Quantitative Flow Ratio Is Associated with Extent and Severity of Ischemia in Non-Culprit Lesions of Patients with Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
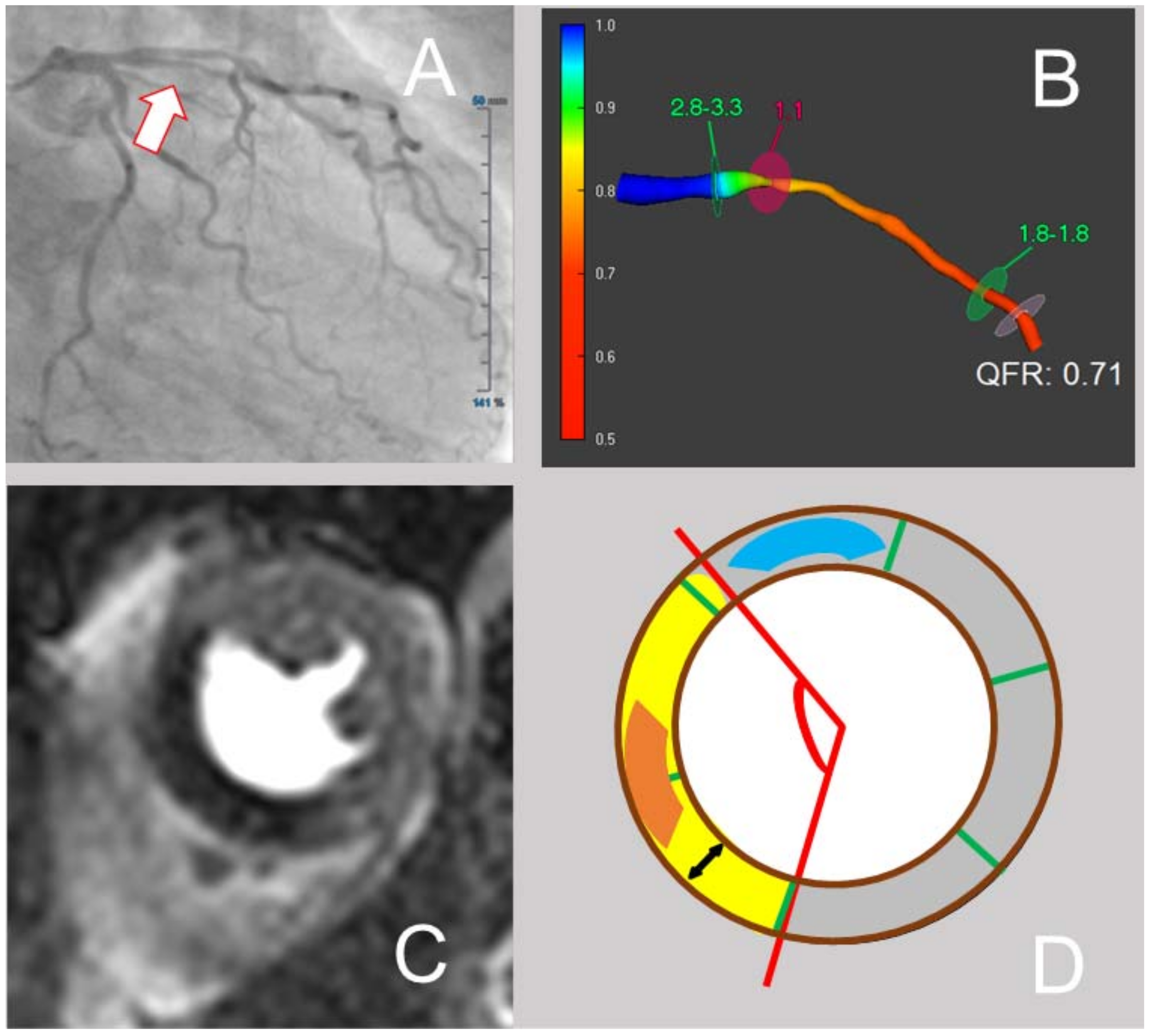
2.2. QFR Analysis
2.3. CMR Image Acquisition and Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
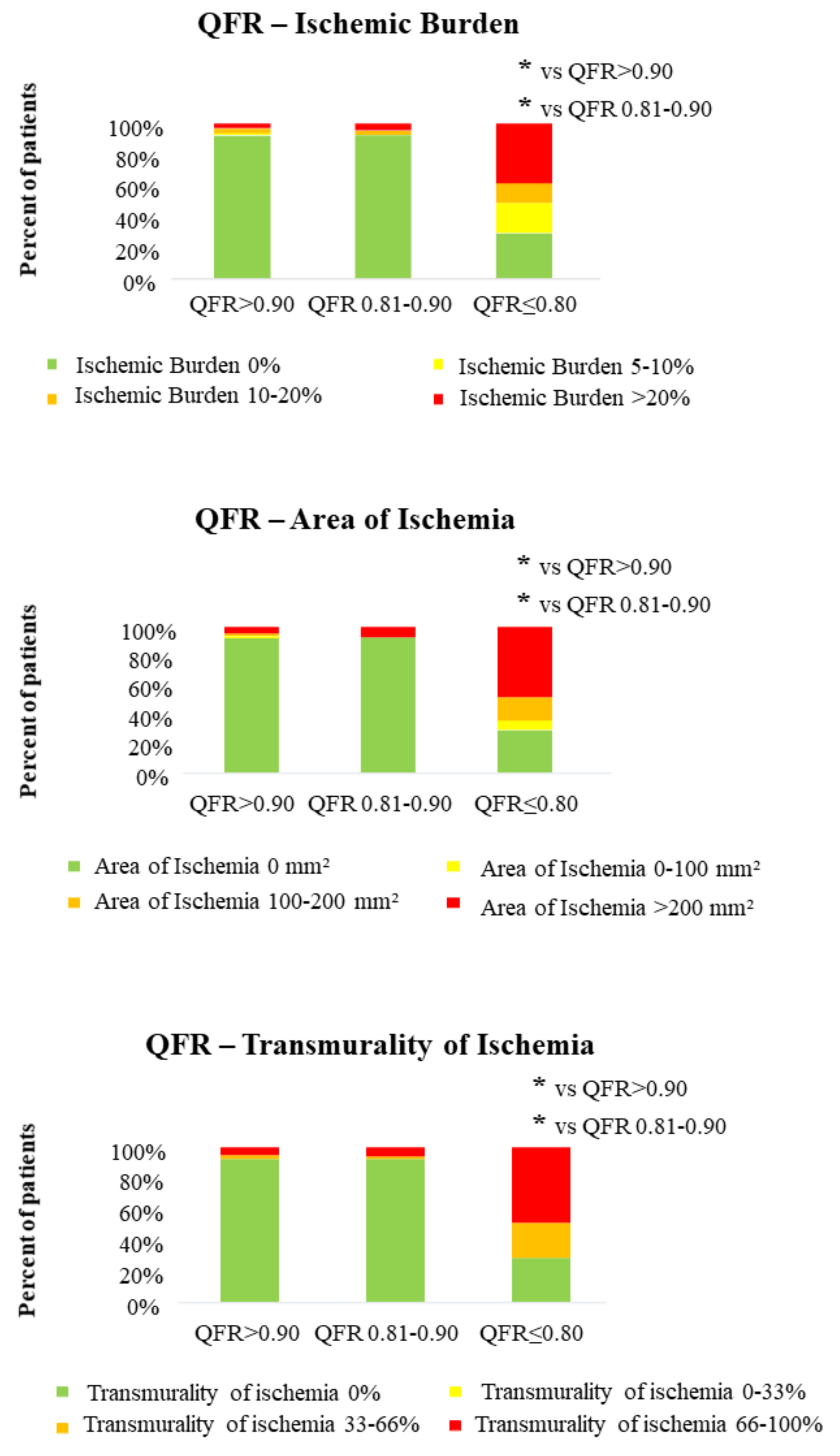
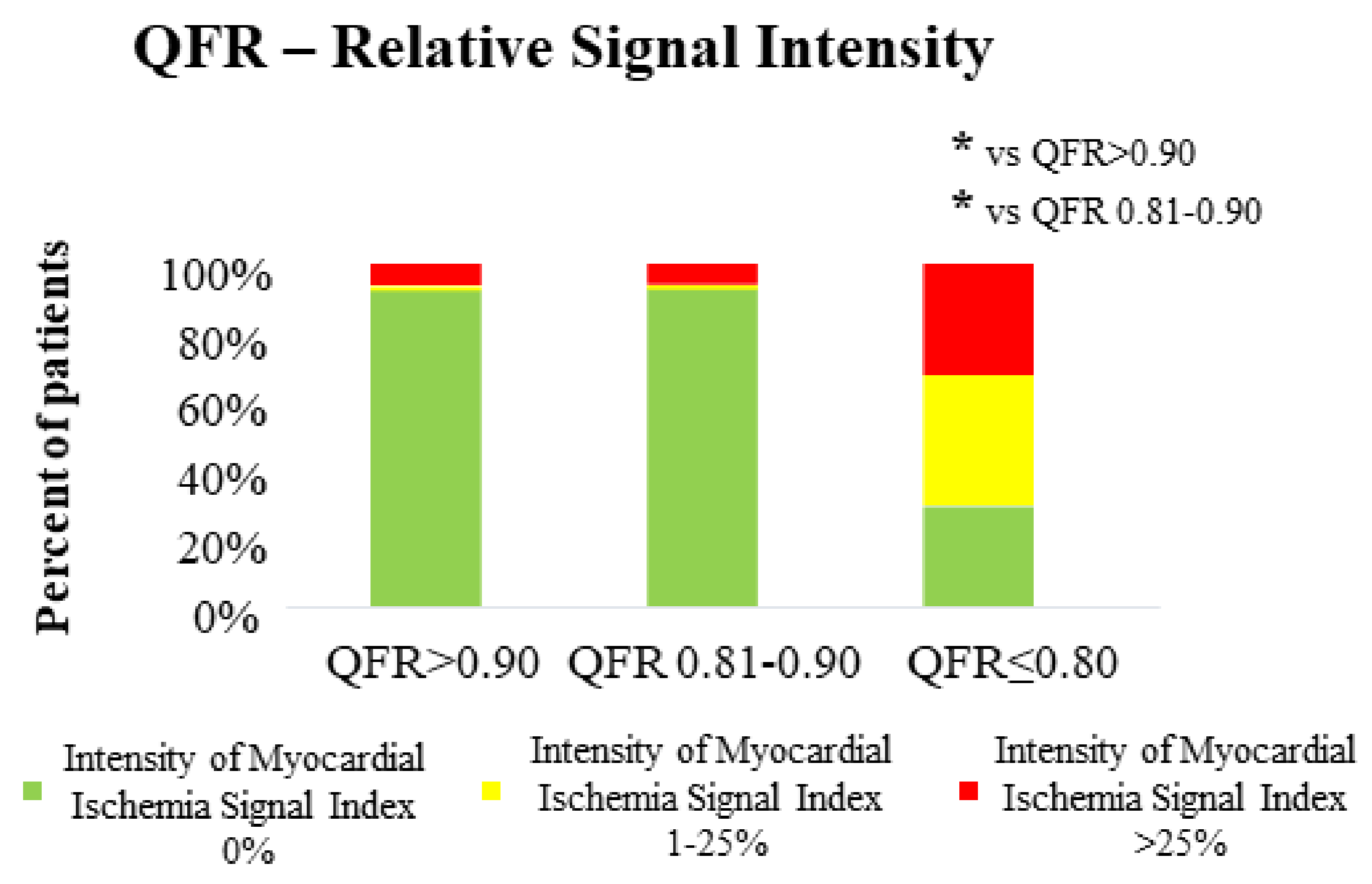
3.2. QFR and CMR Parameters in the Assessment of Both Extent and Severity of Myocardial Ischemia
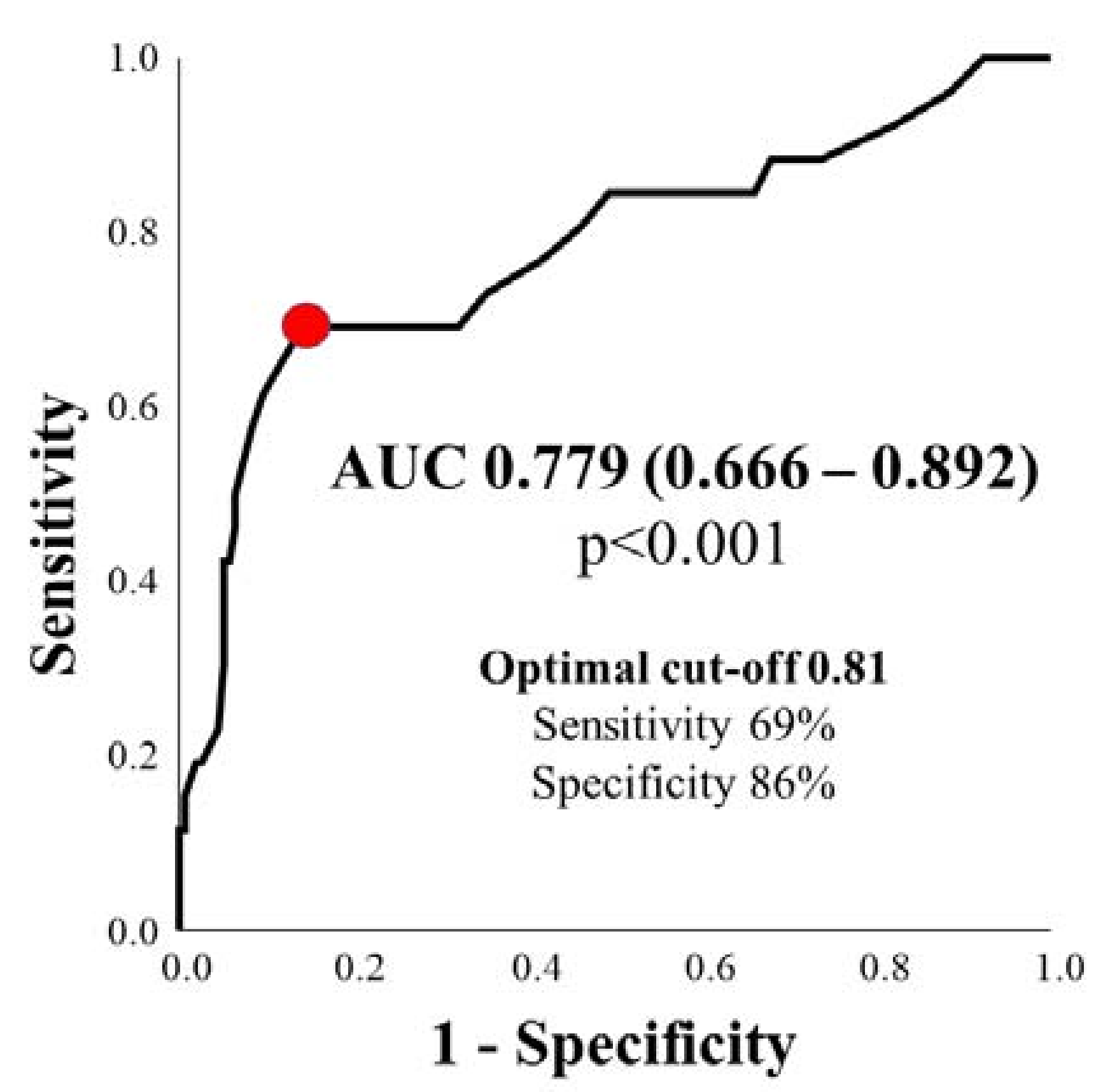
3.3. Diagnostic Efficiency of QFR in Predicting Clinically Relevant Ischemia ≥ 10%
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sejr-Hansen, M.; Westra, J.; Winther, S.; Tu, S.; Nissen, L.; Gormsen, L.; Petersen, S.E.; Ejlersen, J.; Isaksen, C.; Botker, H.E.; et al. Comparison of quantitative flow ratio and fractional flow reserve with myocardial perfusion scintigraphy and cardiovascular magnetic resonance as reference standard. A Dan-NICAD substudy. Int. J. Cardiovasc. Imaging 2020, 36, 395–402. [Google Scholar] [CrossRef]
- Westra, J.; Tu, S.; Winther, S.; Nissen, L.; Vestergaard, M.B.; Andersen, B.K.; Holck, E.N.; Fox Maule, C.; Johansen, J.K.; Andreasen, L.N.; et al. Evaluation of coronary artery stenosis by quantitative flow ratio during invasive coronary angiography: The wifi ii study (wire-free functional imaging II). Circ. Cardiovasc. Imaging 2018, 11, e007107. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Barbato, E.; Kőszegi, Z.; Yang, J.; Sun, Z.; Holm, N.; Tar, B.; Li, Y.; Rusinaru, D.; Wijns, W.; et al. Fractional Flow Reserve Calculation From 3-Dimensional Quantitative Coronary Angiography and TIMI Frame Count. JACC Cardiovasc. Interv. 