Prevalence and Course of SARS-CoV-2 Infection among Immunocompromised Children Hospitalised in the Tertiary Referral Hospital in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Admission Criteria
- Symptomatic COVID-19 in children under constant medical care at Children’s Memorial Health Institute requiring hospitalisation;
- Asymptomatic COVID-19 or quarantine in children under constant medical care at Children’s Memorial Health Institute requiring specialised diagnostics or treatment which could not be postponed prior to recovery from the virus;
- Symptoms of suspected COVID-19 in all children admitted urgently to the Children’s Memorial Health Institute. (The hospitalisation in the COVID-19 Subunit aimed to exclude SARS-CoV-2 infection before the possible transfer to the appropriate ward.)
2.3. Diagnosis of SARS-CoV2 Infection
- Those diagnosed with the infection before hospitalisation;
- Convalescents with a history of illness within the last three months.
2.4. Division of the Subgroups
2.5. Clinical and Laboratory Data
- Clinical data: age, sex, the reasons for admission, COVID-19 status in the patient and the caregiver, final diagnosis, the manifestation of the disease, treatment, incidence of complications, comorbidities, and the presence of immunodeficiency;
- Body temperature, blood oxygen saturation, respiratory rate, heart rate, and blood pressure on hospital admission;
- Laboratory results on admission: white blood cell (WBC) count, neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP), procalcitonin (PCT), erythrocyte sedimentation rate (ESR), level of haemoglobin (Hgb) and platelets (PLT), D-dimers, fibrinogen, lactate dehydrogenase (LDH), transaminases (ALT and AST), creatinine and urea levels, and N-terminal prohormone of brain natriuretic peptide (NT-proBNP);
- Chest X-ray results.
2.6. Fever—Definitions
- Fever—A body temperature above 38 °C;
- Isolated fever—A body temperature above 38 °C without any other accompanying symptoms;
- Febrile neutropenia—A body temperature greater than or equal to 38 °C for at least an hour, with an absolute neutrophil count of less than 1.5 × 109 cells/L.
2.7. Disease Severity—Definitions
- Asymptomatic—No symptoms of COVID-19;
- Mild—Symptoms that generally did not require hospitalisation (i.e., moderate fever or cough that lasted for several days, rhinorrhoea, moderate gastrointestinal symptoms, rash, etc.). However, hospitalisation was required for other indications;
- Moderate—Required hospitalisation in a paediatric ward;
- Severe—Required hospitalisation in the ICU;
- PIMS (Paediatric Inflammatory Multisystem Syndrome)—The diagnosis was based on WHO diagnostic criteria [9].
2.8. Statistical Analysis
3. Results
3.1. Entire Study Group
3.2. Children with Immunodeficiency
3.3. Comparison of Groups of Children with and without Immunodeficiency
3.3.1. Symptoms on Admission
3.3.2. COVID-19 Course
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Risk Assessment: Outbreak of Acute Respiratory Syndrome Associated with a Novel Coronavirus, China; First Cases Imported in the EU/EEA.; Second Update. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/risk-assessment-outbreak-acute-respiratory-syndrome-associated-novel-0 (accessed on 20 June 2021).
- European Centre for Disease Prevention and Control. COVID Situation Update Worldwide, as of Week 23, Updated the 17th of June 2021. 2021. Available online: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (accessed on 20 September 2021).
- Cao, Q.; Chen, Y.C.; Chen, C.L.; Chiu, C.H. SARS-CoV-2 infection in children: Transmission dynamics and clinical characteristics. J. Formos. Med. Assoc. 2020, 119, 670–673. [Google Scholar] [CrossRef]
- Spielberger, B.D.; Goerne, T.; Geweniger, A.; Henneke, P.; Elling, R. Intra-Household and Close-Contact SARS-CoV-2 Transmission Among Children—A Systematic Review. Front Pediatr. 2021, 9, 613292. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef]
- Loske, J.; Röhmel, J.; Lukassen, S.; Stricker, S.; Magalhães, V.G.; Liebig, J.; Chua, R.L.; Thürmann, L.; Messingschlager, M.; Seegebarth, A.; et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Minotti, C.; Tirelli, F.; Barbieri, E.; Giaquinto, C.; Donà, D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J. Infect. 2020, 81, e61–e66. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- World Health Organisation. Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. 2020. Available online: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 20 June 2021).
