Long-Term Outcome of Mechanical and Biological Prostheses in Patients with Left-Side Infective Endocarditis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Collection and Extraction
2.3. Endpoints
2.4. Statistical Analysis
3. Results
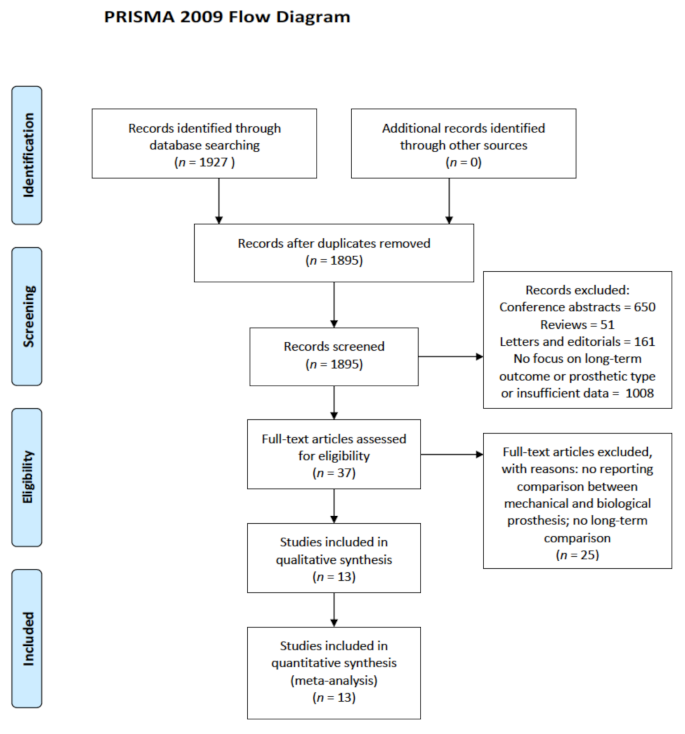
3.1. Study Selection and Literature Search
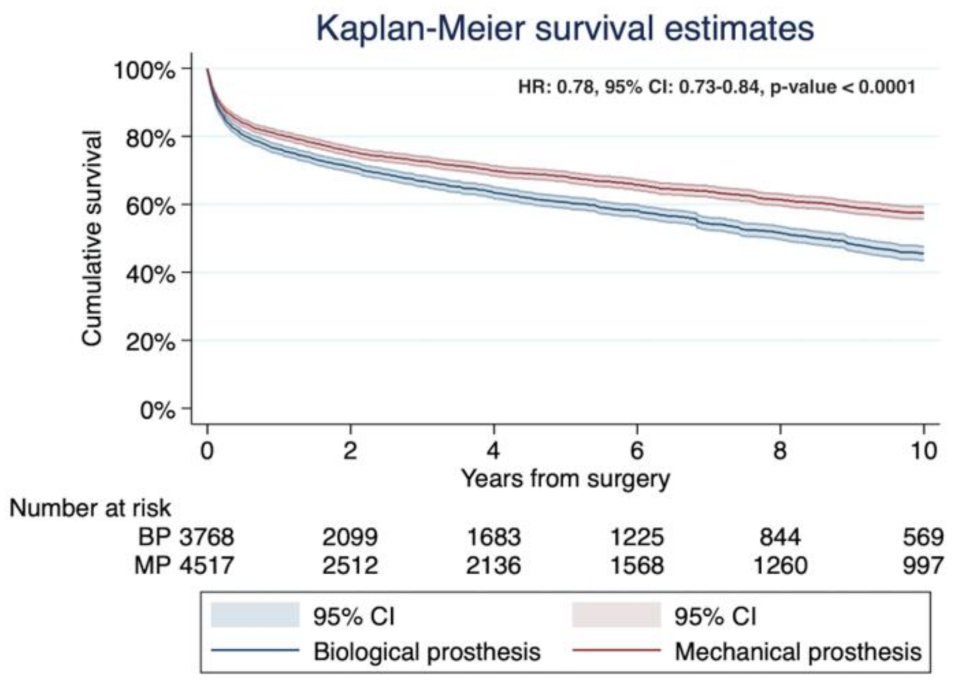
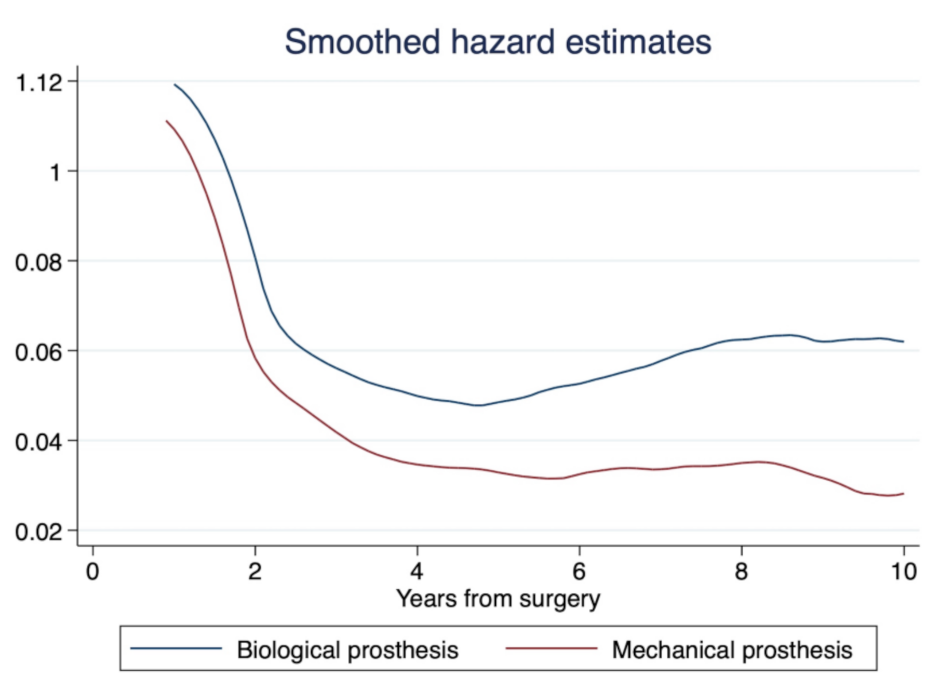
3.2. Study Primary Endpoint: Long-Term Mortality
3.3. Study Secondary Outcome: Early Mortality and Freedom from Both Prosthesis Reinfection and Reintervention
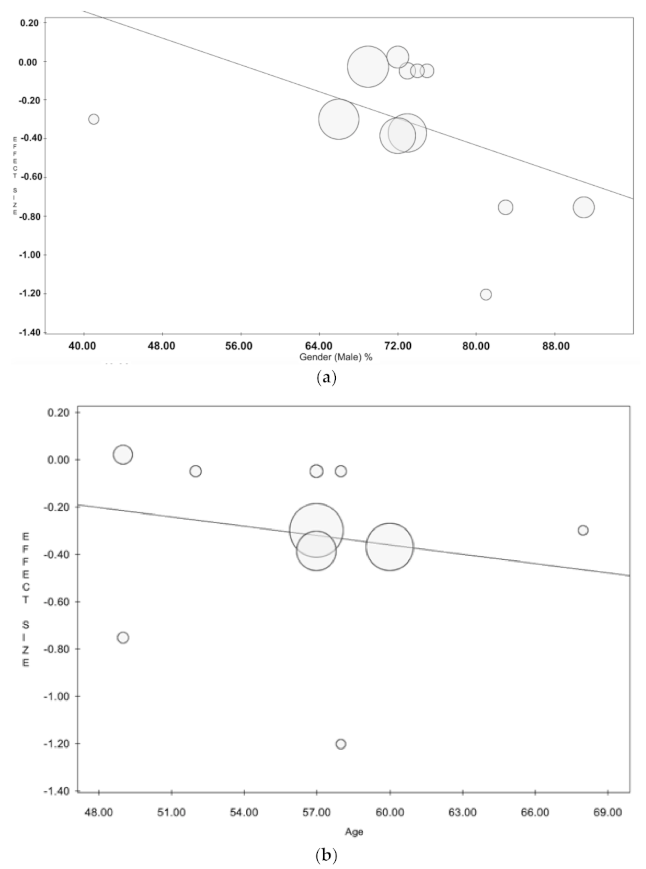
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pettersson, G.B.; Hussain, S.T. Current AATS guidelines on surgical treatment of infective endocarditis. Ann. Cardiothorac. Surg. 2019, 8, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Patel, N.J.; Deshmukh, A.; Golwala, H.; Patel, N.; Badheka, A.; Hirsch, G.A.; Mehta, J.L. Trends in Infective Endocarditis Incidence, Microbiology, and Valve Replacement in the United States from 2000 to 2011. J. Am. Coll. Cardiol. 2015, 65, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Dayer, M.J.; Jones, S.; Prendergast, B.; Baddour, L.M.; Lockhart, P.B.; Thornhill, M.H. Incidence of infective endocarditis in England, 2000–2013: A secular trend, interrupted time-series analysis. Lancet 2015, 385, 1219–1228. [Google Scholar] [CrossRef]
- Olmos, C.; Vilacosta, I.; Fernández-Pérez, C.; Bernal, J.L.; Ferrera, C.; García-Arribas, D.; Pérez-García, C.N.; San Román, J.A.; Maroto, L.; Macaya, C.; et al. The Evolving Nature of Infective Endocarditis in Spain: A Population-Based Study (2003 to 2014). J. Am. Coll. Cardiol. 