2014, 7, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Van Rosendael, A.R.; Koning, G.; Dimitriu-Leen, A.C.; Smit, J.M.; Montero-Cabezas, J.M.; van der Kley, F.; Jukema, J.W.; Reiber, J.H.C.; Bax, J.J.; Scholte, A. Accuracy and reproducibility of fast fractional flow reserve computation from invasive coronary angiography. Int. J. Cardiovasc. Imaging 2017, 33, 1305–1312. [Google Scholar] [CrossRef]
- Tu, S.; Westra, J.; Yang, J.; von Birgelen, C.; Ferrara, A.; Pellicano, M.; Nef, H.; Tebaldi, M.; Murasato, Y.; Lansky, A.; et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: The international multicenter FAVOR pilot study. JACC Cardiovasc. Interv. 2016, 9, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Emori, H.; Kubo, T.; Kameyama, T.; Ino, Y.; Matsuo, Y.; Kitabata, H.; Terada, K.; Katayama, Y.; Taruya, A.; Shimamura, K.; et al. Quantitative flow ratio and instantaneous wave-free ratio for the assessment of the functional severity of intermediate coronary artery stenosis. Coron. Artery Dis. 2018, 29, 611–617. [Google Scholar] [CrossRef]
- Xu, B.; Tu, S.; Qiao, S.; Qu, X.; Chen, Y.; Yang, J.; Guo, L.; Sun, Z.; Li, Z.; Tian, F.; et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J. Am. Coll. Cardiol. 2017, 70, 3077–3087. [Google Scholar] [CrossRef]
- Westra, J.; Andersen, B.K.; Campo, G.; Matsuo, H.; Koltowski, L.; Eftekhari, A.; Liu, T.; Di Serafino, L.; Di Girolamo, D.; Escaned, J.; et al. Diagnostic performance of in-procedure angiography-derived quantitative flow reserve compared to pressure-derived fractional flow reserve: The FAVOR II Europe-Japan study. J. Am. Heart Assoc. 2018, 7, e009603. [Google Scholar] [CrossRef] [PubMed]
