Perceived Challenges to Routine Uptake of the Ankle Brachial Index within Primary Care Practice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Questionnaire Distribution
2.2. Statistical Analysis
2.3. Research Ethics
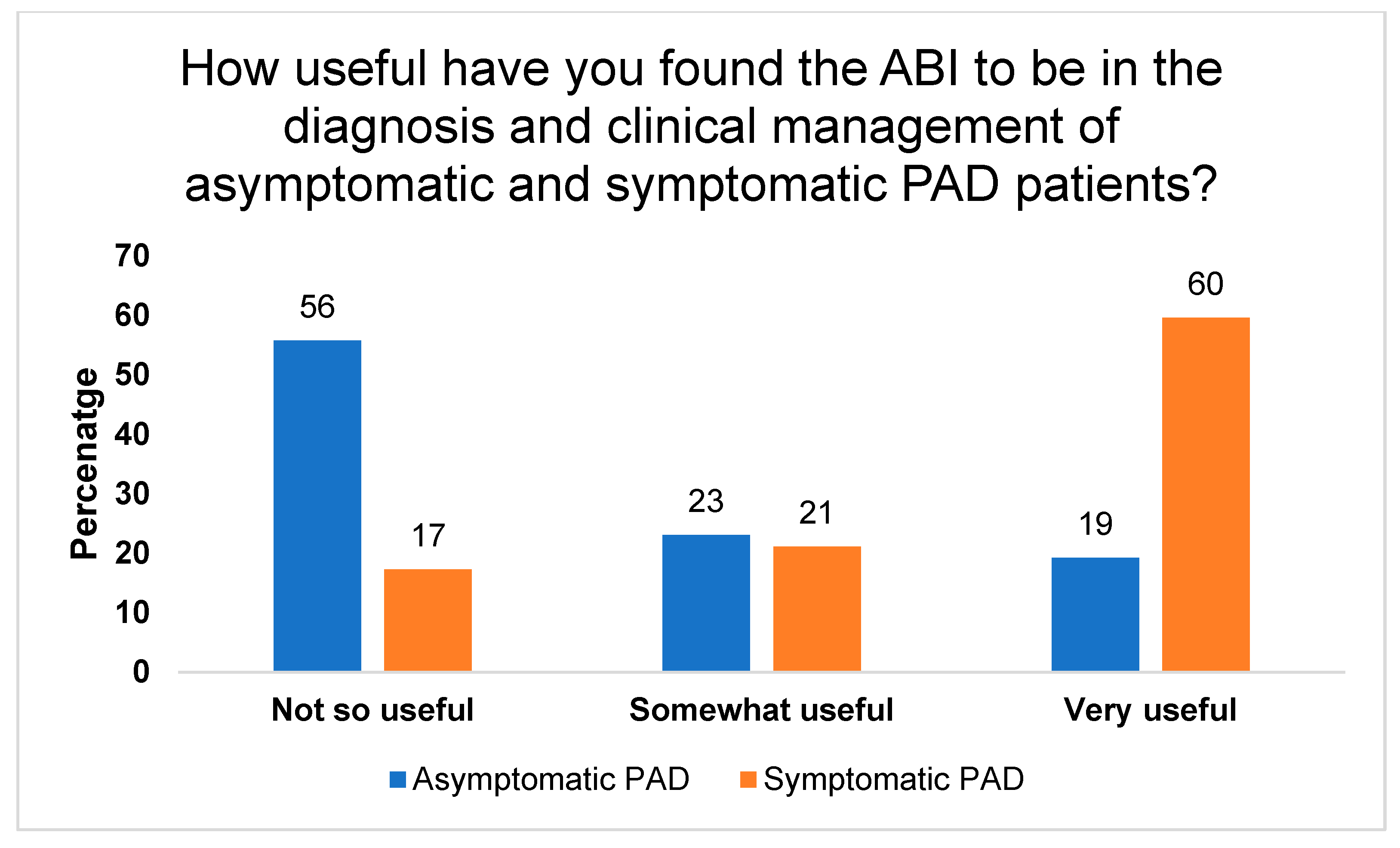
3. Results
3.1. Perceived Barriers, Limitations, and Feasibility of ABI Usage
3.2. Use of ABI in Primary Care Practice
3.3. Factors Important for Diagnosing PAD
3.4. Other Potential Diagnostic Methods/Tools
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannan, R.Y.; Dattani, N.; Sayers, R.; Bown, M. Survey of ankle–brachial pressure index use and its perceived barriers by general practitioners in the UK. Postgrad. Med. J. 2016, 92, 322–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, J.; Santulli, G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018, 275, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.J. A Cost Effectiveness Analysis of Using the Ankle-Brachial Index to Screen for Peripheral Artery Disease; University of California: Los Angeles, CA, USA, 2012. [Google Scholar]
- Guirguis-Blake, J.M.; Evans, C.V.; Redmond, N.; Lin, J.S. Screening for peripheral artery disease using the Ankle-Brachial Index: Updated evidence report and systematic review for the US preventive services task force. JAMA 2018, 320, 184–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkes, S.; Stansby, G.; Sims, A.; Haining, S.; Allen, J. Peripheral arterial disease: Diagnostic challenges and how photoplethysmography may help. Br. J. Gen. Pract. 2015, 65, 323–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, R.; Papia, G. Peripgeral Arterial Disease: A Hidden Danger. Wound Care Can. 2016, 14, 16–20. [Google Scholar]
