contributing to cavity ejection. Constructive MW is defined as shortening of the myocytes during systole added to lengthening of the myocytes during isovolumic relaxation.
- Global Wasted Work (GWW): work performed by the LV that does not contribute to cavity ejection. Wasted MW is defined as lengthening of myocytes (rather than shortening) during systole added to shortening during the isovolumic relaxation phase.
- Global Work Efficiency (GWE): constructive MW/(constructive MW + wasted MW) (these values will not be affected by peak LV pressure).
2.4. 3, 3-diphosphono-1, 2-propanodicarboxylic Acid (DPD) Scintigraphy
2.5. Statistical Analysis
2.6. Study Ethics
3. Results
3.1. Echocardiographic Predictors of DPD Verified ATTR-CA
3.2. Myocardial Work and Biomarkers
3.3. Myocardial Work and All Cause Mortality in ATTR-CA
3.4. Variability Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maleszewski, J.J. Cardiac amyloidosis: Pathology, nomenclature, and typing. Cardiovasc. Pathol. 2015, 24, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.H. Diagnosis and Management of the Cardiac Amyloidoses. Circulation 2005, 112, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.S.; Bavry, A.A. Diagnosis of Transthyretin Amyloid Cardiomyopathy. Cardiol. Ther. 2020, 9, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2021, 23, 512–526. [Google Scholar] [CrossRef] [PubMed]
- AlJaroudi, W.A.; Desai, M.Y.; Tang, W.H.W.; Phelan, D.; Cerqueira, M.D.; Jaber, W.A. Role of imaging in the diagnosis and management of patients with cardiac amyloidosis: State of the art review and focus on emerging nuclear techniques. J. Nucl. Cardiol. 2013, 21, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Jurcuţ, R.; Onciul, S.; Adam, R.; Stan, C.; Coriu, D.; Rapezzi, C.; Popescu, B.A. Multimodality imaging in cardiac amyloidosis: A primer for cardiologists. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Roger-Rolle, A.; Cariou, E.; Rguez, K.; Fournier, P.; Lavie-Badie, Y.; Blanchard, V.; Jérôme Roncalli, J.; Galinier, M.; Carrié, D.; Lairez, O.; et al. Can myocardial work indices contribute to the exploration of patients with cardiac amyloidosis? Open Heart 2020, 7, e001346. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, K.; Pilebro, B.; Sundström, T.; Lindqvist, P. Prevalence of wild type transtyrethin cardiac amyloidosis in a heart failure clinic. ESC Heart Fail. 2020, 8, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.A.; Otto, C.; Stoddard, M.; Waggoner, A.; Zoghbi, W.A. Recommendations for quantification of Doppler echocardiography: A report from the Doppler quantification task force of the nomenclature and standards committee of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2002, 15, 167–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitter, S.S.; Shah, S.J.; Thomas, J.D. A Test in Context: E/A and E/e’ to Assess Diastolic Dysfunction and LV Filling Pressure. J. Am. Coll. Cardiol. 2017, 69, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Donal, E.; Penicka, M.; Sletten, O.J. How to measure left ventricular myocardial work by pressure–strain loops. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Reggiani, M.L.B.; et al. Noninvasive Etiologic Diagnosis of Cardiac Amyloidosis Using 99mTc-3,3-Diphosphono-1,2-Propanodicarboxylic Acid Scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, E.; Hubert, A.; Le Rolle, V.; Hernandez, A.; Smiseth, O.A.; Mabo, P.; Leclercq, C.; Donal, E. Myocardial constructive work and cardiac mortality in resynchronization therapy candidates. Am. Heart J. 2019, 212, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemmensen, T.S.; Eiskjær, H.; Ladefoged, B.; Mikkelsen, F.; Sørensen, J.; Granstam, S.-O.; Rosengren, S.; Flachskampf, F.A.; Poulsen, S.H. Prognostic implications of left ventricular myocardial work indices in cardiac amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, T.S.; Eiskjær, H.; Mikkelsen, F.; Granstam, S.-O.; Flachskampf, F.A.; Sørensen, J.; Poulsen, S.H. Left Ventricular Pressure-Strain–Derived Myocardial Work at Rest and during Exercise in Patients with Cardiac Amyloidosis. J. Am. Soc. Echocardiogr. 2020, 33, 573–582. [Google Scholar] [CrossRef] [PubMed]

