Quality of Life in Electrochemotherapy for Cutaneous and Mucosal Head and Neck Tumors
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bar-Ad, V.; Palmer, J.; Yang, H.; Cognetti, D.; Curry, J.; Luginbuhl, A.; Tuluc, M.; Campling, B.; Axelrod, R. Current Management of Locally Advanced Head and Neck Cancer: The Combination of Chemotherapy with Locoregional Treatments. Semin. Oncol. 2014, 41, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Strojan, P.; Grošelj, A.; Serša, G.; Plaschke, C.; Vermorken, J.; Nuyts, S.; de Bree, R.; Eisbruch, A.; Mendenhall, W.; Smee, R.; et al. Electrochemotherapy in Mucosal Cancer of the Head and Neck: A Systematic Review. Cancers 2021, 13, 1254. [Google Scholar] [CrossRef] [PubMed]
- Plaschke, C.C.; Bertino, G.; McCaul, J.A.; Grau, J.J.; de Bree, R.; Sersa, G.; Occhini, A.; Groselj, A.; Langdon, C.; Heuveling, D.A.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results from the treatment of mucosal cancers. Eur. J. Cancer 2017, 87, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Sersa, G.; De Terlizzi, F.; Occhini, A.; Plaschke, C.C.; Groselj, A.; Langdon, C.; Grau, J.J.; McCaul, J.A.; Heuveling, D.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer 2016, 63, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Clover, A.; de Terlizzi, F.; Bertino, G.; Curatolo, P.; Odili, J.; Campana, L.; Kunte, C.; Muir, T.; Brizio, M.; Sersa, G.; et al. Electrochemotherapy in the treatment of cutaneous malignancy: Outcomes and subgroup analysis from the cumulative results from the pan-European International Network for Sharing Practice in Electrochemotherapy database for 2482 lesions in 987 patients (2008–2019). Eur. J. Cancer 2020, 138, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Ojo, B.; Genden, E.M.; Teng, M.S.; Milbury, K.; Misiukiewicz, K.J.; Badr, H. A systematic review of head and neck cancer quality of life assessment instruments. Oral Oncol. 