- Spitaleri, G.; Tebaldi, M.; Biscaglia, S.; Westra, J.; Brugaletta, S.; Erriquez, A.; Passarini, G.; Brieda, A.; Leone, A.M.; Picchi, A.; et al. Quantitative flow ratio identifies nonculprit coronary lesions requiring revascularization in patients with ST-Segment-Elevation myocardial infarction and multivessel disease. Circ. Cardiovasc. Interv. 2018, 11, e006023. [Google Scholar] [CrossRef] [PubMed]
- Lauri, F.M.; Macaya, F.; Mejia-Renteria, H.; Goto, S.; Yeoh, J.; Nakayama, M.; Quiros, A.; Liontou, C.; Pareek, N.; Fernandez-Ortiz, A.; et al. Angiography-derived functional assessment of non-culprit coronary stenoses in primary percutaneous coronary intervention. EuroIntervention 2020, 15, e1594–e1601. [Google Scholar] [CrossRef]
- Sejr-Hansen, M.; Westra, J.; Thim, T.; Christiansen, E.H.; Eftekhari, A.; Kristensen, S.D.; Jakobsen, L.; Gotberg, M.; Frobert, O.; van der Hoeven, N.W.; et al. Quantitative flow ratio for immediate assessment of nonculprit lesions in patients with ST-segment elevation myocardial infarction-An iSTEMI substudy. Catheter. Cardiovasc. Interv. 2019, 94, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Milzi, A.; Dettori, R.; Marx, N.; Reith, S.; Burgmaier, M. Quantitative flow ratio (QFR) identifies functional relevance of non-culprit lesions in coronary angiographies of patients with acute myocardial infarction. Clin. Res. Cardiol. 2021. [Google Scholar] [CrossRef]
- Milzi, A.; Dettori, R.; Burgmaier, K.; Marx, N.; Reith, S.; Burgmaier, M. Quantitative flow ratio is related to intraluminal coronary stenosis parameters as assessed with optical coherence tomography. J. Clin. Med. 2021, 10, 1856. [Google Scholar] [CrossRef]
