Abstract
Background: Nearly two decades have passed since a paradoxical reaction in the orofacial region to some bone modifying agents and other drugs was recognized, namely medication-related osteonecrosis of the jaw (MRONJ). Purpose: The aim of this manuscript was to critically review published data on MRONJ to provide an update on key terminology, concepts, and current trends in terms of prevention and diagnosis. In addition, our objective was to examine and evaluate the therapeutic options available for MRONJ. Methods: The authors perused the most relevant literature relating to MRONJ through a search in textbooks and published articles included in several databases for the years 2003–2021. Results and conclusions: A comprehensive update of the current understanding of these matters was elaborated, addressing these topics and identifying relevant gaps of knowledge. This review describes our updated view of the previous thematic blocks, highlights our current clinical directions, and emphasizes controversial aspects and barriers that may lead to extending the accumulating body of evidence related to this severe treatment sequela.
1. Introduction
Avascular necrosis, also known as osteonecrosis, is a generic term referring to the ischemic death of the constituents of bone [1,2]. Osteonecrosis has a wide variety of causes and can affect nearly any bone in the human body, counterpart individually each osteonecrosis-affected bone has unique clinical, etiologic, and prognostic factors. Bone infarction begins when the blood supply is discontinued. Once this infarct is established, a central necrotic core surrounded by an ischemic zone is commonly found [3,4]. Osteomyelitis of the jaw is a kind of avascular necrosis, characterized by infection and inflammation of the bone marrow in the bones of the jaw (i.e., maxilla and/or the mandible) [5,6]. In contrast to avascular osteonecrosis in other bones, in jaws, the main cause is the spread of adjacent odontogenic infection, whereas the second most common cause is trauma. During the 19th and early 20th centuries, two historic occupational diseases brought on by the ingestion and subsequent absorption of radium and white phosphorus into the bones were described [7,8]. A related condition, initially named bisphosphonate-associated osteonecrosis of the jaw (BRONJ), was initially described as a side-effect of amino-bisphosphonates by Marx in 2003 [6], a class of phosphorus-based drugs that inhibit bone resorption which were and are used widely for treating osteoporosis, bone metastases in cancer and some other conditions mainly to reduce fractures incidence fractures and other skeletal-related events [9].
Five years later, new oncology drugs and other types of antiresorptive therapies were progressively linked to this avascular necrosis from case reports to adverse events of long-term follow-up of randomized trials [10,11]. In addition, anti-angiogenic agents such as monoclonal antibodies against VEGF (i.e., Bevacizumab, Aflibercept), tyrosine kinase inhibitors (i.e., Sunitinib, Sorafenib, Imatinib, Axitinib, and Cabozantinib), and mTOR inhibitors (i.e., Temsirolimus and Everolimus) [12,13,14]. Although, some of these cases are related to a concomitant consumption of antiresorptive drugs or to a previous discontinuation [12,15]. Other drugs were also related to this type of jaw avascular necrosis such as anti-TNF factors (Etanercept, Adalimumab, Infliximab), anti-CD20 antibodies (Rituximab), and other immunosuppressive drugs such as methotrexate, prednisolone, or tocilizumab. Even some selective estrogen receptor modulator such as tamoxifen and raloxifene has been related to MRONJ [13,16]. The list of agents responsible for this outcome continues to rise but a poor level of evidence is generalized due to this level being based mainly on case reports and case series which jeopardize the current understanding of this adverse event [17]. Moreover, it is important to remember that exposed bone or sequestra can occur in patients not exposed to these drugs [18,19].
The explained rationale switched the definition to medication-related osteonecrosis of the jaw (MRONJ). This term is supported by the American Association of Oral and Maxillofacial Surgeons (AAOMS) in its latest position paper update [20]; although it is worth mentioning that the 2015 International Taskforce on Osteonecrosis of the Jaw consensus paper removed anti-angiogenics due to a lack of evidence at the time [21]. Two previous position papers, one by the American Society for Bone and Mineral Research (ASBMR) back in 2007 and another previous one by the AAOMS started touting this treatment sequela [20,22]. So was conceived a severe bone disease currently known as MRONJ.
The objectives of this study were: to critically review published data on MRONJ to provide an update on key terminology and concepts and current trends in terms of prevention and diagnosis. Finally, we evaluate the possible usefulness of current treatment strategies in MRONJ.
2. Methods
All papers and clinical reviews of MRONJ in the electronic databases (Medline, Embase, Scopus, and the Cochrane Library) published from January 2003 to January 2021 in any language have been evaluated. We also perused relevant textbooks and abstracts from our institution’s library catalog. Independent research in relevant related-content journals was also performed, including Bone; British Journal of Oral and Maxillofacial Surgery; International Journal of Oral and Maxillofacial Surgery; Journal of Bone and Mineral Research; Journal of Oral Maxillofacial Surgery; Journal of Oral Pathology and Medicine; Journal of Cranio-Maxillofacial Surgery; Oral Diseases; Oral Oncology and Oral Surgery, Oral Medicine Oral Pathology, and Oral Radiology. We also examined the references of every article retrieved and those of recent reviews to trace further publications or reports.
3. Definition
AAOMS update of 2014, reported that a patient is considered to have MRONJ if all the following conditions are met: (i) current or past treatment with antiresorptive or antiangiogenic drugs; (ii) exposed bone or intra- or extraoral fistulisation in the maxillofacial region communicating with the bone and persisting for more than 8 weeks; (iii) no history of maxillary radiotherapy or clear maxillary metastatic disease [20].
It is worth mentioning that AAOMS and ASBMR definitions slightly differ. AAOMS introduces the statement “obvious metastatic disease”, which is not included in the article from the ASBMR position paper [20,21].
As stated, the diagnosis is essentially clinical-driven, although with nuances [23,24]. AAOMS did introduce a possible definition of this outcome (referred to as stage 0) as some authors suggested, corresponding to patients with symptoms but no exposed bone [25,26]. In this vein, AAOMS at the time of its latest position paper considered this variant as prodromal, and that over time up to 50% of these patients will progress to MRONJ stages 1, 2, or 3 [20]. In fact, later research confirmed this progression rate to frank bone exposure may be plausible [27]. Nonetheless, some authors considered this point-of-view erroneous due to it neglects of up to a quarter of affected individuals, so on being precipitant for delayed diagnosis and ultimately contributing to poorer control [28]. In this sense, this group of experts suggests that radiological examination via orthopantomography, cone-beam computed tomography, and magnetic resonance imaging are mandatory exploratory measures to complement clinical examination to detect plausible stage 0 cases but also on other stages to assess their extent and involvement of neighboring tissues [29]. AAOMS stated that this stage may overestimate the true disease frequency by including false-positive values due to its based on nonspecific clinical findings, radiographic changes, and symptoms that may overlap with other jawbone alterations such as osteomyelitis, osteoradionecrosis, alveolar osteitis, sinusitis, fibro-osseous lesions, chronic sclerosing osteomyelitis or oral ulceration and bone sequestration [20]. This ambiguity emphasizes the need for a more precise definition of non-exposed-MRONJ to address this complex differential diagnosis [30].
4. Pathogenesis
Despite the increasing amount of literature generated over the years, the pathogenesis of MRONJ is still not completely elucidated [31]. Many theories have been proposed for the pathogenesis of MRONJ [32]. Taken together, this research suggests it is probably multifactorial, with important roles for infection, inflammation, and trauma to the bone or soft tissue amplified by an altered bone remodeling or over suppression of bone resorption and angiogenesis inhibition [23,33,34]. Other alternative theories or others trying to integrate all these factors were also postulated or even partially studied [30]. These three main etiological fractions are discussed in-depth.
4.1. Infection, Inflammation, and Trauma
Invasive dental treatments (IDTs) and periodontal disease (PD) have been considered as potential risk factors of MRONJ; however, the association between these exposures and MRONJ remains controversial [35]. Dental treatments are considered IDTs when the treatments may cause bleeding and introduce oral bacteria into the bloodstream, such as extraction, scaling and root planning, implant placement, and any kind of oral surgery. IDTs as PD can yield temporary bacteraemia able to cause a microbial immune subversion that triggers systemic inflammation. In this vein, scanning electron microscope analysis from MRONJ lesions has revealed microbial biofilm formation on sequestered bone, and in this dysbiosis seems to be the regular presence of Actinomyces species [36]. The critical role of bacterial infection in the pathogenesis of MRONJ may be justified by its decreased incidence in patients following improvement in their dental hygiene [35,37,38]. Several animal models have shown that inflammation or bacterial infection and systemic antiresorptive drugs are sufficient to induce MRONJ and that at the same time the presence of previous necrosis seems not to be a prerequisite [39,40]. In this context, some of the bacteria responsible for this dysbiosis can produce lipopolysaccharides that are able to increase cytokine production or directly regulate the production of receptor activator of nuclear factor κB ligand (RANKL). These circumstances can modify the bone matrix by osteoclast reprogramming. These cells may produce an exacerbated number of osteolytic proteins such as H+-ATPases, V-ATPase, and chloride channel 7 [41,42]. This process alters natural homeostasis triggering acidification processes able to alter bone turnover [23]. In this vein, Otto et al. postulated that localized change in pH caused by dentoalveolar infections or IDTs may be the initial context of MRONJ onset [43]. This group later demonstrated in vitro that bisphosphonates and a local acidic milieu reduce cell viability and activity of immortalized mesenchymal stem cells [44]. Dayisoylu et al. also demonstrated that an alkaline environment can prevent MRONJ by an in vivo study [45]. It is also worth mentioning that recently indigenous microbiota seems to protect against MRONJ onset according to a mice-based study [46].