2012, 48, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, A.; Noronha, V.; Joshi, A.; Patil, V.M.; Menon, N.; Prabhash, K. Palliative chemotherapy in head and neck cancer: Balancing between beneficial and adverse effects. Expert Rev. Anticancer Ther. 2020, 20, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Potenza, I.; Schena, M.; Riva, G.; Pecorari, G.; Demo, P.G.; Fasolis, M.; Moretto, F.; Garzaro, M.; Di Muzio, J.; et al. Induction Chemotherapy and Sequential Concomitant Chemo-radiation in Locally Advanced Head and Neck Cancers: How Induction-phase Intensity and Treatment Breaks May Impact on Clinical Outcomes. Anticancer Res. 2015, 35, 6247–6254. [Google Scholar] [PubMed]
- Schwartz, L.H.; Seymour, L.; Litière, S.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur. J. Cancer 2016, 62, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Nolte, S.; Liegl, G.; Petersen, M.; Aaronson, N.; Costantini, A.; Fayers, P.; Groenvold, M.; Holzner, B.; Johnson, C.; Kemmler, G.; et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur. J. Cancer 2019, 107, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Orlowski, S.; Belehradek, J.; Paoletti, C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur. J. Cancer Clin. Oncol. 1991, 27, 68–72. [Google Scholar] [CrossRef]
- Gehl, J.; Skovsgaard, T.; Mir, L.M. Enhancement of cytotoxicity by electropermeabilization: An improved method for screening drugs. Anticancer Drugs 1998, 9, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Enokida, T.; Tahara, M. Electrochemotherapy in the Treatment of Head and Neck Cancer: Current Conditions and Future Directions. Cancers 2021, 13, 1418. [Google Scholar] [CrossRef] [PubMed]
- Allegra, E.; Domanico, R.; Trapasso, S.; Santoro, M.; Pingitore, D. Electrochemotherapy in combination with chemoradiotherapy in the treatment of oral carcinomas in advanced stages of disease: Efficacy, safety, and clinical outcomes in a small number of selected cases. Drug Des. Dev. Ther. 2015, 9, 1185–1191. [Google Scholar] [CrossRef][Green Version]