- Nicastro, E.; Verdoni, L.; Bettini, L.R.; Zuin, G.; Balduzzi, A.; Montini, G.; Biondi, A.; D’Antiga, L. COVID-19 in Immunosuppressed Children. Front Pediatr. 2021, 9, 629240. [Google Scholar] [CrossRef] [PubMed]
- Niehues, T. The febrile child: Diagnosis and treatment. Dtsch Arztebl. Int. 2013, 110, 764–773. [Google Scholar] [PubMed] [Green Version]
- Worrall, G. Acute cough in children. Can Fam. Physician. 2011, 57, 315–318. [Google Scholar]
- Tregoning, J.S.; Schwarze, J. Respiratory viral infections in infants: Causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010, 23, 74–98. [Google Scholar] [CrossRef] [Green Version]
- Millen, G.C.; Arnold, R.; Cazier, J.B.; Curley, H.; Feltbower, R.G.; Gamble, A.; Glaser, A.W.; Grundy, R.G.; Lee, L.Y.W.; McCabe, M.G.; et al. Severity of COVID-19 in children with cancer: Report from the United Kingdom Paediatric Coronavirus Cancer Monitoring Project. Br. J. Cancer. 2021, 124, 754–759. [Google Scholar] [CrossRef]
- Ferrari, A.; Zecca, M.; Rizzari, C.; Porta, F.; Provenzi, M.; Marinoni, M.; Schumacher, R.F.; Luksch, R.; Terenziani, M.; Casanova, M.; et al. Children with cancer in the time of COVID-19: An 8-week report from the six pediatric onco-hematology centers in Lombardia, Italy. Pediatr. Blood Cancer. 2020, 67, e28410. [Google Scholar] [CrossRef] [PubMed]
- Madhusoodhan, P.P.; Pierro, J.; Musante, J.; Kothari, P.; Gampel, B.; Appel, B.; Levy, A.; Tal, A.; Hogan, L.; Sharma, A.; et al. Characterisation of COVID-19 disease in pediatric oncology patients: The New York-New Jersey regional experience. Pediatr. Blood Cancer. 2021, 68, e28843. [Google Scholar] [CrossRef] [PubMed]
- Tirupathi, R.; Muradova, V.; Shekhar, R.; Salim, S.A.; Al-Tawfiq, J.A.; Palabindala, V. COVID-19 disparity among racial and ethnic minorities in the US: A cross sectional analysis. Travel. Med. Infect Dis. 2020, 38, 101904. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, E.; Di Giorgio, A.; Zambelli, M.; Ginammi, M.; Bravi, M.; Stroppa, P.; Casotti, V.; Palladino, R.; Colledan, M.; D’Antiga, L. Impact of the Severe Acute Respiratory Syndrome Coronavirus 2 Outbreak on Pediatric Liver Transplant Recipients in Lombardy, Northern Italy. Liver Transpl. 2020, 26, 1359–1362. [Google Scholar] [CrossRef] [PubMed]