| ATTR-CA (n = 86) | HFnCA (n = 30) | p-Value | |
|---|---|---|---|
| NT-proBNP log, ng/L | 3.1 ± 0.6 | 2.9 ± 0.7 | 0.007 |
| Troponin, ng/L | 35 (36) | 23 (24) | 0.148 |
| SBP, mmHg | 130 ± 20 | 142 ± 18 | 0.020 |
| DBP, mmHg | 80 (14) | 85 (17) | 0.004 |
| Height, cm | 175 ± 8 | 173 ± 9 | 0.201 |
| Weight, kg | 78 ± 13 | 87 ± 21 | 0.015 |
| BMI, kg/m2 | 25 ± 4 | 29 ± 6 | <0.001 |
| Female gender (%) | 19 (22%) | 11 (35%) | 0.06 |
| ATTR-CA (86) | HFnCA (30) | p-Value ATTR-CA vs. HFnCA | |
|---|---|---|---|
| Age, years | 77 (42) | 75 (8) | 0.712 |
| HR, bpm | 72 ± 12 | 66 ± 13 | 0.05 |
| IVS, mm | 18 (5) | 15 (2) | 0.005 |
| LVDD, mm | 44 ± 5 | 48 ± 7 | 0.004 |
| PWT, mm | 14.0 ± 2.9 | 10.5 ± 1.8 | <0.001 |
| LAVI, mL/m2 | 39 (18) | 38 (17) | 0.943 |
| RWT, mm | 0.65 ± 0.18 | 0.45 ± 0.13 | <0.001 |
| RV-RA, mmHg | 25 (10) | 25 (10) | 0.321 |
| E velocity, cm/s | 80 (22) | 60 (30) | 0.002 |
| RELAPS | 2.2 (1.0) | 0.7 (0.4) | <0.001 |
| AF, % | 27 | 3 | <0.001 |
| GLS, % | 12.7 ± 3.9 | 12.6 ± 4.5 | 0.910 |
| GWI, mmHg% | 1180 ± 473 | 1453 ± 647 | 0.040 |
| GCW, mmHg% | 1560 ± 547 | 1908 ± 772 | 0.026 |
| GWW, mmHg/% | 210 (89) | 380 (268) | <0.001 |
| GWE, % | 82 (9) | 83 (13) | 0.0497 |
| ATTR-CA (28) | HFnCA (22) | p-Value ATTR-CA vs. HFnCA | |
|---|---|---|---|
| GLS, % | 14.1 ± 2.9 | 12.4 ± 4.5 | 0.102 |
| GWI mmHg% | 1446 ± 384 | 1449 ± 668 | 0.984 |
| GCW mmHg% | 1865 ± 440 | 1876 ± 791 | 0.985 |
| GWW mmHg% | 319 ± 190 | 409 ± 173 | 0.091 |
| GWE, % | 83 ± 7 | 80 ± 7 | 0.118 |
| RELAPS | 1.9 (1.1) | 0.8 (0.73) | <0.001 |
| RWT, mm | 0.65 ± 0.17 | 0.46 ± 0.13 | <0.001 |
| NT-proBNP All Patients | Troponin All Patients | NT-proBNP ATTR-CA | Troponin ATTR-CA | |
|---|---|---|---|---|
| GLS | R = −0.18, p = ns | R = −0.38, p = 0.001 | R = −0.28, p = 0.044 | R = −0.47, p < 0.001 |
| GWI | R = −0.27, p = 0.019 | R = 0.40, p = 0.001 | R = −0.37, p = 0.006 | R = −0.44, p = 0.001 |
| GCW | R = −0.24, p = 0.038 | R = −0.35, p = 0.002 | R= −0.38, p = 0.005 | R = −0.41, p = 0.003 |
| GWW | R = −0.12, p = ns | R = −0.21, p = 0.067 | R = −0.085, p = ns | R = −0.140, p = ns |
| GWE | R = −0.34, p = 0.003 | R = −0.22, p = 0.054 | R = −0.085, p = ns | R = −0.134, p = ns |
| RELAPS | R = 0.19, p = ns | R = 0.22, p = 0.026 | R = 0.21, p = ns | R = 0.17, p = ns |
| RWT | R = 0.26, p = 0.006 | R = 0.26, p = 0.006 | 0.27, p = 0.014 | R = 0.21, p = 0.070 |
| N | Mean/Median * | SD/IQR * | p-Value | ||
|---|---|---|---|---|---|
| GLS | Dead | 19 | 11.3 * | 4.0 * | 0.043 * |
| Alive | 34 | 13.5 * | 3.6 * | ||
| GWI | Dead | 19 | 977 * | 462 * | 0.018 * |
| Alive | 34 | 1295 * | 777 * | ||
| GCW | Dead | 19 | 1301 | 543 | 0.009 |
| Alive | 34 | 1705 | 501 | ||
| GWW | Dead | 19 | 209 * | 169 * | 0.911 * |
| Alive | 34 | 221 * | 211 * | ||
| GWE | Dead | 19 | 83 * | 12 * | 0.043 * |
| Alive | 34 | 83 * | 11 * | ||
| RWT | Dead | 30 | 0.66 | 0.26 | 0.039 |
| Alive | 54 | 0.57 | 0.30 | ||
| RELAPS | Dead | 28 | 2.4 * | 1.4 * | 0.856 * |
| Alive | 52 | 2.0 * | 1.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henein, M.Y.; Lindqvist, P. Myocardial Work Does Not Have Additional Diagnostic Value in the Assessment of ATTR Cardiac Amyloidosis. J. Clin. Med. 2021, 10, 4555. https://doi.org/10.3390/jcm10194555
Henein MY, Lindqvist P. Myocardial Work Does Not Have Additional Diagnostic Value in the Assessment of ATTR Cardiac Amyloidosis. Journal of Clinical Medicine. 2021; 10(19):4555. https://doi.org/10.3390/jcm10194555
Chicago/Turabian StyleHenein, Michael Y., and Per Lindqvist. 2021. "Myocardial Work Does Not Have Additional Diagnostic Value in the Assessment of ATTR Cardiac Amyloidosis" Journal of Clinical Medicine 10, no. 19: 4555. https://doi.org/10.3390/jcm10194555
APA StyleHenein, M. Y., & Lindqvist, P. (2021). Myocardial Work Does Not Have Additional Diagnostic Value in the Assessment of ATTR Cardiac Amyloidosis. Journal of Clinical Medicine, 10(19), 4555. https://doi.org/10.3390/jcm10194555