2017, 70, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Von Bardeleben, R.S.; Ostad, M.A.; Hobohm, L.; Munzel, T.; Konstantinides, S.; Lankeit, M. Temporal Trends in the Prevalence of Infective Endocarditis in Germany between 2005 and 2014. Am. J. Cardiol. 2017, 119, 317–322. [Google Scholar] [CrossRef]
- Khan, M.Z.; Munir, M.B.; Khan, M.U.; Khan, S.U.; Vasudevan, A.; Balla, S. Contemporary Trends and Outcomes of Prosthetic Valve Infective Endocarditis in the United States: Insights from the Nationwide Inpatient Sample. Am. J. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Salem, M.; Friedrich, C.; Saad, M.; Frank, D.; Salem, M.; Puehler, T.; Schoettler, J.; Schoeneich, F.; Cremer, J.; Haneya, A. Active Infective Native and Prosthetic Valve Endocarditis: Short- and Long-Term Outcomes of Patients after Surgical Treatment. J. Clin. Med. 2021, 10, 1868. [Google Scholar] [CrossRef] [PubMed]
- Elgharably, H.; Hussain, S.T.; Shrestha, N.K.; Blackstone, E.H.; Pettersson, G.B. Current Hypotheses in Cardiac Surgery: Biofilm in Infective Endocarditis. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Hear. J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Pettersson, G.B.; Coselli, J.S.; Hussain, S.T.; Griffin, B.; Blackstone, E.H.; Gordon, S.M.; LeMaire, S.A.; Woc-Colburn, L.E.; AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J. Thorac. Cardiovasc. Surg. 2017, 153, 1241–1258.e29. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Royston, P. Recostructing time-to-event data from published Kaplan-Meier curves. Stata J. 2017, 17, 786–802. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.R.; Miller, D.C.; Moore, K.A.; Oyer, P.E.; Mitchell, R.S.; Robbins, R.C.; Stinson, E.B.; Shumway, N.E.; Reitz, B.A. Treatment of endocarditis with valve replacement: The question of tissue versus mechanical prosthesis. Ann. Thorac. Surg. 2001, 71, 1164–1171. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Delahaye, F.; Obadia, J.-F.; Duval, X.; Selton-Suty, C.; Carteaux, J.-P.; Hoen, B.; Alla, F. for the AEPEI Study Group. Aortic valve replacement for active infective endocarditis: 5-year survival comparison of bioprostheses, homografts and mechanical prostheses. Eur. J. Cardio-Thorac. Surg. 2010, 37, 1025–1032. [Google Scholar] [CrossRef]
- Fedoruk, L.M.; Jamieson, W.R.E.; Ling, H.; MacNab, J.S.; Germann, E.; Karim, S.S.; Lichtenstein, S.V. Predictors of recurrence and reoperation for prosthetic valve endocarditis after valve replacement surgery for native valve endocarditis. J. Thorac. Cardiovasc. Surg. 2009, 137, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Musci, M.; Hübler, M.; Amiri, A.; Stein, J.; Kosky, S.; Meyer, R.; Weng, Y.; Hetzer, R. Surgical treatment for active infective prosthetic valve endocarditis: 22-year single-centre experience. Eur. J. Cardio-Thorac. Surg. 2010, 38, 528–538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jassar, A.S.; Bavaria, J.E.; Szeto, W.Y.; Moeller, P.J.; Maniaci, J.; Milewski, R.K.; Gorman, J.H., 3rd; Desai, N.D.; Gorman, R.C.; Pochettino, A. Graft Selection for Aortic Root Replacement in Complex Active Endocarditis: Does It Matter? Ann. Thorac. Surg. 2012, 93, 480–487. [Google Scholar] [CrossRef]