- Belli, L.S.; Fondevila, C.; Cortesi, P.A.; Conti, S.; Karam, V.; Adam, R.; Coilly, A.; Ericzon, B.G.; Loinaz, C.; Cuervas-Mons, V.; et al. Protective Role of Tacrolimus, Deleterious Role of Age and Comorbidities in Liver Transplant Recipients with Covid-19: Results From the ELITA/ELTR Multi-center European Study. Gastroenterology 2021, 160, 1151–1163. [Google Scholar] [CrossRef]
- Dorfman, L.; Nassar, R.; Binjamin Ohana, D.; Oseran, I.; Matar, M.; Shamir, R.; Assa, A. Pediatric inflammatory bowel disease and the effect of COVID-19 pandemic on treatment adherence and patients’ behavior [published online ahead of print, 2021 the 14th of January]. Pediatr. Res. 2021, 1–5. [Google Scholar] [CrossRef]
- Reinsch, S.; Stallmach, A.; Grunert, P.C. The COVID-19 Pandemic: Fears and Overprotection in Pediatric Patients with Inflammatory Bowel Disease and Their Families. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 65–74. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Axelrad, J.; Halfvarson, J.; Khalili, H.; Larsson, E.; Lochhead, P.; Roelstraete, B.; Simon, T.G.; Söderling, J.; Olén, O. Inflammatory bowel disease and risk of severe COVID-19: A nationwide population-based cohort study in Sweden. United Eur. Gastroenterol. J. 2021, 9, 177–192. [Google Scholar] [CrossRef]
- Balduzzi, A.; Brivio, E.; Rovelli, A.; Rizzari, C.; Gasperini, S.; Melzi, M.L.; Conter, V.; Biondi, A. Lessons after the early management of the COVID-19 outbreak in a pediatric transplant and hemato-oncology center embedded within a COVID-19 dedicated hospital in Lombardia, Italy. Estote parati. Bone Marrow Transplant. 2020, 55, 1900–1905. [Google Scholar] [CrossRef] [Green Version]
- Babaha, F.; Rezaei, N. Primary Immunodeficiency Diseases in COVID-19 Pandemic: A Predisposing or Protective Factor? Am. J. Med. Sci. 2020, 360, 740–741. [Google Scholar] [CrossRef] [PubMed]