- Lenk, K.; Schwarzbach, V.; Antoniadis, M.; Blum, M.; Zeynalova, S.; Hagendorff, A.; Leistner, D.; Landmesser, U.; Lavall, D.; Laufs, U. Angiography-based quantitative coronary contrast-flow ratio measurements correlate with myocardial ischemia assessed by stress MRI. Int. J. Cardiovasc. Imaging 2020, 36, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.M.; Koning, G.; van Rosendael, A.R.; Dibbets-Schneider, P.; Mertens, B.J.; Jukema, J.W.; Delgado, V.; Reiber, J.H.C.; Bax, J.J.; Scholte, A.J. Relationship between coronary contrast-flow quantitative flow ratio and myocardial ischemia assessed by SPECT MPI. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1888–1896. [Google Scholar] [CrossRef]
- Shaw, L.J.; Berman, D.S.; Picard, M.H.; Friedrich, M.G.; Kwong, R.Y.; Stone, G.W.; Senior, R.; Min, J.K.; Hachamovitch, R.; Scherrer-Crosbie, M.; et al. Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc. Imaging 2014, 7, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Hachamovitch, R.; Rozanski, A.; Shaw, L.J.; Stone, G.W.; Thomson, L.E.; Friedman, J.D.; Hayes, S.W.; Cohen, I.; Germano, G.; Berman, D.S. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur. Heart J. 2011, 32, 1012–1024. [Google Scholar] [CrossRef]
- Shaw, L.J.; Berman, D.S.; Maron, D.J.; Mancini, G.B.; Hayes, S.W.; Hartigan, P.M.; Weintraub, W.S.; O’Rourke, R.A.; Dada, M.; Spertus, J.A.; et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: Results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008, 117, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Hachamovitch, R.; Hayes, S.W.; Friedman, J.D.; Cohen, I.; Berman, D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003, 107, 2900–2907. [Google Scholar] [CrossRef]