- Perri, F.; Longo, F.; Fusco, R.; D’alessio, V.; Aversa, C.; Pavone, E.; Pontone, M.; Marciano, M.L.; Villano, S.; Franco, P.; et al. Electrochemotherapy as a first line treatment in recurrent squamous cell carcinoma of the oral cavity and oropharynx pdl-1 negative and/or with evident contraindication to immunotherapy: A randomized multicenter controlled trial. Cancers 2021, 13, 2210. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Perri, F.; Pavone, E.; Aversa, C.; Maglione, M.G.; Guida, A.; Montano, M.; Villano, S.; Daponte, A.; Caponigro, F.; et al. Electrochemotherapy as palliative treatment in patients with advanced head and neck tumours: Outcome analysis in 93 patients treated in a single institution. Oral Oncol. 2019, 92, 77–84. [Google Scholar] [CrossRef]
- Pichi, B.; Pellini, R.; Spriano, G. Electrochemotherapy—A locoregional therapy with well-established palliative effect in patient with large recurrent lesion of head and neck. J. Cranio-Maxillofac. Surg. 2018, 47, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Plaschke, C.C.; Johannesen, H.H.; Hansen, R.H.; Hendel, H.W.; Kiss, K.; Gehl, J.; Wessel, I. The DAHANCA 32 study: Electrochemotherapy for recurrent mucosal head and neck cancer. Head Neck 2018, 41, 329–339. [Google Scholar] [CrossRef]

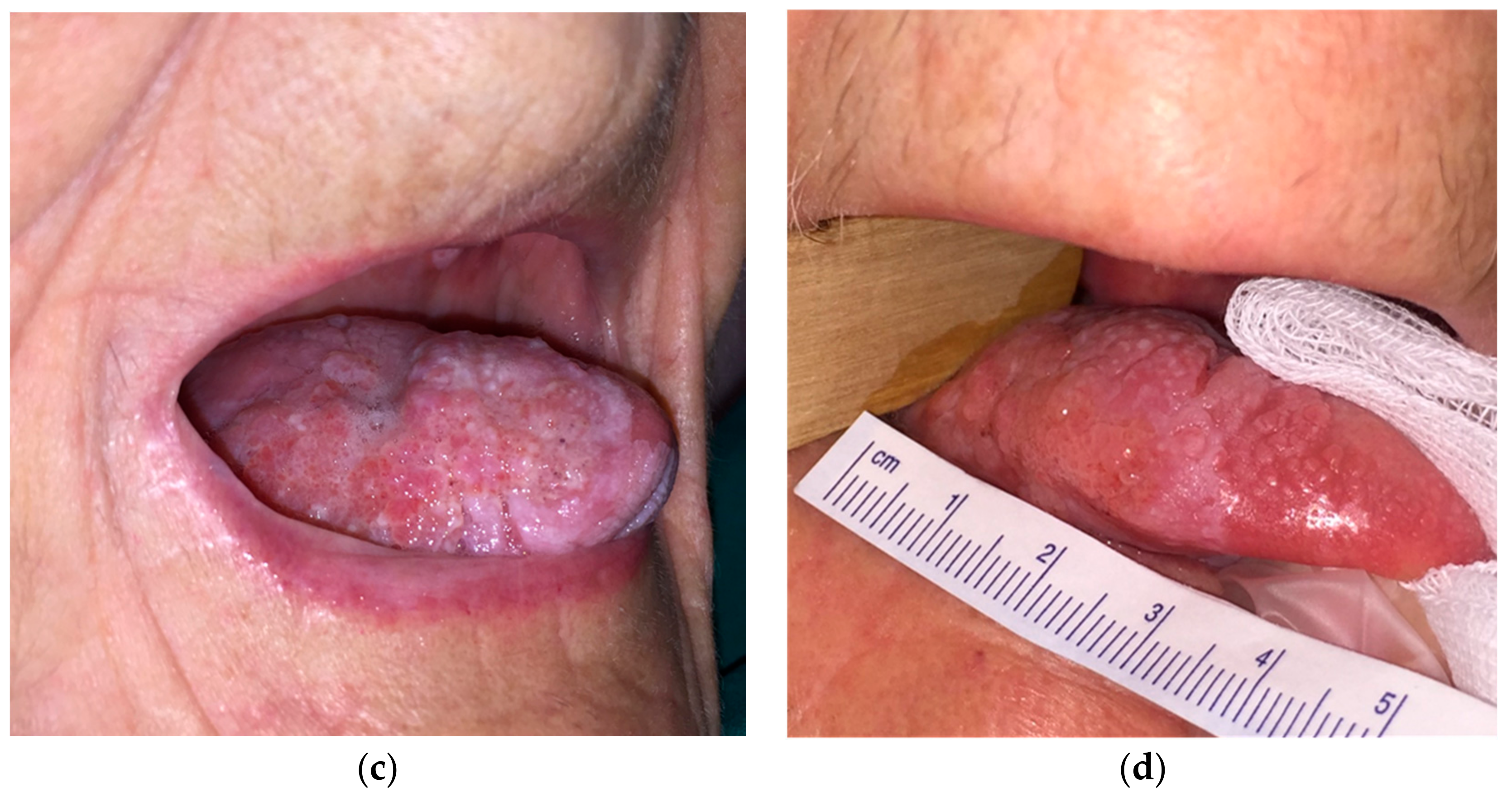
| Characteristics | N (%) |
|---|---|
| Sex | |
| Male | 21 (77.8) |
| Female | 6 (22.2) |
| Smoking | |
| Active | 8 (29.6) |
| Former | 12 (44.4) |
| Never | 7 (25.9) |
| Alcohol consumption | 2 (7.4) |
| Tumor site | |
| Skin | 9 (33.3) |
| Mucosa | 18 (66.7) |
| Tumor type | |
| Primary | 11 (40.7) |
| Relapse | 16 (59.3) |
| Patients | Site of Primary Tumor | Histology | TNM | ECT Site |
|---|---|---|---|---|
| 1 | Skin | Squamous cell carcinoma | T3N3bM0 | Inferior lip + adenopathies |
| 2 | Skin | Basal cell carcinoma | - | Scalp |