- Greason, K.L.; Thomas, M.; Steckelberg, J.M.; Daly, R.C.; Schaff, H.V.; Li, Z.; Dearani, J.A. Outcomes of surgery in the treatment of isolated nonnative mitral valve infective endocarditis. J. Thorac. Cardiovasc. Surg. 2014, 147, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Leither, M.D.; Shroff, G.R.; Ding, S.; Gilbertson, D.T.; Herzog, C.A. Long-term survival of dialysis patients with bacterial endocarditis undergoing valvular replacement surgery in the United States. Circulation 2013, 128, 344–351. [Google Scholar] [CrossRef]
- Delahaye, F.; Chu, V.; Altclas, J.; Barsic, B.; Delahaye, A.; Freiberger, T.; Gordon, D.; Hannan, M.; Hoen, B.; Kanj, S.; et al. One-year outcome following biological or mechanical valve replacement for infective endocarditis. Int. J. Cardiol. 2015, 178, 117–123. [Google Scholar] [CrossRef]
- Kim, J.B.; Ejiofor, J.I.; Yammine, M.; Camuso, J.M.; Walsh, C.W.; Ando, M.; Melnitchouk, S.I.; Rawn, J.D.; Leacche, M.; MacGillivray, T.E.; et al. Are homografts superior to conventional prosthetic valves in the setting of infective endocarditis involving the aortic valve? J. Thorac. Cardiovasc. Surg. 2016, 151, 1239–1248.e2. [Google Scholar] [CrossRef]
- Said, S.M.; Abdelsattar, Z.M.; Schaff, H.V.; Greason, K.L.; Daly, R.C.; Pochettino, A.; Joyce, L.D.; Dearani, J.A. Outcomes of surgery for infective endocarditis: A single-centre experience of 801 patients. Eur. J. Cardio-Thorac. Surg. 2018, 53, 435–439. [Google Scholar] [CrossRef]
- Toyoda, N.; Itagaki, S.; Tannous, H.; Egorova, N.N.; Chikwe, J. Bioprosthetic Versus Mechanical Valve Replacement for Infective Endocarditis: Focus on Recurrence Rates. Ann. Thorac. Surg. 2018, 106, 99–106. [Google Scholar] [CrossRef]
- Kytö, V.; Ahtela, E.; Sipilä, J.; Rautava, P.; Gunn, J. Mechanical versus biological valve prosthesis for surgical aortic valve replacement in patients with infective endocarditis. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 386–392. [Google Scholar] [CrossRef]