- Buckert, D.; Cieslik, M.; Tibi, R.; Radermacher, M.; Rottbauer, W.; Bernhardt, P. Cardiac magnetic resonance imaging derived quantification of myocardial ischemia and scar improves risk stratification and patient management in stable coronary artery disease. Cardiol. J. 2017, 24, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Dettori, R.; Milzi, A.; Frick, M.; Burgmaier, K.; Almalla, M.; Lubberich, R.K.; Marx, N.; Reith, S.; Burgmaier, M. Lesion geometry as assessed by optical coherence tomography is related to myocardial ischemia as determined by cardiac magnetic resonance imaging. J. Clin. Med. 2021, 10, 3342. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S.; et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.; Schuster, A.; Perera, D.; Nagel, E. Cardiac magnetic resonance imaging to guide complex revascularization in stable coronary artery disease. Eur. Heart J. 2010, 31, 2209–2215. [Google Scholar] [CrossRef]
- Simundic, A.M. Measures of diagnostic accuracy: Basic definitions. EJIFCC 2009, 19, 203–211. [Google Scholar]
- Moscarella, E.; Gragnano, F.; Cesaro, A.; Ielasi, A.; Diana, V.; Conte, M.; Schiavo, A.; Coletta, S.; Di Maio, D.; Fimiani, F.; et al. Coronary physiology assessment for the diagnosis and treatment of coronary artery disease. Cardiol. Clin. 2020, 38, 575–588. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Westra, J.; Ding, D.; Zhao, Q.; Yang, J.; Sun, Z.; Huang, J.; Pu, J.; Xu, B.; et al. Automatic coronary blood flow computation: Validation in quantitative flow ratio from coronary angiography. Int. J. Cardiovasc. Imaging 2019, 35, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Lee, S.H.; Lee, J.M.; Hwang, D.; Zhang, J.; Kim, J.; Im, S.Y.; Kim, H.K.; Nam, C.W.; Doh, J.H.; et al. Clinical relevance and prognostic implications of contrast quantitative flow ratio in patients with coronary artery disease. Int. J. Cardiol. 2021, 325, 23–29. [Google Scholar] [CrossRef]
- Tang, J.; Chu, J.; Hou, H.; Lai, Y.; Tu, S.; Chen, F.; Yao, Y.; Ye, Z.; Gao, Y.; Mao, Y.; et al. Clinical implication of QFR in patients with ST-segment elevation myocardial infarction after drug-eluting stent implantation. Int. J. Cardiovasc. Imaging 2021, 37, 755–766. [Google Scholar] [CrossRef]
- Filev, P.D.; Stillman, A.E. Long-Term prognostic value of stress perfusion cardiovascular magnetic resonance imaging. Curr. Treat Options Cardiovasc. Med. 2019, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.T.; Paul, M.; Morton, G.; Schuster, A.; Chiribiri, A.; Perera, D.; Nagel, E. Correlation of fractional flow reserve with ischemic burden measured by cardiovascular magnetic resonance perfusion imaging. Am. J. Cardiol. 2017, 120, 1913–1919. [Google Scholar] [CrossRef][Green Version]
- Puymirat, E.; Cayla, G.; Simon, T.; Steg, P.G.; Montalescot, G.; Durand-Zaleski, I.; le Bras, A.; Gallet, R.; Khalife, K.; Morelle, J.F.; et al. Multivessel PCI guided by FFR or angiography for myocardial infarction. N. Engl. J. Med. 2021, 385, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Biscaglia, S.; Guiducci, V.; Santarelli, A.; Amat Santos, I.; Fernandez-Aviles, F.; Lanzilotti, V.; Varbella, F.; Fileti, L.; Moreno, R.; Giannini, F.; et al. Physiology-guided revascularization versus optimal medical therapy of nonculprit lesions in elderly patients with myocardial infarction: Rationale and design of the FIRE trial. Am. Heart J. 2020, 229, 100–109. [Google Scholar] [CrossRef] [PubMed]