Apart from these mechanisms, altered host immune response sense another as important as the infection itself. The immune cells and macrophages are involved in the wound healing process [47]. It has been suggested that macrophages may initially bond to bisphosphonates instead of osteoclasts and the presence of this antiresorptive significantly alters macrophage viability and morphology in vitro [40,48]. This theory seems valid considering the lack of affinity between bisphosphonates and osteoclast and the superior accumulation of these drugs on jaws in relation to the rest of the skeleton [31].
4.2. Altered Bone Remodelling or Oversuppression of Bone Resorption
Bisphosphonates and other antiresorptive drugs, inhibit osteoclast differentiation and increase cell death. Moreover, adequate bone remodeling capacity is thought to be critical in the defense against infection and accumulating microfractures [49,50]. The increased bone resorption in the setting of oral conditions, coupled with the thin overlying mucosa and a direct pathway through the periodontal ligament with the external environment, make the jaws a suitable breeding ground for MRONJ to develop [51]. It is worth mentioning that BPs can play a pivotal role in mesenchymal stem cells of the oral cavity. Particularly, BPs at periodontal ligament stem cells can cause impairment by inducing apoptosis in a dose-dependent manner [52,53]. To combat the effects of bone turnover suppression at the jawbone, withdrawing antiresorptive medications before tooth extraction of surgical procedures is often advocated to potentially reduce the risk of MRONJ, and in this line, the concept “drug holidays” was made. No human studies have evidence regarding its usefulness [54,55]; nonetheless, an animal-based study showed this method as promising both in the case of denosumab and bisphosphonates [54]. Other alternatives to mitigate this impaired bone remodeling have been proposed such as the use of parathyroid hormone and its derivatives or optimal daily vitamin D intake [20].
4.3. Altered Angiogenesis
Bone becomes necrotic without adequate blood supply [56]. In the case of MRONJ, bisphosphonates can contribute to the pathogenesis of MRONJ due to their ability to reduced blood vessel formation causing delayed mucosal healing [57]. It has been reported that various antiangiogenic agents, such as VEGF antibodies and tyrosine kinase inhibitors, can also cause jaw necrosis and therefore the development of MRONJ [15]. Unfortunately, it is still unclear if and how simultaneous or time-shifted use of bisphosphonates and further antiangiogenic agents increases the risk and the extent of MRONJ development as debated by the AAOMS and ASBMR [20,21]. The bisphosphonate compound zoledronic acid indirectly impaired angiogenesis via targeting MMP9 expressing macrophage, which, curiously, is one of the most altered molecules in the pathogenesis of periodontal diseases. It is plausible that bisphosphonates can block angiogenesis by interfering with endothelial cell proliferation and survival via apoptosis although the level of evidence is low some studies at a genetic and transcriptional level have help for the field to move forward [48,58,59].
In the case of denosumab, this monoclonal antibody seems to not induce soft tissue toxicity so in this vein angiogenesis has not proved to be linked to this kind of lesions [60]. Nevertheless, patients who have taken bisphosphonate drugs at any time in the past and those who have taken denosumab in the last nine months are allocated to a risk group as if they were still taking the drug [61].
5. Incidence
Rare outcomes require an evidence-based estimate of the global point prevalence to inform public policy. Estimating the global prevalence of rare drug adverse events is challenging foremost for the diversity of the data, which are derived from a variety of disparate information sources that are not standardized and difficult to pool [62]. Anecdotally, a Swedish retrospective cohort study reported that a general dentist practitioner may expect to see a case of MRONJ every 62 years in clinical practice [63]. The risk of MRONJ among patients with cancer exposed to antiresorptive or antiangiogenic medication is about 1% (range 0.2–6.7%), whereas this risk is about 0.1% (range 0.004–0.2%) among patients who are being treated for osteoporosis using antiresorptive therapy. The risk of MRONJ is greater in patients with cancer than in those receiving antiresorptive treatments for osteoporosis by a factor of 10 [64]. A recent meta-analysis found that the use of denosumab is associated with a significantly higher risk of developing MRONJ compared to zoledronate in cancer patients, although authors reported serious plausible biases within its report [65]. A recent multicenter retrospective cohort study involving 22 secondary care centers elucidated data stratified by bisphosphonates type, a time of 6.0 and 2.2 years of oral alendronate and intravenous zoledronate therapy, respectively, and a time of 5.3 and 2.2 years of therapy is required for 50% of patients with osteoporosis and cancer to develop MRONJ [66]. To conclude, all these numbers are simply approximations, but they can be promptly translated into clinical application to inform the design of clinical trials, epidemiological studies. Further research, notably through long-term population registries and the implementation of a specific codification in healthcare systems, will help to refine our presented MRONJ estimates in terms of epidemiology [67].
6. Risk Factors
Triggering factors and risk factors for MRONJ, comorbidities, and medications should be explored as examined individually in light of the foregoing considerations.
Triggering factors risk factors, and so the first group, are classically identified as IDT or oral pathologies such as PD. The most reported dental risk factors are classically dental extraction, periodontal disease, other kinds of oral surgeries, dental implant placement, infection/abscess, or trauma derived from ineffective prosthetic solutions [10,35,68,69]. There has been clinical evidence that wearing ill-fitting dentures is also one of the MRONJ risk factors [70]. Nonurgent procedures should be delayed [21]. It is possible that some of the cases described can be linked mainly to just single local risk factors use but to rule out a putative risk factor based on the presence of another appears inappropriate [71]. Risk factors are not etiologic agents, and such an approach would not allow the identification of new risk factors or categorization of the present ones [72]. Therefore, it is reasonable to suggest that optimizing the health of the oral cavity by reducing inflammatory and infection burden to prevent the need for future invasive treatment must be the prudent strategy in at-risk patients [73]. It is worth mentioning that the only independent risk factor linked to MRONJ is tooth extraction despite the advanced identification of other risk factors [33]. Although, some controversies are still discussed due to some authors reporting that underlying pre-existing dental/periodontal infection rather than the surgery per se may act as the real starting point [74,75].
An interdisciplinary and oncologist, rheumatologist general dental professionals are essential [76]. A dentist should also be provided with information about the patient’s medical diagnosis and current therapy, or the future therapy established and its duration. Controllable risk factors (i.e., modifiable) should be minimized, and comprehensive oral care monitoring as recommended by the AAOMS should be established [20].
A recent systematic review pooled 39 different systemic diseases and 14 medical conditions as potential MRONJ risk factors [35]. In the case of systemic diseases, excluding those involving the use of these drugs, cardiovascular diseases and rheumatoid arthritis are highlighted [77,78,79]. Among the medical factors: chemotherapy, corticosteroids, smoking vitamin D deficiency, renal dialysis, anemia, Paget’s disease of bone, erythropoietin therapy, cyclophosphamide therapy, alcohol intake, and obesity are the most frequently reported [80,81,82]. Due to the scarce number of longitudinal studies involving this outcome, results make it difficult to quantitatively assess the thresholds for the level of damage of each of them individually [83].
It has also been well documented that advanced age is one of the significant risk factors for developing MRONJ. Occurring most commonly in sexagenarians and septuagenarians. Thus, in the day-to-day practice of gerodontology, strict surveillance is of paramount importance.
Whether genetic variation is a predictor for the development of medication-related osteonecrosis of the jaws (MRONJ) has been recently addressed in a systematic review finding that all studies have failed to show a single gene as a risk factor for MRONJ [84]. In terms of biomarkers at a translation or post-translational level according to recent reviews, none has achieved prominence or efficiency [85,86]. Marx et al. once identified serum CTX as a useful tool for risk assessment and treatment planning. This proposal has been strongly discarded by further studies [87]; all biomarker studies are invalid because all are made after the time-point of diagnosis, so the notion of prospection is totally absent [23]. Nowadays, it remains impossible to use any available biomarker with predictive or prognostic utility for these outcomes.
7. Prevention
Patients at higher risk for MRONJ should be placed on short follow-up intervals to maintain oral health and identify necrosis at the earliest stage possible. The onset of MRONJ may be subtle; so routine radiographic evaluation for hard tissue radiolucency may indicate the onset of an early stage (i.e., 0). So, in any kind of suspicious jaw region of at-risk patients, they are encouraged [25,27,28,29].