| 3 | Skin | Basal cell carcinoma | - | Nose |
| 4 | Skin | Basal cell carcinoma | - | Nose |
| 5 | Skin | Squamous cell carcinoma | T2N0M0 | Preauricolar region |
| 6 | Skin | Squamous cell carcinoma | T3N0M0 | Preauricolar region |
| 7 | Skin | Squamous cell carcinoma | T3N0M0 | Preauricolar region |
| 8 | Skin | Squamous cell carcinoma | T3N0M0 | Preauricolar region |
| 9 | Skin | Basal cell carcinoma | - | Cheek |
| 10 | Oral cavity | Squamous cell carcinoma | T2N0M0 | Retromolar trigone |
| 11 | Oral cavity | Squamous cell carcinoma | T2N0M0 | Tongue |
| 12 | Oral cavity | Squamous cell carcinoma | T3N0M0 | Tongue |
| 13 | Oral cavity | Squamous cell carcinoma | T0N3bM0 | Adenopathies |
| 14 | Oropharynx | Squamous cell carcinoma | T2N0M0 | Soft palate |
| 15 | Oral cavity | Squamous cell carcinoma | T3N0M0 | Tongue |
| 16 | Oral cavity | Squamous cell carcinoma | T2N0M0 | Cheek mucosa |
| 17 | Oral cavity | Squamous cell carcinoma | T0N3bM0 | Adenopathies |
| 18 | Parotid gland | Adenocarcinoma | T4aN0M0 | Parotid gland + skin |
| 19 | Parotid gland | Adenocarcinoma | T4aN0M0 | Parotid gland + skin |
| 20 | Oral cavity | Adenocarcinoma | T4aN2aM0 | Cheek mucosa + adenopathies |
| 21 | Parotid gland | Squamous cell carcinoma | T4aN0M0 | Parotid gland + skin |
| 22 | Parotid gland | Adenocarcinoma | T4bN3bM0 | Parotid gland + adenopathies |
| 23 | Parotid gland | Adenocarcinoma | T4bN3bM0 | Parotid gland + adenopathies |
| 24 | Larynx | Squamous cell carcinoma | T0N3bM0 | Adenopathies |
| 25 | Oral cavity | Squamous cell carcinoma | T0N3bM0 | Adenopathies |
| 26 | Oral cavity | Squamous cell carcinoma | T0N3bM0 | Adenopathies |
| 27 | Oral cavity | Squamous cell carcinoma | T2N0M0 | Oral floor |
| Scores | T0 | T1 (1 Month) | T2 (3 Months) | T3 (6 Months) | p Value |
|---|---|---|---|---|---|
| Karnofsky performance status | 80.0 (20.0) | 80.0 (20.0) | 90.0 (30.0) | 80.0 (40.0) | 0.539 |
| EORTC QLQ-C30 questionnaire | |||||