- Rubino, A.S.; Della Ratta, E.E.; Galbiati, D.; Ashurov, R.; Galgano, V.L.; Montella, A.P.; De Feo, M.; Della Corte, A. Can prosthesis type influence the recurrence of infective endocarditis after surgery for native valve endocarditis? A propensity weighted comparison. Eur. J. Cardio-Thorac. Surg. 2021. [Google Scholar] [CrossRef]
- Tao, E.; Wan, L.; Wang, W.; Luo, Y.; Zeng, J.; Wu, X. The prognosis of infective endocarditis treated with biological valves versus mechanical valves: A meta-analysis. PLoS ONE 2017, 12, e0174519. [Google Scholar] [CrossRef] [PubMed]
- Flynn, C.D.; Curran, N.P.; Chan, S.; Zegri-Reiriz, I.; Tauron, M.; Tian, D.H.; Pettersson, G.B.; Coselli, J.S.; Misfeld, M.; Antunes, M.J.; et al. Systematic review and meta-analysis of surgical outcomes comparing mechanical valve replacement and bioprosthetic valve replacement in infective endocarditis. Ann. Cardiothorac. Surg. 2019, 8, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.G.; Murdoch, D.J.; Alu, M.C.; Cheung, A.; Crowley, A.; Dvir, D.; Herrmann, H.C.; Kodali, S.K.; Leipsic, J.; Miller, D.C.; et al. 3-Year Outcomes After Valve-in-Valve Transcatheter Aortic Valve Replacement for Degenerated Bioprostheses: The PARTNER 2 Registry. J. Am. Coll. Cardiol. 2019, 73, 2647–2655. [Google Scholar] [CrossRef]
- Klautz, R.J.M.; Vriesendorp, M.D.; Dagenais, F.; Labrousse, L.; Bapat, V.; Moront, M.G.; Misfeld, M.; Gearhart, E.; Kappetein, A.P.; Sabik, J.F., 3rd. Antithrombotic therapy and bleeding events after aortic valve replacement with a novel bioprosthesis. J. Thorac. Cardiovasc. Surg. 2021, 161, 66–75.e4. [Google Scholar] [CrossRef]