Several prophylactic protocols have been proposed for preventing this complication, including antiseptic rinses immediately before extraction and until healing of the socket, antibiotic prophylaxis, alveoloplasty with primary closure, fibrin, or autologous platelet-rich plasma, ozone therapy, limitation of the number of extractions performed in each session [69,76,88]. According to a recent metanalysis including six studies with 2332 cancer patients, dental preventive measures decreased MRONJ incidence by 77.3% (95% CI = 47.4–90.2%; p = 0.001) compared to at-risk control groups [69]. Particularly, the efficacy of autologous platelet concentrate (APC) applications in the prevention of MRONJ together with surgical debridement has not proved sufficient effectiveness for implementation [89].
In terms of non-dentoalveolar-related measures, the drug holiday concept (i.e., temporary discontinuation of the medication) in at-risk patients has been the subject of debate. Only one study to date confirmed this rationale [90] but based on the current body of evidence it seems negligible [55]. Theoretically, the risk of MRONJ diminishes when the frequency of administration of medications is reduced, so a reduced drug schedule may be a useful tool to prevent this severe adverse effect [91]. The half-life of bisphosphonates has been reported to be more than 10 years due to the higher affinity to hydroxyapatite, although that of denosumab is about 26 days after administration [92]. On the other hand, it is well known that suspension of drug therapy can create different cost–benefit balances, according also to the type of medication used; in some cases, even this drug suppression can affect the primary disease that precipitated the use of these drugs triggering an even worse outcome in the well-being of patients. It is important to reinforce the concept that the typical patient with MRONJ, apart from it, generally suffers general frailty. So, each case must be individualized for the benefit of the patient bearing in mind the entire conundrum of pathologies [93]. Taking together available recommendations on the management of individuals using or scheduled for their intake are hindered by controversy and a lack of evidence, and merely a reflection of panels of expert opinions [94,95].
8. Staging and Prognostication
One of the most important issues in the development of a staging system for MRONJ is to aid in the selection of appropriate treatment and foresee a prognosis. The establishment of globally accepted criteria for the management of patients is imperative to achieve proper treatment approaches. However, from the introduction of the initial introduction by Ruggiero et al. [96], new staging systems have been constantly introduced jeopardizing the body of literature produced year after year in terms of longitudinal studies [97]. In this section, we report and discuss the most current accepted and up-to-date staging system to achieve consensus [20] (Figure 1). Therefore, current treatment strategies for MRONJ have been constructed based on clinical aspects rather than scientific evidence. The treatment strategy for MRONJ at each stage is introduced based on several position papers, and other studies including systematic reviews and consensus statements [20,21,23,30,82].
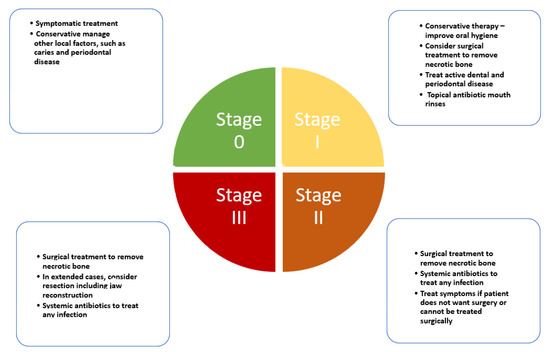
Figure 1.
Clinical pathway for medication-related osteonecrosis of the jaw according to AAOMS latest update.
The following contents are an elaboration of how such staging is designed to combat MRONJ stages and to improve understanding and individual decision-making.
8.1. Stage 0
Stage 0 is characterized as a non-exposed variant of MRONJ and presents with nonspecific clinical signs, symptoms, and radiographic features such as: obscuring of the periodontal ligament, changes to the trabecular pattern, or osteosclerosis [98,99]. Reports have shown that the so-called nonexposed form of MRONJ might represent 13 to 20% of all cases of MRONJ [100]. It has been reported that 50% of patients in Stage 0 have progressed to a worse staging [101]. It is worth mentioning that diagnosing patients who have some symptoms without exposed bone as Stage 0 MRONJ, may also produce overdiagnosis [102]. For this rationale, specifical diagnostic tools are necessary [29]. However, symptomatic treatment and conservative management are recommended for patients with Stage 0 [25].
8.2. Stage 1
AAOMS defined this stage as a clinical scenario where exposed and necrotic bone, or fistulae that probes to be bone coexists in patients who are normally asymptomatic and have no evidence of infection [20]. Less frequently other oral signs are displayed such as: dental mobility; mucosal fistula; swelling; abscess; trismus; mandibular deformity or local hypoesthesia [103]. It may also present with radiographic findings overlapped with those described in Stage 0 [104,105].
8.3. Stage 2
This stage is characterized by exposed and necrotic bone or fistulae that probe to bone, with evidence of infection. These patients are typically symptomatic. These patients may also present with radiographic concordant with Stage 0.
8.4. Stage 3
Clinical signs and symptoms are the same as stage 2. Nonetheless, according to AAOMS one of the following features must also be present to establish diagnosis: exposed necrotic bone extending beyond the region of alveolar bone, i.e., inferior border and ramus; jaw fracture; extra-oral fistula; oral antral/oral–nasal communication; osteolysis extending to the inferior border of the mandible or sinus floor [20]. Some radiographic signs are also characteristic, such as diffuse osteosclerosis, with or without the following signs combined with the prominence of the inferior alveolar nerve canal, periosteal reaction, or sequestra formation.
Any classification should include all clinical possibilities to render studies comparable. The latest AAOMS 2014 update seemed to adopt all the proposals and critics. In this vein, we considered it as a quasi-gold standard able to guide MRONJ research properly [20]. Nevertheless, in our modest opinion, there are a few prevailing concerns about this classification: (i) MRONJ Stages 2 and 3 definitions are difficult to distinguish clearly, since some position papers, clinical reviews, and a clinical guideline do not provide the exact limit to establish exact thresholds [106]; (ii) the importance of the presence or absence of symptoms (i.e., divide each phase into a or a b according to presence or absence [97]; (iii) present classification/staging does not adequately capture the extension and severity of bone affected [30].
9. Management
There is no gold standard therapy defined in the literature, and the successful treatment of MRONJ remains elusive [3]. Expert opinion-based recommendations for the management of MRONJ included in the latest AAOMS position paper are primarily based on staging [20]. The goal of MRONJ therapy should be control of infection, progression of bone necrosis, ease associated pain, and ultimately improving the quality of life of patients [107].
According to the AAOMS report, the treatment modality for the initial stages should always be conservative and elective surgical procedures should be avoided [20]. Added to staging, conservative shall be initiated based on an in-depth microbiological study (i.e., bacterial cultivation and susceptibility testing) and radiological analysis (i.e., CT, MRI, or nuclear imaging) [108,109].
Commonly establish a treatment for 10–15 days with the appropriate antibiotic, in parallel with chlorhexidine rinses (once every 12 h for a month). In the case of normal flora, it is recommended to use amoxicillin/clavulanic acid, clindamycin, although the use of tetracyclines is also accepted [110]. In the case of the presence of bacterial species resistant against β-lactamase inhibitors, fluoroquinolones are the ones to use [111]. The healthcare professional should carry out irrigation of the exposed necrotic bed with 0.12% chlorhexidine—once every 72 h for four weeks. Then, the lesion should be re-evaluated one month later. If an improvement is confirmed the patient should continue with the 0.12% chlorhexidine rinses for another month and the professional’s application every 72 h [111,112]. In the case of a continuum suppuration, a curettage and regularization of the areas with irregular bone anatomy is recommended. In those cases, showing small areas of bone exposure after the first revaluation, the use of a soft laser to treat the necrotic bone to reach the bleeding healthy bone is recommended [113]. This minimally invasive technique allows to create micro-perforations on the bone basis and thus stimulating angiogenesis and mitigating inflammation and infection consequently [114].
Such planning is recommended in cases where there is no obvious disease progression, or uncontrolled pain because of MRONJ. It is essential to consider the individual response to treatment to switch to surgical approaches. In this vein, our opinion differs from the AAOMS mantra that the treatment modality should always be conservative and elective surgical procedures should be avoided due to a risk of extension of the areas of bone exposure or aggravation of the symptoms [115]. As related at a Workshop of the European task force on medication-related osteonecrosis of the jaw position document, a recent body of evidence has evidenced the equal or even better performance of early surgical interventions versus conservative approaches in early stages of MRONJ [30,104,116]. It is also worth mentioning that to surgically treat patients in a bad condition or with a poor life expectancy is not reasonable [33].
Surgery approaches are therefore indicated for patients with MRONJ whose disease does not respond to conservative treatment or is deemed unlikely to respond to conservative approaches from the start due to its advanced stage [117]. We believe that the protocol described by Wilde et al. is the most accurate in terms of our department’s results: “A full-thickness mucoperiosteal flap should be high and extended to reveal the entire area of exposed bone and beyond to disease-free margins; resection of the affected bone should be extended horizontally and inferiorly to reach healthy-appearing, bleeding bone; sharp edges should be smoothed; and primary soft tissue closure achievement” [118]. We defend the use of platelet-rich concentrates, preferable leukocyte platelet-rich fibrin, following Choukroun’s method but always associated with surgical management [119,120]. Although, it is worth mentioning that according to a recent systematic review the application of APCs did not show an unequivocal improvement versus surgical treatment alone [89].