- Quinti, I.; Lougaris, V.; Milito, C.; Cinetto, F.; Pecoraro, A.; Mezzaroma, I.; Mastroianni, C.M.; Turriziani, O.; Bondioni, M.P.; Filippini, M.; et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J. Allergy Clin. Immunol. 2020, 146, 211–213.e4. [Google Scholar] [CrossRef] [PubMed]
- Castano-Jaramillo, L.M.; Yamazaki-Nakashimada, M.A.; O’Farrill-Romanillos, P.M.; Zermeño, D.M.; Mendoza, S.C.S.; Montoya, E.V.; Campos, J.A.G.; Sánchez-Sánchez, L.M.; González, L.B.G.; López, J.M.R.; et al. COVID-19 in the Context of Inborn Errors of Immunity: A Case Series of 31 Patients from Mexico. J. Clin. Immunol. 2021, 10, 1–16. [Google Scholar]
- Samprathi, M.; Jayashree, M. Biomarkers in COVID-19: An Up-To-Date Review. Front Pediatr. 2021, 8, 607647. [Google Scholar] [CrossRef] [PubMed]
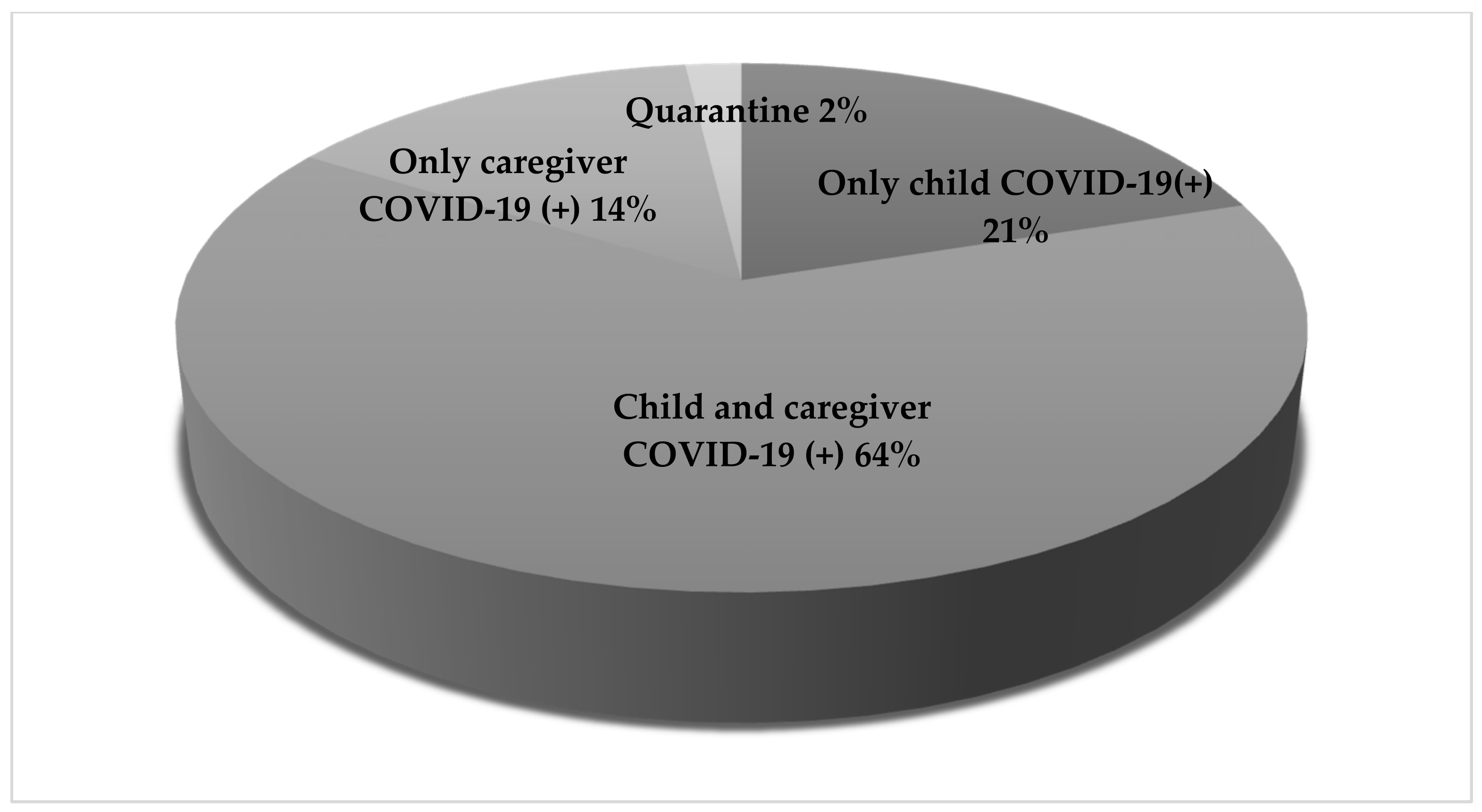
| Reasons for Hospital Admission | ||
|---|---|---|
| Without the initial diagnosis of SARS-CoV-2 infection | ||
| Reason | Number | % |
| Isolated fever | 68 | 21.73 |
| Features of gastroenteritis | 59 | 18.85 |
| Features of respiratory infection (cough, dyspnoea) | 31 | 9.90 |
| Suspected urinary tract infection (UTI) | 20 | 6.39 |
| Suspected sepsis | 18 | 5.75 |
| Suspected Kawasaki Disease/PIMS | 8 | 2.56 |
| Poisoning | 6 | 1.92 |
| Acute abdomen | 6 | 1.92 |
| Epileptic seizures | 4 | 1.28 |
| Sinusitis | 4 | 1.28 |
| Stomatitis | 2 | 0.64 |
| Severe weakness | 2 | 0.64 |
| Declines in blood oxygen saturation | 1 | 0.32 |
| Lymphadenopathy | 1 | 0.32 |
| Other | 15 | 4.79 |
| With the initial diagnosis of SARS-CoV-2 infection | ||
| Reason | Number | % |
| Oncological treatment (mainly CTH) | 41 | 13.10 |
| Newly diagnosed type 1 Diabetes (DM1) | 8 | 2.56 |
| Chemotherapy side effects | 6 | 1.92 |
| Post-transplant problems | 6 | 1.92 |
| Heart defects treatment | 4 | 1.28 |
| Gastrointestinal bleeding | 4 | 1.28 |
| Demyelinating diseases treatment | 4 | 1.28 |
| COVID-19 | 4 | 1.28 |
| Neurosurgical problems | 3 | 0.96 |
| Ulcerative colitis treatment | 2 | 0.64 |
| Treatment or diagnosis of other diseases | 13 | 4.15 |
| Diagnosis | Entire Study Group n = 68 | ID (+) n = 28 | ID (−) n = 40 | p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | N | % | ||