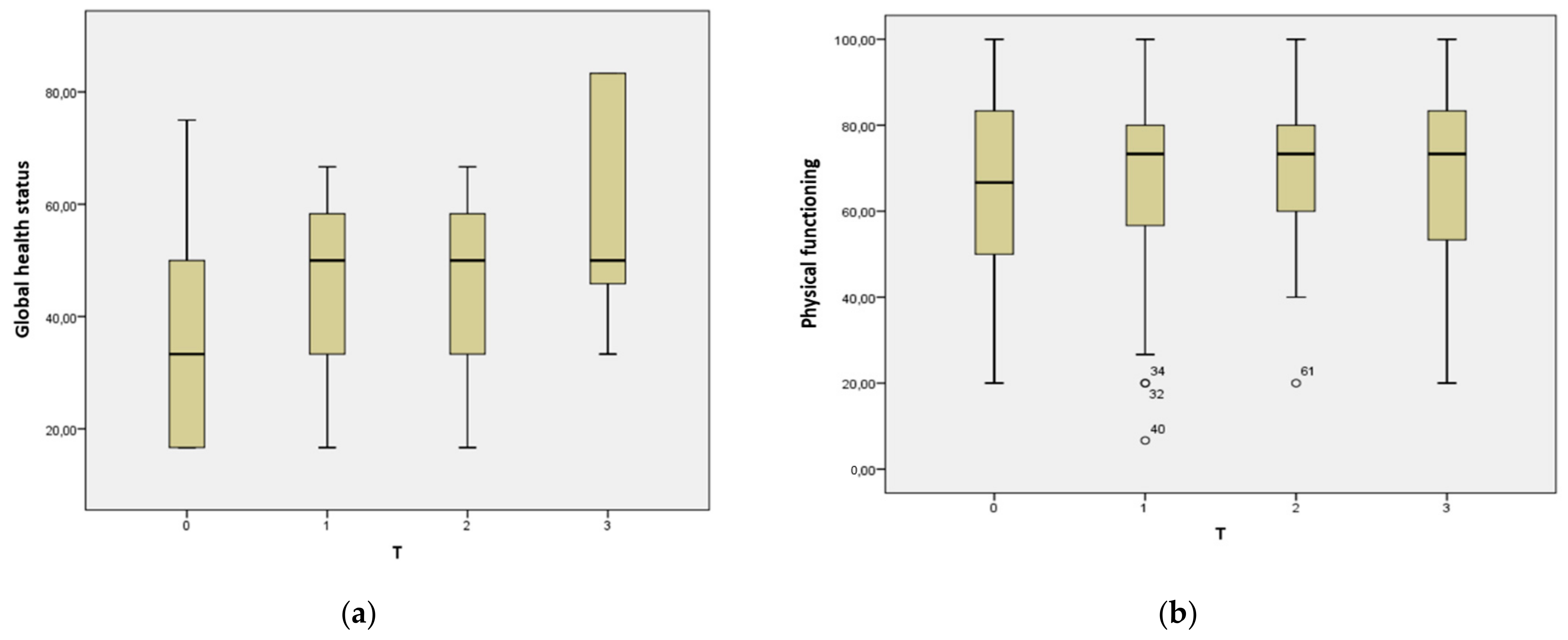
| Global health status | 33.3 (33.3) | 50.0 (25.0) | 50.0 (25.0) | 50.0 (41.6) | 0.026 * |
| Physical functioning | 66.7 (40.0) | 73.3 (26.7) | 73.3 (21.7) | 73.3 (40.0) | 0.596 |
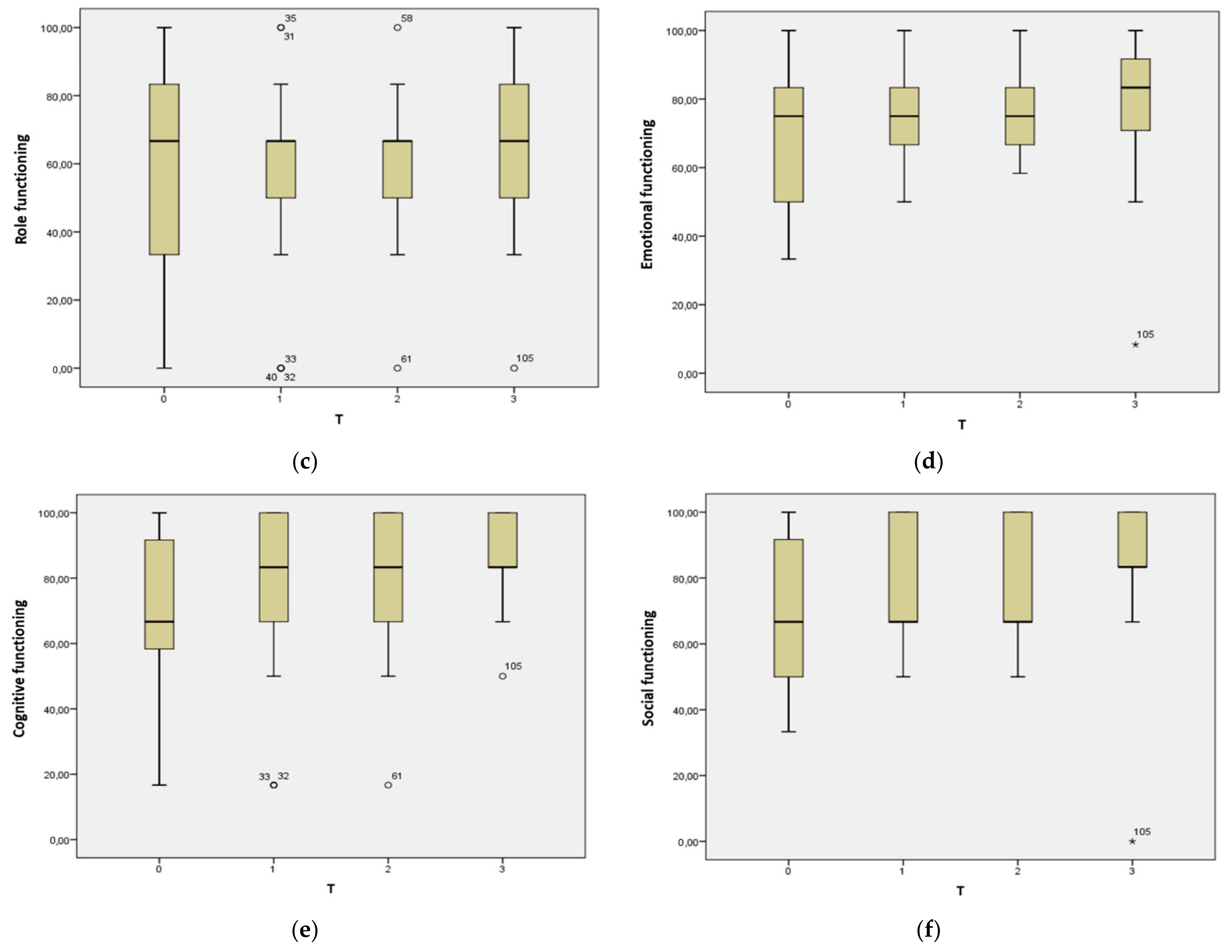
| Role functioning | 66.7 (50.0) | 66.7 (16.7) | 66.7 (16.7) | 66.7 (50.0) | 0.356 |
| Emotional functioning | 75.0 (33.3) | 75.0 (16.7) | 75.0 (16.7) | 33.3 (33.3) | 0.243 |
| Cognitive functioning | 66.7 (50.0) | 33.3 (33.3) | 33.3 (33.3) | 83.3 (16.7) | 0.297 |
| Social functioning | 66.7 (50.0) | 66.7 (33.3) | 66.7 (33.3) | 83.3 (16.7) | 0.043 * |
| Fatigue | 44.4 (55.6) | 33.3 (44.5) | 33.3 (25.0) | 22.2 (55.6) | 0.768 |
| Nausea and vomiting | 0.0 (16.7) | 0.0 (16.7) | 0.0 (16.7) | 0.0 (0.0) | 0.589 |
| Pain | 16.7 (66.7) | 16.7 (33.3) | 16.7 (33.3) | 16.7 (50.0) | 0.045 * |
| Dyspnea | 33.3 (33.3) | 33.3 (33.3) | 33.3 (33.3) | 0.0 (33.3) | 0.533 |
| Insomnia | 33.3 (66.7) | 33.3 (33.3) | 16.7 (33.3) | 0.0 (33.3) | 0.138 |
| Appetite loss | 33.3 (66.7) | 0.0 (33.3) | 0.0 (33.3) | 0.0 (33.3) | 0.002 * |
| Constipation | 0.0 (33.3) | 0.0 (33.3) | 0.0 (33.3) | 0.0 (33.3) | 0.179 |
| Diarrhea | 0.0 (33.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (33.3) | 0.072 |
| Financial difficulties | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (33.3) | 0.697 |
| H&N35 questionnaire | |||||
| Pain | 16.7 (16.7) | 16.7 (25.0) | 16.7 (27.1) | 0.0 (16.7) | 0.049 * |
| Swallowing | 8.3 (25.0) | 8.3 (41.7) | 8.3 (35.4) | 0.0 (25.0) | 0.642 |
| Senses problems | 16.7 (33.3) | 16.7 (33.3) | 16.7 (33.3) | 0.0 (16.7) | 0.436 |
| Speech problems | 22.2 (33.3) | 22.2 (33.3) | 22.2 (36.1) | 22.2 (22.2) | 0.742 |
| Trouble with social eating | 8.3 (25.0) | 16.7 (33.3) | 16.7 (33.3) | 16.7 (25.0) | 0.869 |
| Trouble with social contact | 6.7 (20.0) | 13.3 (33.3) | 10.0 (33.3) | 6.7 (20.0) | 0.452 |
| Less sexuality | 0.0 (66.7) | 33.3 (66.7) | 33.3 (66.7) | 33.3 (66.7) | 0.392 |