According to a recent systematic review, a diversity of promising flap options was reported by several authors with promising results (for an extended overview please see [121]). Particularly our group, in cases of advanced MRONJ stages, opts to perform the bone resection and, when necessary, reconstruction of the area with a microvascularized flap [122]. We also reported that surgical neurolysis of the inferior alveolar nerve may be considered as the choice therapeutic technique to treat neuropathic pain when presented after initial surgery [123]. It is worth mentioning that the use of obturators of MRONJ cases allocated at the upper jaw has been also certified as a promising intervention [124]. There are also some pioneering experiences performed by a limited group of research groups such as the use of fluorescence-guided surgery or piezoelectric surgery which have shown promising results in terms of complete mucosal healing [125,126]. During surgical debridement, a fluorescence lamp can be used intraoperatively as a guide for delimiting resection margins of necrotic bone. The most often used light device was the VELScope®® system [127]. According to a recent systematic review, further prospective studies with larger samples are still required to ascertain its clinical validity [128].
Figure 2 shows two examples of management for a spontaneous stage I case, where also shows the treatment for a stage II MRONJ case related to a dental extraction both cases treated in our facilities. Figure 3 displays the management of a stage III case related to dental implants placement.
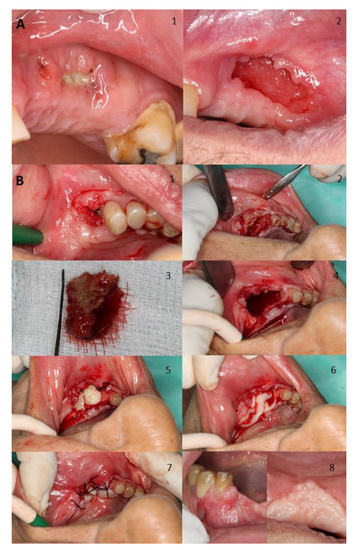
Figure 2.
(A) Man aged 77 years with prostate cancer without symptomatology developed stage I MRONJ in his left maxilla with bone exposure after receiving zoledronate for suspected bone metastases. Image 1 relates to initial presentation, whilst the second one shows the outcome of treatment with antibiotics and chlorhexidine treatment for 2 months; bone gradually sequestered over time and the soft tissue as the soft tissue eventually closed. (B) Woman aged 81 years with osteoporosis who developed stage II MRONJ with bone exposure in her left mandible with severe pain. The patient was initially treated with risedronate but was eventually switched to denosumab. Triggers of necrosis were mainly severe chronic periodontal disease and a dental extraction (Image 1). Initially we performed an incision and flap elevation to visualize affected area (Image 2). Then resection of necrotic bone was performed (Images 3 and 4). Then, an application of L-PRF by Choukroun’s method was performed and primary wound closure was achieved (Image 5, 6, and 7). A month after treatment total resolution was achieved (Image 8).
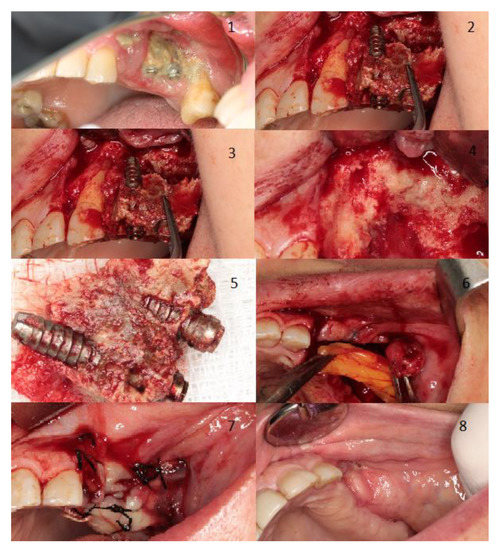
Figure 3.
Woman aged 81 years with osteoporosis treat with alendronate. The patient developed stage 3 MRONJ with large areas of bone exposure and pus exudation on the left maxilla plus a chronic oroantral fistula on the left maxillary sinus. The patient experienced severe pain and had severe signs of infection. The trigger factor was dental implants placement (Image 1). Initially, we performed an incision and flap elevation to visualize the affected area (Image 2). Then, two extractions were performed due to the poor dental prognosis, implant extraction, and resection of necrotic bone was also performed (Images 3–5). To close the oroantral fistula, an intraoral bichectomy was performed to harvest tissue. Obtained tissue was placed in the resection area and primary wound closure was achieved (Images 6 and 7). Two months after treatment, total resolution was achieved (Image 8).
On the other hand, adjunctive or alternative options for conservative and more radical options have also been proposed such as α-tocopherol, pentoxifylline, ozone therapy, hyperbaric oxygen treatment, or the use of laser therapy with Er:YAG or low light laser therapy [129,130]. Systemic administration of teriparatide with or without a local delivery of recombinant human bone morphogenic growth factor 2 has also shown promising results [131,132]. It is important to emphasize that most of this body of literature relies on case series, retrospective or prospective case-control studies, and a reduced number of clinical trials with a limited number of cases. This fact, added to the diversity of the populations under investigation, specific at-risk drug therapy, and the variety of definitions of successful outcomes, creates a major obstacle to drawing insightful evidence.
10. Conclusions
The present review shows relevant shortcomings in almost all MRONJ-related issues critically discussed. Further basic, observational, and interventional studies are necessary to comprehensively understand this complex adverse drug reaction in the orofacial region. Bearing in mind the classical aphorism “there are no diseases, there are ill people”. An individualized evaluation of the patient’s general health and a thorough investigation of the underlying local risk factors are essential, since its underestimation might contribute not only to the onset or lack of response to treatment for MRONJ but also, and more importantly, affect the underlying disease that motivated the prescription of these drugs.
Author Contributions
Conceptualization, A.I.L.-P., J.B., L.B. and M.P.-S.; methodology, P.G.-V., C.M.C.-P. and P.C.-B.; software, J.C.; validation, Ó.Á.-C., A.I.L.-P.; formal analysis, A.I.L.-P., A.B.-C. and M.P.-S.; investigation, A.I.L.-P., L.B. and J.B.; data curation, J.C., M.Á.B.-F. and J.C.; writing—original draft preparation, A.I.L.-P. and M.P.-S.; writing—review and editing, all authors; supervision, J.B., M.P.-S.; project administration, A.B.-C. and J.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
Data available on request due to restrictions e.g., privacy or ethical.