- Bartus, K.; Litwinowicz, R.; Bilewska, A.; Stapor, M.; Bochenek, M.; Rozanski, J.; Sadowski, J.; Filip, G.; Kusmierczyk, M.; Kapelak, B. Final 5-year outcomes following aortic valve replacement with a RESILIA™ tissue bioprosthesis. Eur. J. Cardio-Thorac. Surg. 2021, 59, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Puskas, J.D.; Gerdisch, M.; Nichols, D.; Fermin, L.; Rhenman, B.; Kapoor, D.; Copeland, J.; Quinn, R.; Hughes, G.C.; Azar, H.; et al. Anticoagulation and Antiplatelet Strategies After On-X Mechanical Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 2717–2726. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]


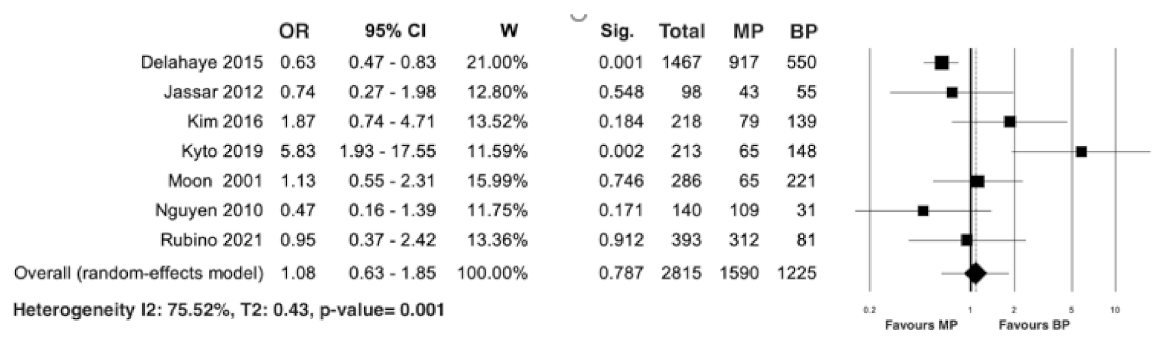
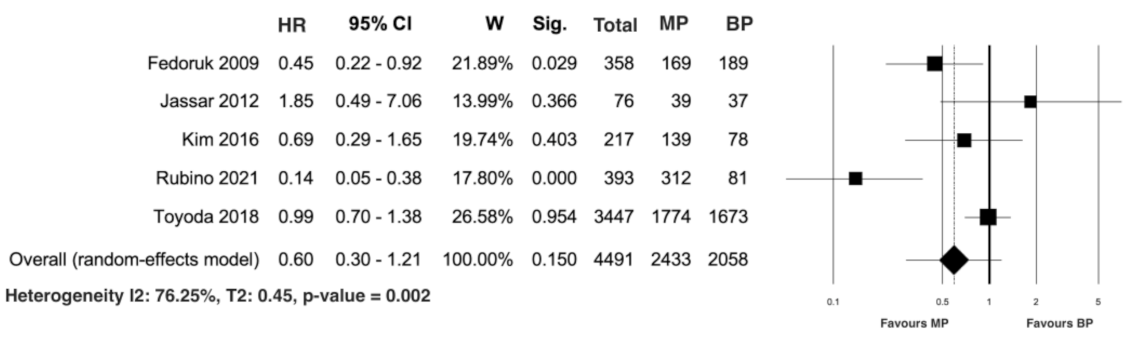
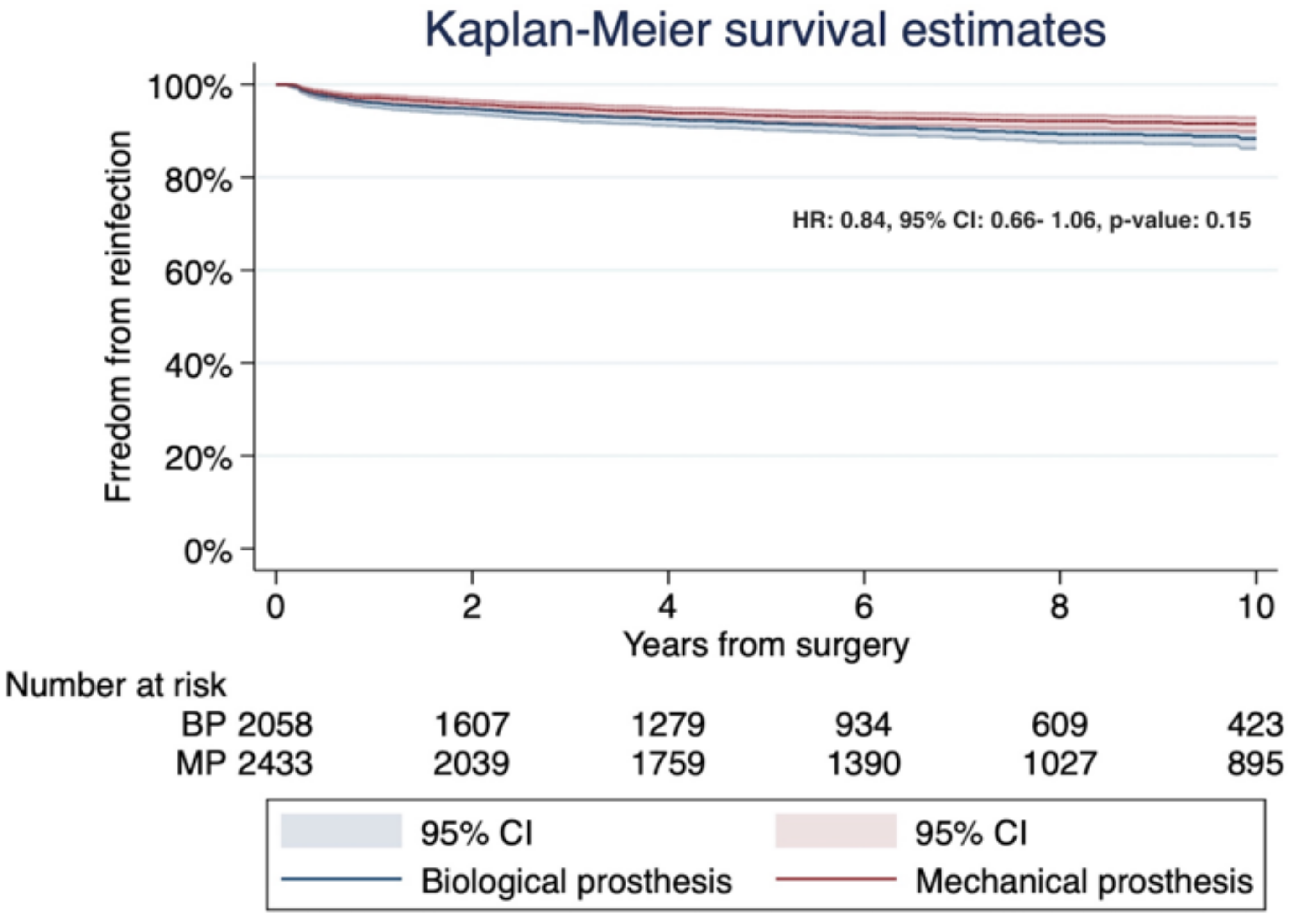

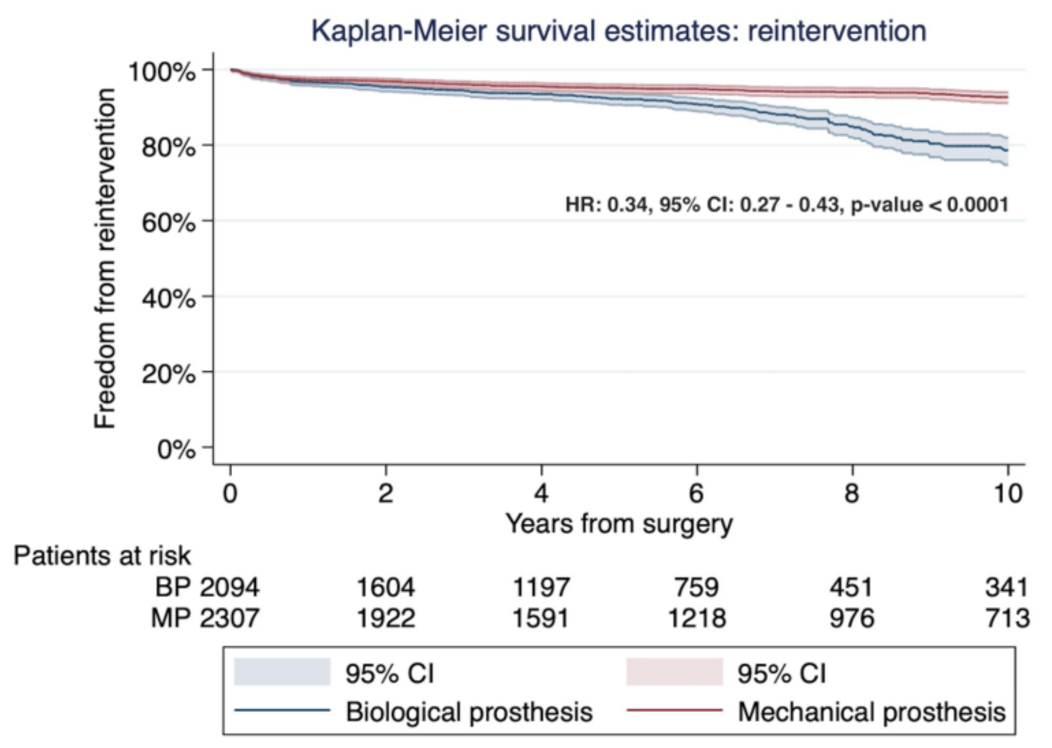
| Authors (Years/Country/Study Design) | Number of Patients (%) | Male Gender (%) | Mean Age | DM (%) | RF (%) |
|---|---|---|---|---|---|
| a Moon et al. [20] (2001/USA/ROS) | Total 286 | Total: 221 (72) | Total: 49 | Total: 32 (10.4) | Total: 78 (25.5) |
| MP: 65 (22.7) | MP: n.a. | MP: n.a. | MP: n.a. | MP: n.a. | |
| BP: 221 (77.3) | BP: n.a. | BP: n.a. | BP: n.a. | BP: n.a. | |
| b Nguyen et al. [21] (2009/France/ROS) | Total: 140 | Total: 113 (80.7) | Total: 57.9 | Total: 17 (12.1) | Total: 15 (10.7) |
| MP: 109 (77.8) | MP: 88 (80.7) | MP: 57.3 | MP: 14 (12.8) | MP: 11 (10.1) | |
| BP: 31 (22.2) | BP: 25 (80.6) | BP: 63.2 | BP: 3 (9.7) | BP: 4 (12.9) | |
| Fedoruk et al. [22] (2009/Canada/ROS) | Total: 358 | Total: 257 (71.8) | Total: 41.8 | Total: n.a. | Total: n.a. |
| MP: 169 (47.2) | MP: n.a. | MP: 45.6 | MP: n.a. | MP: n.a. | |
| BP: 189 (52.8) | BP: n.a. | BP: 51.6 | BP: n.a. | BP: n.a. | |