| Teeth | 0.0 (33.3) | 0.0 (33.3) | 0.0 (33.3) | 0.0 (33.3) | 0.585 |
| Opening mouth | 33.3 (66.7) | 33.3 (33.3) | 16.7 (41.7) | 33.3 (33.3) | 0.632 |
| Dry mouth | 0.0 (33.3) | 0.0 (33.3) | 0.0 (0.0) | 33.3 (33.3) | 0.145 |
| Sticky saliva | 33.3 (33.3) | 33.3 (33.3) | 16.7 (33.3) | 33.3 (33.3) | 0.768 |
| Coughing | 0.0 (33.3) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.190 |
| Felt ill | 0.0 (0.0) | 0.0 (33.3) | 0.0 (33.3) | 0.0 (0.0) | 0.787 |
| Pain killers | 100.0 (100.0) | 100.0 (100.0) | 100.0 (100.0) | 0.0 (100.0) | 0.025 * |
| Nutritional supplements | 0.0 (100.0) | 0.0 (100.0) | 0.0 (100.0) | 0.0 (100.0) | 0.066 |
| Feeding tube | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.392 |
| Weight loss | 0.0 (100.0) | 0.0 (100.0) | 0.0 (100.0) | 0.0 (100.0) | 0.234 |
| Weight gain | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.330 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riva, G.; Salonia, L.; Fassone, E.; Sapino, S.; Piano, F.; Pecorari, G. Quality of Life in Electrochemotherapy for Cutaneous and Mucosal Head and Neck Tumors. J. Clin. Med. 2021, 10, 4366. https://doi.org/10.3390/jcm10194366
Riva G, Salonia L, Fassone E, Sapino S, Piano F, Pecorari G. Quality of Life in Electrochemotherapy for Cutaneous and Mucosal Head and Neck Tumors. Journal of Clinical Medicine. 2021; 10(19):4366. https://doi.org/10.3390/jcm10194366
Chicago/Turabian StyleRiva, Giuseppe, Laura Salonia, Elisabetta Fassone, Silvia Sapino, Fabrizio Piano, and Giancarlo Pecorari. 2021. "Quality of Life in Electrochemotherapy for Cutaneous and Mucosal Head and Neck Tumors" Journal of Clinical Medicine 10, no. 19: 4366. https://doi.org/10.3390/jcm10194366
APA StyleRiva, G., Salonia, L., Fassone, E., Sapino, S., Piano, F., & Pecorari, G. (2021). Quality of Life in Electrochemotherapy for Cutaneous and Mucosal Head and Neck Tumors. Journal of Clinical Medicine, 10(19), 4366. https://doi.org/10.3390/jcm10194366