Acknowledgments
Our sincere apologies to all the authors whose work could not be cited due to space constraints.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AAOMS | American association of oral and maxillofacial surgeons; |
| APC | Autologous platelet concentrate; |
| ASBMR | American society for bone and mineral research; |
| BP | Bisphosphonate; |
| BRONJ | Biphosphonate-related osteonecrosis of the jaw; |
| CI | Confidential interval; |
| CT | Computed tomography; |
| CTX | Collagen type I c-telopeptide; |
| Er:YAG | Erbium YAG; |
| IDT | Invasive dental treatment; |
| MMP9 | Matrix metallopeptidase 9; |
| MRI | Magnetic resonance imaging; |
| MRONJ | Medication-related osteonecrosis of the jaw; |
| mTOR | Mammalian Target of Rapamycin; |
| PD | Periodontal disease; |
| RANKL | Receptor activator for nuclear factor κB ligand; |
| VEGF | Vascular endothelial growth factor. |
References
- Lafforgue, P. Pathophysiology and natural history of avascular necrosis of bone. Jt. Bone Spine 2006, 73, 500–507. [Google Scholar] [CrossRef]
- Goodman, S.B.; Maruyama, M. Inflammation, bone healing and osteonecrosis: From bedside to bench. J. Inflamm. Res. 2020, 13, 913–923. [Google Scholar] [CrossRef]
- Hayashida, S.; Soutome, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; et al. Evaluation of the treatment strategies for medication-related osteonecrosis of the jaws (MRONJ) and the factors affecting treatment outcome: A multicenter retrospective study with propensity score matching analysis. J. Bone Miner. Res. 2017, 32, 2022–2029. [Google Scholar] [CrossRef] [Green Version]
- James, J.; Steijn-Myagkaya, G.L. Death of osteocytes. Electron microscopy after in vitro ischaemia. J. Bone Jt. Surg. Br. 1986, 68, 620–624. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; Engroff, S.L. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J. Oral Maxillofac. Surg. 2004, 62, 527–534. [Google Scholar] [CrossRef]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Vance, M.A. Osteonecrosis of the jaw and bisphosphonates: A comparison with white phosphorus, radium, and osteopetrosis. Clin. Toxicol. 2007, 45, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, J. Bisphosphonates and phossy-jaw: Breathing new life into an old problem. Lancet Oncol. 2006, 7, 447–449. [Google Scholar] [CrossRef]
- Russell, R.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef]
- Gaudin, E.; Seidel, L.; Bacevic, M.; Rompen, E.; Lambert, F. Occurrence and risk indicators of medication-related osteonecrosis of the jaw after dental extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Nogueras Gonzalez, G.M.; Geng, Y.; Won, A.M.; Cabanillas, M.E.; Naing, A.; Myers, J.N.; Li, Y.; Chambers, M.S. Prevalence of medication related osteonecrosis of the jaw in patients treated with sequential antiresorptive drugs: Systematic review and meta-analysis. Support. Care Cancer 2021, 29, 2305–2317. [Google Scholar] [CrossRef]
- Migliorati, C.A.; Brennan, M.T.; Peterson, D.E. Medication-related osteonecrosis of the jaws. J. Natl. Cancer. Inst. Monogr. 2019, 2019, lgz009. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, N.; Bakr, M.M.; Meer, M.; Siddiqi, A. Emerging therapies with potential risks of medicine-related osteonecrosis of the jaw: A review of the literature. Br. Dent. J. 2020, 228, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Tetradis, S. Osteonecrosis of the jaw in the absence of antiresorptive or antiangiogenic exposure: A series of 6 cases. J. Oral Maxillofac. Surg. 2017, 75, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Fantasia, J.E. The role of antiangiogenic therapy in the development of osteonecrosis of the jaw. Oral Maxillofac. Surg. Clin. North. Am. 2015, 27, 547–553. [Google Scholar] [CrossRef]
- Bennardo, F.; Buffone, C.; Giudice, A. New therapeutic opportunities for COVID-19 patients with Tocilizumab: Possible correlation of interleukin-6 receptor inhibitors with osteonecrosis of the jaws. Oral Oncol. 2020, 106, 104659. [Google Scholar] [CrossRef] [PubMed]
- Eguia, A.; Bagan-Debon, L.; Cardona, F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e71–e83. [Google Scholar] [CrossRef]
- Koorbusch, G.F.; Deatherage, J.R.; Cure, J.K. How can we diagnose and treat osteomyelitis of the jaws as early as possible? Oral Maxillofac. Surg. Clin. N. Am. 2011, 23, 557–567. [Google Scholar] [CrossRef]
- Farah, C.S.; Savage, N.W. Oral ulceration with bone sequestration. Aust. Dent. J. 2003, 48, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F.; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef] [Green Version]
- Yarom, N.; Shapiro, C.L.; Peterson, D.E.; Van Poznak, C.H.; Bohlke, K.; Ruggiero, S.L.; Migliorati, C.A.; Khan, A.; Morrison, A.; Anderson, H.; et al. Medication-related osteonecrosis of the jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2270–2290. [Google Scholar] [CrossRef]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-related osteonecrosis of jaws (MRONJ) prevention and diagnosis: Italian consensus update 2020. Int. J. Environ. Res. Public. Health. 2020, 17, 5998. [Google Scholar] [CrossRef]
- Fedele, S.; Porter, S.R.; D’Aiuto, F.; Aljohani, S.; Vescovi, P.; Manfredi, M.; Arduino, P.G.; Broccoletti, R.; Musciotto, A.; Di Fede, O.; et al. Nonexposed variant of bisphosphonate-associated osteonecrosis of the jaw: A case series. Am. J. Med. 2010, 123, 1060–1064. [Google Scholar] [CrossRef] [Green Version]
- Bagan, J.V.; Hens-Aumente, E.; Leopoldo-Rodado, M.; Poveda-Roda, R.; Bagan, L. Bisphosphonate-related osteonecrosis of the jaws: Study of the staging system in a series of clinical cases. Oral Oncol. 2012, 48, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Yarom, N.; Fedele, S.; Lazarovici, T.S.; Elad, S. Is exposure of the jawbone mandatory for establishing the diagnosis of bisphosphonate-related osteonecrosis of the jaw? J. Oral Maxillofac. Surg. 2010, 68, 705. [Google Scholar] [CrossRef] [PubMed]
- Fedele, S.; Bedogni, G.; Scoletta, M.; Favia, G.; Colella, G.; Agrillo, A.; Bettini, G.; Di Fede, O.; Oteri, G.; Fusco, V.; et al. Up to a quarter of patients with osteonecrosis of the jaw associated with antiresorptive agents remain undiagnosed. Br. J. Oral Maxillofac. Surg. 2015, 53, 13–17. [Google Scholar] [CrossRef]
- Bedogni, A.; Fedele, S.; Bedogni, G.; Scoletta, M.; Favia, G.; Colella, G.; Agrillo, A.; Bettini, G.; Di Fede, O.; Oteri, G.; et al. Staging of osteonecrosis of the jaw requires computed tomography for accurate definition of the extent of bony disease. Br. J. Oral Maxillofac. Surg. 2014, 52, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Schiodt, M.; Otto, S.; Fedele, S.; Bedogni, A.; Nicolatou-Galitis, O.; Guggenberger, R.; Herlofson, B.B.; Ristow, O.; Kofod, T. Workshop of European task force on medication-related osteonecrosis of the jaw-Current challenges. Oral Dis. 2019, 25, 1815–1821. [Google Scholar] [CrossRef] [Green Version]
- Pazianas, M. Osteonecrosis of the jaw and the role of macrophages. J. Natl. Cancer Inst. 2011, 103, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Hakam, A.E.; McCauley, L.K. Current understanding of the pathophysiology of osteonecrosis of the jaw. Curr. Osteoporos. Rep. 2018, 16, 584–595. [Google Scholar] [CrossRef]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiodt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef]
- Otto, S.; Aljohani, S.; Fliefel, R.; Ecke, S.; Ristow, O.; Burian, E.; Troeltzsch, M.; Pautke, C.; Ehrenfeld, M. Infection as an important factor in medication-related osteonecrosis of the jaw (MRONJ). Medicina 2021, 57, 463. [Google Scholar] [CrossRef]
- McGowan, K.; McGowan, T.; Ivanovski, S. Risk factors for medication-related osteonecrosis of the jaws: A systematic review. Oral Dis. 2018, 24, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Zirk, M.; Wenzel, C.; Buller, J.; Zoller, J.E.; Zinser, M.; Peters, F. Microbial diversity in infections of patients with medication-related osteonecrosis of the jaw. Clin. Oral Investig. 2019, 23, 2143–2151. [Google Scholar] [CrossRef]