| c Musci et al. [23] (2010/Germany/ROS) | Total 122 | Total: 255 (73.1) | Total: 57.3 | Total: 76 (21.8) | Total: 130 (37) |
| MP: 29 (23.8) | MP: n.a. | MP: n.a. | MP: n.a. | MP: n.a. | |
| BP: 93 (76.2) | BP: n.a. | BP: n.a. | BP: n.a. | BP: n.a. | |
| d Jassar et al. [24] (2012/USA/ROS) | Total: 98 | Total: 73 (74.5) | Total: 58.3 | Total: 17 (17.3) | Total: 13 (13.3) |
| MP: 43 (43.9) | MP: 38 (88.3) | MP: 51.8 | MP: 9 (20.9) | MP: 3 (7) | |
| BP: 55 (56.1) | BP: 35 (63.6) | BP: 62.7 | BP: 10 (18.2) | BP: 10 (18.2) | |
| Leither et al. [25] (2013/USA/ROS) | Total: 1267 | Total: 763 (69.2) | Total: n.a. | Total: 454 (35.8) | Total: 1267 (100) |
| MP: 761 (60) | MP: 417 (61.7) | MP: n.a. | MP: 241 (34.1) | MP: 761 (100) | |
| BP: 506 (40) | BP: 346 (59.1) | BP: n.a. | BP: 212 (37.8 | BP: 506 (100) | |
| Greason et al. [26] (2014/USA/ROS) | Total: 39 | Total: 16 (41) | Total: 68 | Total: n.a. | Total: 6 (15) |
| MP: 23 (59) | MP: n.a. | MP: n.a. | MP: n.a. | MP: n.a. | |
| BP: 16 (41) | BP: n.a. | BP: n.a. | BP: n.a. | BP: n.a. | |
| Delahaye et al. [27] (2014/Multicenter/ROS) | Total: 1467 | Total: 1053 (71.9) | Total: 56.6 | Total: n.a. | Total: 88 (12.6) |
| MP: 917 (62.5) | MP: 665 (72.7) | MP: 53.6 | MP: n.a. | MP: 39 (9.5) | |
| BP: 550 (37.5) | BP: 388 (70.5) | BP: 61.6 | BP: n.a. | BP: 49 (17.1) | |
| e Kim et al. [28] (2016/South Korea/ROS) | Total: 218 | Total: 165 (75.8) | Total: 52.3 | Total: 36 (16.5) | Total: n.a. |
| MP: 79 (36.2) | MP: 60 (75.5) | MP: 47.2 | MP: 6 (5.6) | MP: n.a. | |
| BP: 139 (63.8) | BP: 105 (75.9) | BP: 59.8 | BP: 30 (21.6) | BP: n.a. | |
| f Said et al. [29] (2017/USA(ROS) | Total: 595 | Total: n.a. | Total: n.a. | Total: n.a. | Total: n.a. |
| MP: 312 (52.4) | MP: n.a | MP: n.a | MP: n.a | MP: n.a | |