- Schröder, F.; Diehm, N.; Kareem, S.; Ames, M.; Pira, A.; Zwettler, U.; Lawall, H.; Diehm, C. A modified calculation of ankle-brachial pressure index is far more sensitive in the detection of peripheral arterial disease. J. Vasc. Surg. 2006, 44, 531–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaï, S.P.; Kruidenier, L.M.; Rouwet, E.V.; Bartelink, M.-L.E.; Prins, M.H.; Teijink, J.A. Ankle brachial index measurement in primary care: Are we doing it right? Br. J. Gen. Pract. 2009, 59, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease). J. Am. Coll. Cardiol. 2006, 47, e1–e192. [Google Scholar]
- Davies, J.H.; Kenkre, J.; Williams, E.M. Current utility of the ankle-brachial index (ABI) in general practice: Implications for its use in cardiovascular disease screening. BMC Fam. Pract. 2014, 15, 69. [Google Scholar] [CrossRef] [Green Version]
- Hageman, D.; Pesser, N.; Gommans, L.N.; Willigendael, E.M.; van Sambeek, M.R.; Huijbers, E.; Snoeijen, A.; Scheltinga, M.R.; Teijink, J.A. Limited adherence to peripheral arterial disease guidelines and suboptimal ankle brachial index reliability in Dutch primary care. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 867–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Finance. Ontario’s Long-Term Report on the Economy. 2020. Available online: https://www.ontario.ca/page/ontarios-long-term-report-economy (accessed on 10 July 2021).