- De Bruyn, L.; Coropciuc, R.; Coucke, W.; Politis, C. Microbial population changes in patients with medication-related osteonecrosis of the jaw treated with systemic antibiotics. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Mawardi, H.; Giro, G.; Kajiya, M.; Ohta, K.; Almazrooa, S.; Alshwaimi, E.; Woo, S.B.; Nishimura, I.; Kawai, T. A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw. J. Dent. Res. 2011, 90, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, X.L.; Ma, J.X.; Sun, L.; Lu, B.; Wang, Y.; Xing, G.S.; Wang, Y.; Dong, B.C.; Xu, L.Y.; et al. TET3 mediates alterations in the epigenetic marker 5hmC and Akt pathway in steroid-associated osteonecrosis. J. Bone Miner. Res. 2017, 32, 319–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Yu, W.; Lee, S.; Xu, Q.; Naji, A.; Le, A.D. Bisphosphonate induces osteonecrosis of the jaw in diabetic mice via NLRP3/Caspase-1-dependent IL-1beta mechanism. J. Bone Miner. Res. 2015, 30, 2300–2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghaloo, T.L.; Cheong, S.; Bezouglaia, O.; Kostenuik, P.; Atti, E.; Dry, S.M.; Pirih, F.Q.; Tetradis, S. RANKL inhibitors induce osteonecrosis of the jaw in mice with periapical disease. J. Bone Miner. Res. 2014, 29, 843–854. [Google Scholar] [CrossRef]
- Ribet, A.B.P.; Ng, P.Y.; Pavlos, N.J. Membrane transport proteins in osteoclasts: The ins and outs. Front. Cell. Dev. Biol. 2021, 9, 644986. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Hafner, S.; Mast, G.; Tischer, T.; Volkmer, E.; Schieker, M.; Sturzenbaum, S.R.; von Tresckow, E.; Kolk, A.; Ehrenfeld, M.; et al. Bisphosphonate-related osteonecrosis of the jaw: Is pH the missing part in the pathogenesis puzzle? J. Oral Maxillofac. Surg. 2010, 68, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Pautke, C.; Opelz, C.; Westphal, I.; Drosse, I.; Schwager, J.; Bauss, F.; Ehrenfeld, M.; Schieker, M. Osteonecrosis of the jaw: Effect of bisphosphonate type, local concentration, and acidic milieu on the pathomechanism. J. Oral Maxillofac. Surg. 2010, 68, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- Dayisoylu, E.H.; Ungor, C.; Tosun, E.; Ersoz, S.; Kadioglu Duman, M.; Taskesen, F.; Senel, F.C. Does an alkaline environment prevent the development of bisphosphonate-related osteonecrosis of the jaw? An experimental study in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Vuong, H.E.; Kim, S.; Lenon, A.; Ho, K.; Hsiao, E.Y.; Sung, E.C.; Kim, R.H. Indigenous microbiota protects against inflammation-induced osteonecrosis. J. Dent. Res. 2020, 99, 676–684. [Google Scholar] [CrossRef]
- Kalyan, S.; Wang, J.; Quabius, E.S.; Huck, J.; Wiltfang, J.; Baines, J.F.; Kabelitz, D. Systemic immunity shapes the oral microbiome and susceptibility to bisphosphonate-associated osteonecrosis of the jaw. J. Transl. Med. 2015, 13, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Atsuta, I.; Liu, S.; Chen, C.; Shi, S.; Shi, S.; Le, A.D. IL-17-mediated M1/M2 macrophage alteration contributes to pathogenesis of bisphosphonate-related osteonecrosis of the jaws. Clin. Cancer Res. 2013, 19, 3176–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.W.; Landayan, M.E.; Lee, J.Y.; Tatad, J.C.; Kim, S.J.; Kim, M.R.; Cha, I.H. Role of microcracks in the pathogenesis of bisphosphonate-related osteonecrosis of the jaw. Clin. Oral Investig. 2016, 20, 2251–2258. [Google Scholar] [CrossRef]
- Schoenhof, R.; Munz, A.; Yuan, A.; ElAyouti, A.; Boesmueller, H.; Blumenstock, G.; Reinert, S.; Hoefert, S. Microarchitecture of medication-related osteonecrosis of the jaw (MRONJ); a retrospective micro-CT and morphometric analysis. J. Cranio-Maxillofac. Surg. 2021, 49, 508–517. [Google Scholar] [CrossRef]
- Aghaloo, T.; Hazboun, R.; Tetradis, S. Pathophysiology of osteonecrosis of the jaws. Oral Maxillofac. Surg. Clin. North. Am. 2015, 27, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudice, A.; Antonelli, A.; Chiarella, E.; Baudi, F.; Barni, T.; Di Vito, A. The case of medication-related osteonecrosis of the jaw addressed from a pathogenic point of view: Innovative therapeutic strategies: Focus on the most recent discoveries on oral mesenchymal stem cell-derived exosomes. Pharmaceuticals 2020, 13, 423. [Google Scholar] [CrossRef]
- Di Vito, A.; Chiarella, E.; Baudi, F.; Scardamaglia, P.; Antonelli, A.; Giudice, D.; Barni, T.; Fortunato, L.; Giudice, A. Dose-dependent effects of zoledronic acid on human periodontal ligament stem cells: An In Vitro pilot study. Cell Transplant. 2020, 29. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Pautke, C.; Arens, D.; Poxleitner, P.; Eberli, U.; Nehrbass, D.; Zeiter, S.; Stoddart, M.J. A drug holiday reduces the frequency and severity of medication-related osteonecrosis of the jaw in a minipig model. J. Bone Miner. Res. 2020, 35, 2179–2192. [Google Scholar] [CrossRef]
- Ottesen, C.; Schiodt, M.; Gotfredsen, K. Efficacy of a high-dose antiresorptive drug holiday to reduce the risk of medication-related osteonecrosis of the jaw (MRONJ): A systematic review. Heliyon 2020, 6, e03795. [Google Scholar] [CrossRef] [PubMed]
- Gkouveris, I.; Hadaya, D.; Soundia, A.; Bezouglaia, O.; Chau, Y.; Dry, S.M.; Pirih, F.Q.; Aghaloo, T.L.; Tetradis, S. Vasculature submucosal changes at early stages of osteonecrosis of the jaw (ONJ). Bone 2019, 123, 234–245. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Nicolatou-Galitis, O.; Papassotiriou, I.; Linardou, H.; Karagianni, A.; Tsixlakis, K.; Tarampikou, A.; Michalakakou, K.; Vardas, E.; Bafaloukos, D. The use of crevicular fluid to assess markers of inflammation and angiogenesis, IL-17 and VEGF, in patients with solid tumors receiving zoledronic acid and/or bevacizumab. Support. Care Cancer 2020, 28, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Basi, D.L.; Hughes, P.J.; Thumbigere-Math, V.; Sabino, M.; Mariash, A.; Lunos, S.A.; Jensen, E.; Gopalakrishnan, R. Matrix metalloproteinase-9 expression in alveolar extraction sockets of Zoledronic acid-treated rats. J. Oral Maxillofac. Surg. 2011, 69, 2698–2707. [Google Scholar] [CrossRef]
- Fliefel, R.M.; Entekhabi, S.A.; Ehrenfeld, M.; Otto, S. Geranylgeraniol (GGOH) as a mevalonate pathway activator in the rescue of bone cells treated with zoledronic acid: An in Vitro study. Stem Cells Int. 2019, 2019, 4351327. [Google Scholar] [CrossRef]
- de Molon, R.S.; Shimamoto, H.; Bezouglaia, O.; Pirih, F.Q.; Dry, S.M.; Kostenuik, P.; Boyce, R.W.; Dwyer, D.; Aghaloo, T.L.; Tetradis, S. OPG-Fc but not zoledronic acid discontinuation reverses osteonecrosis of the jaws (ONJ) in mice. J. Bone Miner. Res. 2015, 30, 1627–1640. [Google Scholar] [CrossRef] [Green Version]
- Rosella, D.; Papi, P.; Giardino, R.; Cicalini, E.; Piccoli, L.; Pompa, G. Medication-related osteonecrosis of the jaw: Clinical and practical guidelines. J. Int. Soc. Prev. Community Dent. 2016, 6, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodwell, C.; Ayme, S. Rare disease policies to improve care for patients in Europe. Biochim. Biophys. Acta 2015, 1852, 2329–2335. [Google Scholar] [CrossRef] [Green Version]
- Ulmner, M.; Jarnbring, F.; Torring, O. Osteonecrosis of the jaw in Sweden associated with the oral use of bisphosphonate. J. Oral Maxillofac. Surg. 2014, 72, 76–82. [Google Scholar] [CrossRef]
- Dodson, T.B. The frequency of medication-related osteonecrosis of the jaw and its associated risk factors. Oral Maxillofac. Surg. Clin. North. Am. 2015, 27, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Limones, A.; Saez-Alcaide, L.M.; Diaz-Parreno, S.A.; Helm, A.; Bornstein, M.M.; Molinero-Mourelle, P. Medication-related osteonecrosis of the jaws (MRONJ) in cancer patients treated with denosumab VS. zoledronic acid: A systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e326–e336. [Google Scholar] [CrossRef] [PubMed]
- Fung, P.; Bedogni, G.; Bedogni, A.; Petrie, A.; Porter, S.; Campisi, G.; Bagan, J.; Fusco, V.; Saia, G.; Acham, S.; et al. Time to onset of bisphosphonate-related osteonecrosis of the jaws: A multicentre retrospective cohort study. Oral Dis. 2017, 23, 477–483. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.W.; Kim, S.J.; Lee, S.H.; Lee, H.S. Uncertainty of current algorithm for bisphosphonate-related osteonecrosis of the jaw in population-based studies: A systematic review. J. Bone Miner. Res. 2017, 32, 584–591. [Google Scholar] [CrossRef]
- Vahtsevanos, K.; Kyrgidis, A.; Verrou, E.; Katodritou, E.; Triaridis, S.; Andreadis, C.G.; Boukovinas, I.; Koloutsos, G.E.; Teleioudis, Z.; Kitikidou, K.; et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J. Clin. Oncol. 2009, 27, 5356–5362. [Google Scholar] [CrossRef] [PubMed]
- Karna, H.; Gonzalez, J.; Radia, H.S.; Sedghizadeh, P.P.; Enciso, R. Risk-reductive dental strategies for medication related osteonecrosis of the jaw among cancer patients: A systematic review with meta-analyses. Oral Oncol. 2018, 85, 15–23. [Google Scholar] [CrossRef]
- Niibe, K.; Ouchi, T.; Iwasaki, R.; Nakagawa, T.; Horie, N. Osteonecrosis of the jaw in patients with dental prostheses being treated with bisphosphonates or denosumab. J. Prosthodont. Res. 2015, 59, 3–5. [Google Scholar] [CrossRef]