| n = 145 | |
|---|---|
| Age (years) | 64.2 ± 15.0 |
| Male sex (n, %) | 114 (78.6) |
| STEMI at initial presentation (n, %) | 58 (40) |
| NSTEMI at initial presentation (n,%) | 87 (60) |
| Detection of ischemia (n, %) | 26 (17.9) |
| LV-EF (%) | 49.9 ± 8.0 |
| CV Risk profile | |
| Diabetes mellitus (n, %) | 38 (27.0) |
| BMI (kg/m2) | 26.9 ± 3.5 |
| Hypertension (n, %) | 86 (61.4) |
| Current smoking (n, %) | 49 (36.0) |
| Pack Years (PY) | 27.3 ± 24.5 |
| Lab values | |
| Cholesterol (mg/dL) | 212.2 ± 147.0 |
| LDLc (mg/dL) | 140.9 ± 47.6 |
| HDLc (mg/dL) | 46.6 ± 13.5 |
| Triglycerides (mg/dL) | 109.9 ± 95.2 |
| HbA1c (%) | 6.1 ± 1.3 |
| Non-culprit lesion (vessel) | |
| LAD (n, %) | 67 (46.2) |
| LCx (n, %) | 35 (24.1) |
| RCA (n, %) | 33 (22.8) |
| Diagonal branch (n, %) | 4 (2.8) |
| Obtuse branch (n, %) | 5 (3.4) |
| RIM (n, %) | 1 (0.7) |
| n = 182 | |
|---|---|
| QFR-derived stenosis parameters | |
| QFR | 0.86 ± 0.08 |
| MLD (mm) | 1.36 ± 0.39 |
| Percent area stenosis (%) | 45.4 ± 9.0 |
| Lesion length (mm) | 23.9 ± 13.8 |
| Group 1 | Group 2 | Group 3 | p | pGroup3vs.1 | |
|---|---|---|---|---|---|
| QFR ≥ 0.90 | QFR 0.81–0.90 | QFR ≤ 0.80 | |||
| Area of ischemia (mm2) | 21.8 ± 85.1 | 26.3 ± 101.8 | 265.6 ± 249.0 | <0.001 | <0.001 |
| Ischemic burden (%) | 1.3 ± 5.5 | 1.8 ± 7.3 | 16.6 ± 15.6 | <0.001 | <0.001 |
| Transmurality of ischemia (%) | 5.6 ± 20.2 | 5.7 ± 21.1 | 53.5 ± 37.2 | <0.001 | <0.001 |