| Febrile neutropenia | 14 | 20.59 | 14 | 50.00 | 0 | 0.00 | <0.01* |
| UTI | 10 | 14.70 | 1 | 3.57 | 9 | 22.50 | 0.03* |
| FUO | 8 | 11.76 | 3 | 10.71 | 5 | 12.50 | 0.20 |
| COVID-19 | 8 | 11.76 | 2 | 7.14 | 6 | 15.00 | 0.47 |
| URTI | 6 | 8.82 | 3 | 10.71 | 3 | 7.50 | 0.65 |
| Gastroenteritis | 3 | 4.41 | 1 | 3.57 | 2 | 5.00 | 0.78 |
| Sepsis | 3 | 4.41 | 1 | 3.57 | 2 | 5.00 | 0.50 |
| Pneumonia | 3 | 4.41 | 0 | 0.00 | 3 | 7.50 | 0.14 |
| PFAPA | 2 | 2.94 | 0 | 0.00 | 2 | 5.00 | 0.23 |
| Roseola | 2 | 2.94 | 0 | 0.00 | 2 | 5.00 | 0.23 |
| Cholangitis | 2 | 2.94 | 1 | 3.57 | 1 | 3.50 | 0.80 |
| PIMS | 1 | 1.47 | 1 | 3.57 | 0 | 0.00 | 0.23 |
| Mononucleosis | 1 | 1.47 | 0 | 0.00 | 1 | 2.50 | 0.40 |
| Sinusitis | 1 | 1.47 | 0 | 0.00 | 1 | 2.50 | 0.40 |
| Stomatitis | 1 | 1.47 | 0 | 0.00 | 1 | 2.50 | 0.40 |
| Acute pancreatitis | 1 | 1.47 | 0 | 0.00 | 1 | 2.50 | 0.40 |
| Postoperative wound infection | 1 | 1.47 | 0 | 0.00 | 1 | 2.50 | 0.40 |
| Scarlet fever | 1 | 1.47 | 0 | 0.00 | 1 | 2.50 | 0.40 |
| Parotitis | 1 | 1.47 | 0 | 0.00 | 1 | 2.50 | 0.40 |
| Diagnosis | Entire Study Group n = 31 | ID (+) n = 5 | ID (−) n = 26 | p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Pneumonia | 17 | 54.84 | 2 | 40.00 | 15 | 57.69 | 0.41 |
| URTI | 10 | 32.26 | 2 | 40.00 | 8 | 30.77 | 0.69 |
| Bronchiolitis | 2 | 6.45 | 0 | 0.00 | 2 | 7.69 | 0.52 |
| COVID-19 | 1 | 3.23 | 1 | 20.00 | 0 | 0.00 | 0.02* |
| Mononucleosis | 1 | 3.23 | 0 | 0.00 | 1 | 3.85 | 0.65 |
| Diagnosis | Entire Study Group n = 59 | ID (+) n = 14 | ID (−) n = 45 | p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Gastroenteritis | 34 | 57.63 | 5 | 35.71 | 29 | 64.44 | 0.63 |
| COVID-19 | 10 | 16.95 | 4 | 28.57 | 6 | 13.33 | 0.17 |
| UTI | 8 | 13.56 | 5 | 35.71 | 3 | 6.67 | 0.26 |
| Pneumonia | 4 | 6.78 | 0 | 0.00 | 4 | 8.89 | 0.32 |
| URTI | 3 | 5.08 | 0 | 0.00 | 3 | 6.67 | 0.48 |
| PIMS/Kawasaki disease | 2 | 3.39 | 0 | 0.00 | 2 | 4.44 | 0.77 |
| DM1 | 2 | 3.39 | 0 | 0.00 | 2 | 4.44 | 0.21 |
| Sepsis | 2 | 3.39 | 0 | 0.00 | 2 | 4.44 | 0.48 |
| Acute abdomen | 2 | 3.39 | 0 | 0.00 | 2 | 4.44 | 0.48 |
| Gastrointestinal bleeding | 2 | 3.39 | 0 | 0.00 | 1 | 2.22 | 0.27 |