- Ripamonti, C.I.; Maniezzo, M.; Campa, T.; Fagnoni, E.; Brunelli, C.; Saibene, G.; Bareggi, C.; Ascani, L.; Cislaghi, E. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann. Oncol. 2009, 20, 137–145. [Google Scholar] [CrossRef]
- Kannel, W.B.; Dawber, T.R.; Kagan, A.; Revotskie, N.; Stokes, J. Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann. Intern. Med. 1961, 55, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Barasch, A.; Cunha-Cruz, J.; Curro, F.A.; Hujoel, P.; Sung, A.H.; Vena, D.; Voinea-Griffin, A.E.; CONDOR Collaborative Group; Beadnell, S.; Craig, R.G.; et al. Risk factors for osteonecrosis of the jaws: A case-control study from the CONDOR dental PBRN. J. Dent. Res. 2011, 90, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, B.; Nehrbass, D.; Arens, D.; Stadelmann, V.A.; Zeiter, S.; Otto, S.; Kircher, P.; Stoddart, M.J. Medication-related osteonecrosis of the jaw in a minipig model: Parameters for developing a macroscopic, radiological, and microscopic grading scheme. J. Cranio-Maxillofac. Surg. 2019, 47, 1162–1169. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Razis, E.; Galiti, D.; Galitis, E.; Labropoulos, S.; Tsimpidakis, A.; Sgouros, J.; Karampeazis, A.; Migliorati, C. Periodontal disease preceding osteonecrosis of the jaw (ONJ) in cancer patients receiving antiresorptives alone or combined with targeted therapies: Report of 5 cases and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ruiz, M.M.; Romero-Serrano, M.; Serrano-Gonzalez, A.; Serrera-Figallo, M.A.; Gutierrez-Perez, J.L.; Torres-Lagares, D. Proposal for a preventive protocol for medication-related osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e314–e326. [Google Scholar] [CrossRef]
- Peer, A.; Khamaisi, M. Diabetes as a risk factor for medication-related osteonecrosis of the jaw. J. Dent. Res. 2015, 94, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Di Fede, O.; Bedogni, A.; Giancola, F.; Saia, G.; Bettini, G.; Toia, F.; D’Alessandro, N.; Firenze, A.; Matranga, D.; Fedele, S.; et al. BRONJ in patients with rheumatoid arthritis: A multicenter case series. Oral Dis. 2016, 22, 543–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Various therapeutic methods for the treatment of medication-related osteonecrosis of the jaw (MRONJ) and their limitations: A narrative review on new molecular and cellular therapeutic approaches. Antioxidants 2021, 10, 680. [Google Scholar] [CrossRef]
- Fusco, V.; Cabras, M.; Erovigni, F.; Dell’Acqua, A.; Arduino, P.G.; Pentenero, M.; Appendino, P.; Basano, L.; Ferrera, F.D.; Fasciolo, A.; et al. A multicenter observational study on Medication-Related Osteonecrosis of the Jaw (MRONJ) in advanced cancer and myeloma patients of a cancer network in North-Western Italy. Med. Oral Patol. Oral Cir. Bucal 2020, 26, e466–e473. [Google Scholar] [CrossRef]
- Owosho, A.A.; Liang, S.T.Y.; Sax, A.Z.; Wu, K.; Yom, S.K.; Huryn, J.M.; Estilo, C.L. Medication-related osteonecrosis of the jaw: An update on the memorial sloan kettering cancer center experience and the role of premedication dental evaluation in prevention. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Japanese Allied Committee on Osteonecrosis of the Jaw; Yoneda, T.; Hagino, H.; Sugimoto, T.; Ohta, H.; Takahashi, S.; Soen, S.; Taguchi, A.; Nagata, T.; Urade, M.; et al. Antiresorptive agent-related osteonecrosis of the jaw: Position Paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw. J. Bone Miner. Metab. 2017, 35, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Kun-Darbois, J.D.; Fauvel, F. Medication-related osteonecrosis and osteoradionecrosis of the jaws: Update and current management. Morphologie 2021, 105, 170–187. [Google Scholar] [CrossRef]
- Sandro Pereira da Silva, J.; Pullano, E.; Raje, N.S.; Troulis, M.J.; August, M. Genetic predisposition for medication-related osteonecrosis of the jaws: A systematic review. Int. J. Oral Maxillofac. Surg. 2019, 48, 1289–1299. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; Perez-Sayans, M.; Gonzalez-Palanca, S.; Chamorro-Petronacci, C.; Bagan, J.; Garcia-Garcia, A. Biomarkers to predict the onset of biphosphonate-related osteonecrosis of the jaw: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e26–e36. [Google Scholar] [CrossRef]
- Moraschini, V.; de Almeida, D.C.F.; Figueredo, C.M.; Calasans-Maia, M.D. Association between biomarkers and medication-related osteonecrosis of the jaws: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 504–515. [Google Scholar] [CrossRef]
- Awad, M.E.; Sun, C.; Jernigan, J.; Elsalanty, M. Serum C-terminal cross-linking telopeptide level as a predictive biomarker of osteonecrosis after dentoalveolar surgery in patients receiving bisphosphonate therapy: Systematic review and meta-analysis. J. Am. Dent. Assoc. 2019, 150, 664–675.e8. [Google Scholar] [CrossRef] [PubMed]
- El-Rabbany, M.; Sgro, A.; Lam, D.K.; Shah, P.S.; Azarpazhooh, A. Effectiveness of treatments for medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2017, 148, 584–594.e2. [Google Scholar] [CrossRef]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Cranio-Maxillofac. Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Ri, S.; Umeda, M.; Komatsubara, H.; Kobayashi, M.; Shigeta, T.; Yoshitomi, I.; Ikeda, H.; Shibuya, Y.; Asahina, I.; et al. The observational study of delayed wound healing after tooth extraction in patients receiving oral bisphosphonate therapy. J. Cranio-Maxillofac. Surg. 2013, 41, 558–563. [Google Scholar] [CrossRef]
- Corso, A.; Varettoni, M.; Zappasodi, P.; Klersy, C.; Mangiacavalli, S.; Pica, G.; Lazzarino, M. A different schedule of zoledronic acid can reduce the risk of the osteonecrosis of the jaw in patients with multiple myeloma. Leukemia 2007, 21, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Langdahl, B.; Cohen-Solal, M.; Aubry-Rozier, B.; Eriksen, E.F.; Guanabens, N.; Obermayer-Pietsch, B.; Ralston, S.H.; Eastell, R.; Zillikens, M.C. Discontinuation of Denosumab therapy for osteoporosis: A systematic review and position statement by ECTS. Bone 2017, 105, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Mannion, C.J. Medication-related osteonecrosis of the jaws and quality of life: Review and structured analysis. Br. J. Oral Maxillofac. Surg. 2020, 58, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Freitas, M.; Limeres, J. Prevention of medication-related osteonecrosis of the jaws secondary to tooth extractions. A systematic review. Med. Oral Patol. Oral Cir. Bucal 2016, 21, 250. [Google Scholar] [CrossRef]
- Fedele, S.; Kumar, N.; Davies, R.; Fiske, J.; Greening, S.; Porter, S. Dental management of patients at risk of osteochemonecrosis of the jaws: A critical review. Oral Dis. 2009, 15, 527–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, S.L.; Faia, J.; Carlson, E. Bisphosphonate-related osteonecrosis of the jaw: Background and guidelines for diagnosis, staging and management. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 433–441. [Google Scholar] [CrossRef]
- Bedogni, A.; Fusco, V.; Agrillo, A.; Campisi, G. Learning from experience. Proposal of a refined definition and staging system for bisphosphonate-related osteonecrosis of the jaw (BRONJ). Oral Dis. 2012, 18, 621–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamoto, H.; Grogan, T.R.; Tsujimoto, T.; Kakimoto, N.; Murakami, S.; Elashoff, D.; Aghaloo, T.L.; Tetradis, S. Does CBCT alter the diagnostic thinking efficacy, management and prognosis of patients with suspected Stage 0 medication-related osteonecrosis of the jaws? Dentomaxillofac. Radiol. 2018, 47, 20170290. [Google Scholar] [CrossRef]
- Bagan, L.; Leopoldo-Rodado, M.; Poveda-Roda, R.; Murillo-Cortes, J.; Diaz-Fernandez, J.M.; Bagan, J. Grade of sclerosis in the contralateral mandibular area in osteonecrosis of the jaws. Int. J. Oral Maxillofac. Surg. 2017, 46, 167–172. [Google Scholar] [CrossRef]
- Schiodt, M.; Reibel, J.; Oturai, P.; Kofod, T. Comparison of nonexposed and exposed bisphosphonate-induced osteonecrosis of the jaws: A retrospective analysis from the Copenhagen cohort and a proposal for an updated classification system. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 204–213. [Google Scholar] [CrossRef]
- O’Ryan, F.S.; Khoury, S.; Liao, W.; Han, M.M.; Hui, R.L.; Baer, D.; Martin, D.; Liberty, D.; Lo, J.C. Intravenous bisphosphonate-related osteonecrosis of the jaw: Bone scintigraphy as an early indicator. J. Oral Maxillofac. Surg. 2009, 67, 1363–1372. [Google Scholar] [CrossRef]