| Circumferential extent of ischemia (°) | 3.4 ± 12.3 | 5.8 ± 22.3 | 47.3 ± 35.2 | <0.001 | <0.001 |
| Intensity of myocardial ischemia index (%) | 3.0 ± 12.0 | 2.0 ± 9.3 | 22.0 ± 20.1 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dettori, R.; Frick, M.; Burgmaier, K.; Lubberich, R.K.; Hellmich, M.; Marx, N.; Reith, S.; Burgmaier, M.; Milzi, A. Quantitative Flow Ratio Is Associated with Extent and Severity of Ischemia in Non-Culprit Lesions of Patients with Myocardial Infarction. J. Clin. Med. 2021, 10, 4535. https://doi.org/10.3390/jcm10194535
Dettori R, Frick M, Burgmaier K, Lubberich RK, Hellmich M, Marx N, Reith S, Burgmaier M, Milzi A. Quantitative Flow Ratio Is Associated with Extent and Severity of Ischemia in Non-Culprit Lesions of Patients with Myocardial Infarction. Journal of Clinical Medicine. 2021; 10(19):4535. https://doi.org/10.3390/jcm10194535
Chicago/Turabian StyleDettori, Rosalia, Michael Frick, Kathrin Burgmaier, Richard Karl Lubberich, Martin Hellmich, Nikolaus Marx, Sebastian Reith, Mathias Burgmaier, and Andrea Milzi. 2021. "Quantitative Flow Ratio Is Associated with Extent and Severity of Ischemia in Non-Culprit Lesions of Patients with Myocardial Infarction" Journal of Clinical Medicine 10, no. 19: 4535. https://doi.org/10.3390/jcm10194535
APA StyleDettori, R., Frick, M., Burgmaier, K., Lubberich, R. K., Hellmich, M., Marx, N., Reith, S., Burgmaier, M., & Milzi, A. (2021). Quantitative Flow Ratio Is Associated with Extent and Severity of Ischemia in Non-Culprit Lesions of Patients with Myocardial Infarction. Journal of Clinical Medicine, 10(19), 4535. https://doi.org/10.3390/jcm10194535