- Wilson, A.M.; Kimura, E.; Harada, R.K.; Nair, N.; Narasimhan, B.; Meng, X.-Y.; Zhang, F.; Beck, K.R.; Olin, J.W.; Fung, E.T. β2-Microglobulin as a biomarker in peripheral arterial disease: Proteomic profiling and clinical studies. Circulation 2007, 116, 1396–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckman, J.A.; Preis, O.; Ridker, P.M.; Gerhard-Herman, M. Comparison of usefulness of inflammatory markers in patients with versus without peripheral arterial disease in predicting adverse cardiovascular outcomes (myocardial infarction, stroke, and death). Am. J. Cardiol. 2005, 96, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Arpegård, J.; Östergren, J.; De Faire, U.; Hansson, L.O.; Svensson, P. Cystatin C—A marker of peripheral atherosclerotic disease? Atherosclerosis 2008, 199, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Ho, L.A.; Denenberg, J.O.; Ridker, P.M.; Wassel, C.L.; McDermott, M.M. Biomarkers in peripheral arterial disease patients and near-and longer-term mortality. J. Vasc. Surg. 2010, 52, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; Stampfer, M.J.; Rifai, N. Novel risk factors for systemic atherosclerosis: A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein (a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001, 285, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.H.; Zamzam, A.; Khan, H.; Singh, K.; Forbes, T.L.; Rotstein, O.; Abdin, R.; Eikelboom, J.; Qadura, M. Fatty acid binding protein 3 is associated with peripheral arterial disease. JVS Vasc. Sci. 2020, 1, 168–175. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Zakharyan, A.; Fung, E.T.; Crutcher, G.; Smith, A.; Stanford, C.; Cooke, J. A validated biomarker panel to identify peripheral artery disease. Vasc. Med. 2012, 17, 386–393. [Google Scholar] [CrossRef] [PubMed]
| Limitations | Major Limitation n (%) | Minor Limitation n (%) | No Limitation n (%) |
|---|---|---|---|
| Time Constraint | 40 (77%) | 8 (15%) | 4 (8%) |
| Financial Constraint | 18 (35%) | 15 (29%) | 19 (36%) |
| Clinical Significance | 12 (23%) | 14 (27%) | 24 (46%) |
| Staff Availability | 34 (65%) | 13 (25%) | 4 (8%) |
| Patient Willingness | 8 (15%) | 11 (21%) | 33 (64%) |
| Presence of Wounds | 16 (31%) | 15 (29%) | 20 (39%) |
| ABI Interpretation | 31 (60%) | 11 (21%) | 10 (19%) |
| Patient Population | Yes n (%) | No n (%) |
|---|---|---|
| Healthy Patients | 5 (10%) | 47 (90%) |
| Diabetics | 43 (83%) | 9 (17%) |
| Chronic Renal Failure | 26 (50%) | 26 (50%) |
| Elderly (>65 Years old) | 31 (60%) | 21 (40%) |
| Factors | Agree n (%) | Disagree n (%) |
|---|---|---|
| Risk Factors | 48 (92%) | 4 (8%) |
| Pulse Examination | 44 (85%) | 8 (15%) |
| Questionnaires | 8 (15%) | 44 (85%) |
| Ankle-Brachial Index (ABI) | 45 (87%) | 7 (13%) |
| Toe Brachial Index (TBI) | 6 (12%) | 46 (88%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, L.Y.C.; Syed, M.H.; Zamzam, A.; Rotstein, O.D.; Abdin, R.; Laraya, N.; Qadura, M. Perceived Challenges to Routine Uptake of the Ankle Brachial Index within Primary Care Practice. J. Clin. Med. 2021, 10, 4371. https://doi.org/10.3390/jcm10194371
Chiu LYC, Syed MH, Zamzam A, Rotstein OD, Abdin R, Laraya N, Qadura M. Perceived Challenges to Routine Uptake of the Ankle Brachial Index within Primary Care Practice. Journal of Clinical Medicine. 2021; 10(19):4371. https://doi.org/10.3390/jcm10194371
Chicago/Turabian StyleChiu, Lily Y. C., Muzammil H. Syed, Abdelrahman Zamzam, Ori D. Rotstein, Rawand Abdin, Nadine Laraya, and Mohammad Qadura. 2021. "Perceived Challenges to Routine Uptake of the Ankle Brachial Index within Primary Care Practice" Journal of Clinical Medicine 10, no. 19: 4371. https://doi.org/10.3390/jcm10194371
APA StyleChiu, L. Y. C., Syed, M. H., Zamzam, A., Rotstein, O. D., Abdin, R., Laraya, N., & Qadura, M. (2021). Perceived Challenges to Routine Uptake of the Ankle Brachial Index within Primary Care Practice. Journal of Clinical Medicine, 10(19), 4371. https://doi.org/10.3390/jcm10194371