- Junquera, L.; Gallego, L. Nonexposed bisphosphonate-related osteonecrosis of the jaws: Another clinical variant? J. Oral Maxillofac. Surg. 2008, 66, 1516–1517. [Google Scholar] [CrossRef]
- Migliorati, C.A.; Epstein, J.B.; Abt, E.; Berenson, J.R. Osteonecrosis of the jaw and bisphosphonates in cancer: A narrative review. Nat. Rev. Endocrinol. 2011, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ristow, O.; Ruckschloss, T.; Muller, M.; Berger, M.; Kargus, S.; Pautke, C.; Engel, M.; Hoffmann, J.; Freudlsperger, C. Is the conservative non-surgical management of medication-related osteonecrosis of the jaw an appropriate treatment option for early stages? A long-term single-center cohort study. J. Cranio-Maxillofac. Surg. 2019, 47, 491–499. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Claeys, T.; Huizing, M.T.; Vermorken, J.B.; Fossion, E. Initial experience with conservative treatment in cancer patients with osteonecrosis of the jaw (ONJ) and predictors of outcome. Ann. Oncol. 2009, 20, 331–336. [Google Scholar] [CrossRef]
- Kim, K.M.; Rhee, Y.; Kwon, Y.D.; Kwon, T.G.; Lee, J.K.; Kim, D.Y. Medication related osteonecrosis of the jaw: 2015 position statement of the Korean Society for Bone and Mineral Research and the Korean Association of Oral and Maxillofacial Surgeons. J. Bone Metab. 2015, 22, 151–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagan, J.; Blade, J.; Cozar, J.M.; Constela, M.; Garcia Sanz, R.; Gomez Veiga, F.; Lahuerta, J.J.; Lluch, A.; Massuti, B.; Morote, J.; et al. Recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw (ONJ) in cancer patients treated with bisphosphonates. Med. Oral Patol. Oral Cir. Bucal 2007, 12, 336. [Google Scholar]
- Ewald, F.; Wuesthoff, F.; Koehnke, R.; Friedrich, R.E.; Gosau, M.; Smeets, R.; Rohde, H.; Assaf, A.T. Retrospective analysis of bacterial colonization of necrotic bone and antibiotic resistance in 98 patients with medication-related osteonecrosis of the jaw (MRONJ). Clin. Oral Investig. 2021, 25, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Fedele, S.; Fusco, V.; Pizzo, G.; Di Fede, O.; Bedogni, A. Epidemiology, clinical manifestations, risk reduction and treatment strategies of jaw osteonecrosis in cancer patients exposed to antiresorptive agents. Future Oncol. 2014, 10, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Russmueller, G.; Seemann, R.; Weiss, K.; Stadler, V.; Speiss, M.; Perisanidis, C.; Fuereder, T.; Willinger, B.; Sulzbacher, I.; Steininger, C. The association of medication-related osteonecrosis of the jaw with Actinomyces spp. infection. Sci. Rep. 2016, 6, 31604. [Google Scholar] [CrossRef] [Green Version]
- Hadaya, D.; Soundia, A.; Freymiller, E.; Grogan, T.; Elashoff, D.; Tetradis, S.; Aghaloo, T.L. Nonsurgical management of medication-related osteonecrosis of the jaws using local wound care. J. Oral Maxillofac. Surg. 2018, 76, 2332–2339. [Google Scholar] [CrossRef]
- Pippi, R.; Giuliani, U.; Tenore, G.; Pietrantoni, A.; Romeo, U. What is the risk of developing medication-related osteonecrosis in patients with extraction sockets left to heal by secondary intention? A retrospective case series study. J. Oral Maxillofac. Surg. 2021. [Google Scholar] [CrossRef]
- Favia, G.; Tempesta, A.; Limongelli, L.; Crincoli, V.; Maiorano, E. Medication-related osteonecrosis of the jaw: Surgical or non-surgical treatment? Oral Dis. 2018, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Pouso, A.I.; Perez-Sayans, M.; Chamorro-Petronacci, C.; Gandara-Vila, P.; Lopez-Jornet, P.; Carballo, J.; Garcia-Garcia, A. Association between periodontitis and medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. J. Oral Pathol. Med. 2020, 49, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Kohn, N. Disease stage and mode of therapy are important determinants of treatment outcomes for medication-related osteonecrosis of the jaw. J. Oral Maxillofac. Surg. 2015, 73, S94–S100. [Google Scholar] [CrossRef]
- Giudice, A.; Barone, S.; Diodati, F.; Antonelli, A.; Nocini, R.; Cristofaro, M.G. Can surgical management improve resolution of medication-related osteonecrosis of the jaw at early stages? A prospective cohort study. J. Oral Maxillofac. Surg. 2020, 78, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B.; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J. Oral Maxillofac. Surg. 2009, 67, 2–12. [Google Scholar] [CrossRef]
- Wilde, F.; Heufelder, M.; Winter, K.; Hendricks, J.; Frerich, B.; Schramm, A.; Hemprich, A. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 153–163. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M. How to optimize the preparation of leukocyte- and platelet-rich fibrin (L-PRF, Choukroun’s technique) clots and membranes: Introducing the PRF Box. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Sanchez Perez, A.; Amaral Mendes, R.; Tobias, A. Medication-related osteonecrosis of the jaw: Is autologous platelet concentrate application effective for prevention and treatment? A systematic review. J. Craniomaxillofac. Surg. 2016, 44, 1067–1072. [Google Scholar] [CrossRef]
- Goker, F.; Grecchi, E.; Grecchi, F.; Francetti, L.; Del Fabbro, M. Treatment of medication-related osteonecrosis of the jaw (MRONJ). A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2662–2673. [Google Scholar] [PubMed]
- Vidal-Real, C.; Perez-Sayans, M.; Suarez-Penaranda, J.M.; Gandara-Rey, J.M.; Garcia-Garcia, A. Osteonecrosis of the jaws in 194 patients who have undergone intravenous bisphosphonate therapy in Spain. Med. Oral Patol. Oral Cir. Bucal 2015, 20, 267. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, A.; Somoza-Martin, M.; Gandara-Rey, J.M.; Perez-Sayans, M. Surgical neurolysis for the treatment of neuropathic pain in 2 postmenopausal women with mandibular necrosis resulting from oral bisphosphonates. J. Craniofac. Surg. 2014, 25, 1369–1371. [Google Scholar] [CrossRef]
- Aljohani, S.; Troeltzsch, M.; Hafner, S.; Kaeppler, G.; Mast, G.; Otto, S. Surgical treatment of medication-related osteonecrosis of the upper jaw: Case series. Oral Dis. 2019, 25, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Schnodt, E.M.; Haidari, S.; Brunner, T.F.; Aljohani, S.; Mosleh, M.; Ristow, O.; Troeltzsch, M.; Pautke, C.; Ehrenfeld, M.; et al. Autofluorescence-guided surgery for the treatment of medication-related osteonecrosis of the jaw (MRONJ): A retrospective single-center study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 519–526. [Google Scholar] [CrossRef]
- Blus, C.; Giannelli, G.; Szmukler-Moncler, S.; Orru, G. Treatment of medication-related osteonecrosis of the jaws (MRONJ) with ultrasonic piezoelectric bone surgery. A case series of 20 treated sites. Oral Maxillofac. Surg. 2017, 21, 41–48. [Google Scholar] [CrossRef]
- Giudice, A.; Bennardo, F.; Barone, S.; Antonelli, A.; Figliuzzi, M.M.; Fortunato, L. Can autofluorescence guide surgeons in the treatment of medication-related osteonecrosis of the jaw? A prospective feasibility study. J. Oral Maxillofac. Surg. 2018, 76, 982–995. [Google Scholar] [CrossRef]
- Tomo, S.; da Cruz, T.M.; Figueira, J.A.; Cunha, J.L.S.; Miyahara, G.I.; Simonato, L.E. Fluorescence-guided surgical management of medication-related osteonecrosis of the jaws. Photodiagnosis Photodyn. Ther. 2020, 32, 102003. [Google Scholar] [CrossRef]
- Vescovi, P.; Merigo, E.; Meleti, M.; Fornaini, C.; Nammour, S.; Manfredi, M. Nd:YAG laser biostimulation of bisphosphonate-associated necrosis of the jawbone with and without surgical treatment. Br. J. Oral Maxillofac. Surg. 2007, 45, 628–632. [Google Scholar] [CrossRef]
- Altay, M.A.; Tasar, F.; Tosun, E.; Kan, B. Low-level laser therapy supported surgical treatment of bisphosphonate related osteonecrosis of jaws: A retrospective analysis of 11 cases. Photomed. Laser Surg. 2014, 32, 468–475. [Google Scholar] [CrossRef]
- Kakehashi, H.; Ando, T.; Minamizato, T.; Nakatani, Y.; Kawasaki, T.; Ikeda, H.; Kuroshima, S.; Kawakami, A.; Asahina, I. Administration of teriparatide improves the symptoms of advanced bisphosphonate-related osteonecrosis of the jaw: Preliminary findings. Int. J. Oral Maxillofac. Surg. 2015, 44, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Yoo, H.Y.; Kim, G.T.; Lee, J.W.; Lee, Y.A.; Kim, D.Y.; Kwon, Y.D. Short-term teriparatide and recombinant human bone morphogenetic protein-2 for regenerative approach to medication-related osteonecrosis of the Jaw: A preliminary study. J. Bone Miner. Res. 2017, 32, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).