| Entire Study Group, n= 88 | ID (+) n = 55 | ID (−) n = 33 | p | ||||
|---|---|---|---|---|---|---|---|
| Symptoms on Admission | n | % | n | % | n | % | |
| Fever | 22 | 25.00 | 11 | 20 | 11 | 33.33 | 0.17 |
| Cough | 9 | 10.23 | 4 | 7.27 | 5 | 15.15 | 0.24 |
| Dyspnoea | 4 | 4.55 | 3 | 5.45 | 1 | 3.03 | 0.60 |
| Rhinorrhoea | 9 | 10.23 | 4 | 7.27 | 5 | 15.15 | 0.25 |
| Diarrhoea | 8 | 9.09 | 2 | 3.63 | 6 | 18.18 | 0.02* |
| Vomiting | 10 | 11.36 | 3 | 5.45 | 7 | 21.21 | 0.02* |
| Weakness | 3 | 3.41 | 2 | 3.63 | 1 | 3.03 | 0.13 |
| Declines in blood oxygen saturation | 2 | 2.27 | 2 | 3.63 | 0 | 0 | 0.27 |
| Rash | 2 | 2.27 | 1 | 1.81 | 1 | 3.03 | 0.29 |
| Course of the disease | n | % | n | % | n | % | |
| Asymptomatic | 58 | 65.91 | 40 | 72.72 | 19 | 57.58 | 0.55 |
| Mild | 17 | 19.32 | 12 | 21.81 | 5 | 15.15 | 0.44 |
| Moderate | 4 | 4.55 | 1 | 1.82 | 3 | 9.09 | 0.12 |
| Severe | 3 | 3.41 | 1 | 1.82 | 2 | 6.06 | 0.71 |
| PIMS | 5 | 5.68 | 1 | 1.82 | 4 | 12.12 | 0.04* |
| Variable | Q1 | Median | Q3 | Association Type | Association | p | |
|---|---|---|---|---|---|---|---|
| WBC | |||||||
| (×109/L) | ID (+) | 3.72 | 5.40 | 8.49 | Negative | −2.65 | 0.01 * |
| ID (−) | 5.40 | 7.72 | 11.38 | ||||
| NEUT | |||||||
| (×109/L) | ID (+) | 1.49 | 2.32 | 3.96 | N/A | −0.36 | 0.72 |
| ID (−) | 1.52 | 2.30 | 4.14 | ||||
| LYMPH | |||||||
| (×109/L) | ID (+) | 0.97 | 1.40 | 3.23 | Negative | −3.75 | <0.01 * |
| ID (−) | 2.06 | 4.00 | 5.78 | ||||
| NLR | |||||||
| ID (+) | 0.49 | 1.16 | 3.44 | Positive | 2.07 | 0.04 * | |
| ID (−) | 0.37 | 0.58 | 1.17 | ||||
| HGB | |||||||
| (g/dL) | ID (+) | 9.90 | 10.80 | 12.55 | Negative | −1.98 | 0.05 * |
| ID (−) | 10.75 | 12.00 | 13.10 | ||||
| PLT | |||||||
| (×109/L) | ID (+) | 182.00 | 281.00 | 395.50 | N/A | 1.32 | 0.19 |
| ID (−) | 231.50 | 319.00 | 433.5 | ||||
| CRP | |||||||
| (mg/dL) | ID (+) | 0.07 | 0.10 | 2.18 | N/A | 0.41 | 0.68 |
| ID (−) | 0.09 | 0.10 | 0.80 | ||||
| PCT | |||||||
| (ng/mL) | ID (+) | 0.04 | 0.08 | 0.50 | N/A | −0.39 | 0.69 |
| ID (−) | 0.03 | 0.13 | 0.79 | ||||
| ESR | |||||||
| (mm/h) | ID (+) | 16.00 | 21.00 | 55.00 | N/A | −0.18 | 0.85 |
| ID (−) | 35.00 | 63.00 | 64.50 | ||||
| D-dimers | |||||||
| (µg/L) | ID (+) | 194.00 | 426.50 | 2035.50 | N/A | 0.69 | 0.49 |
| ID (−) | 566.00 | 950.00 | 1264.00 | ||||
| Fibrinogen | |||||||
| (g/L) | ID (+) | 2.44 | 2.85 | 3.86 | N/A | −1.06 | 0.29 |
| ID (−) | 2.07 | 2.30 | 3.42 | ||||
| LDH | |||||||
| (U/L) | ID (+) | 248.00 | 315.50 | 386.75 | N/A | −0.49 | 0.63 |
| ID (−) | 225.50 | 288.50 | 306.00 | ||||
| ALT | |||||||
| (U/L) | ID (+) | 13.75 | 18.00 | 61.50 | N/A | 0.02 | 0.98 |
| ID (−) | 13.25 | 25.00 | 31.50 | ||||
| AST | |||||||
| (U/L) | ID (+) | 23.00 | 27.50 | 35.25 | N/A | 0.87 | 0.38 |
| ID (−) | 22.50 | 34.00 | 40.00 | ||||
| Creatinine | |||||||
| (mg/dL) | ID (+) | 0.38 | 0.47 | 0.61 | N/A | −0.77 | 0.44 |
| ID (−) | 0.40 | 0.44 | 0.53 | ||||
| Urea | |||||||
| (mg/dL) | ID (+) | 16.18 | 22.15 | 30.75 | Positive | 2.05 | 0.04 * |
| ID (−) | 14.45 | 19.70 | 23.50 | ||||
| NT-proBNP | |||||||
| (pg/mL) | ID (+) | 347.15 | 399.40 | 403.85 | N/A | −1.31 | 0.19 |
| ID (−) | 118.83 | 138.90 | 232.15 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuczborska, K.; Książyk, J. Prevalence and Course of SARS-CoV-2 Infection among Immunocompromised Children Hospitalised in the Tertiary Referral Hospital in Poland. J. Clin. Med. 2021, 10, 4556. https://doi.org/10.3390/jcm10194556
Kuczborska K, Książyk J. Prevalence and Course of SARS-CoV-2 Infection among Immunocompromised Children Hospitalised in the Tertiary Referral Hospital in Poland. Journal of Clinical Medicine. 2021; 10(19):4556. https://doi.org/10.3390/jcm10194556
Chicago/Turabian StyleKuczborska, Karolina, and Janusz Książyk. 2021. "Prevalence and Course of SARS-CoV-2 Infection among Immunocompromised Children Hospitalised in the Tertiary Referral Hospital in Poland" Journal of Clinical Medicine 10, no. 19: 4556. https://doi.org/10.3390/jcm10194556
APA StyleKuczborska, K., & Książyk, J. (2021). Prevalence and Course of SARS-CoV-2 Infection among Immunocompromised Children Hospitalised in the Tertiary Referral Hospital in Poland. Journal of Clinical Medicine, 10(19), 4556. https://doi.org/10.3390/jcm10194556