| BP: 283 (47.6) | BP: n.a | BP: n.a | BP: n.a | BP: n.a | |
| Toyoda et al. [30] (2018/USA/ROS) | Total: 3247 | Total: 2295 (70.7) | Total: n.a. | Total: 332 (10.2) | Total: 738 (22.7) |
| MP: 1574 (48.5) | MP: 1207 (70.7) | MP: 53.4 | MP: 163 (10.3) | MP: 365 (23.2) | |
| BP: 1673 (51.5) | BP: 1086 (64.9) | BP: 60.4 | BP: 169 (10.1) | BP: 373 (22.3) | |
| Kyto et al. [31] (2019/Finland/ROS) | Total: 213 | Total: 177 (83.1) | Total: 49.1 | Total: n.a. | Total: n.a. |
| MP: 148 (69.5) | MP: 130 (87.8) | MP: 47.1 | MP: n.a. | MP: n.a. | |
| BP: 65 (30.5) | BP: 47 (72.3) | BP: 53.7 | BP: n.a. | BP: n.a. | |
| Rubino et al. [32] (2021/Italy/ROS) | Total: 395 | Total: 296 (74.9) | Total: n.a. | Total: 46 (11.6) | Total: 21 (5.3) |
| MP: 314 | MP: 235 (74.8) | MP: 50.1 | MP: 39 (12.4) | MP: 19 (6.1) | |
| BP: 81 | BP: 61/75.3) | BP: 56.3 | BP: 7 (8.6) | BP: 2(2.5) |
| Authors (Years/Country) | Active Endocarditis (%) | Cardiogenic Shock (%) | Stroke (%) | Septic Shock (%) |
|---|---|---|---|---|
| Moon et al. [20] (2001/USA/ROS) | Total: 211 (68.9) | Totale: 218 (71.2) | n.a. | Total: 20 (6.5) |
| MP: n.a. | MP: n.a. | MP: n.a. | MP: n.a. | |
| BP: n.a. | BP: n.a. | BP: n.a. | BP: n.a. | |
| Nguyen et al. [21] (2009/France/ROS) | Total: 140 (100) | Total: 38 (27.1) | Total: 20 (14.2) | Total: 13 (9.3) |
| MP: 109 (100) | MP: 31 (28.4) | MP: 19 (17.4) | MP: 9 (8.2) | |
| BP: 31 (100) | BP: 7 (22.6) | BP: 1 (14.3) | BP: 4 (12.9) | |
| Fedoruk et al. [22] (2009/Canada/ROS) | Total: n.a. | Total: n.a. | Total: n.a. | Total: n.a. |
| MP: n.a. | MP: n.a. | MP: n.a. | MP: n.a. | |
| BP: n.a. | BP: n.a. | BP: n.a. | BP: n.a. | |
| Musci et al. [23] (2010/Germany/ROS) | Total: 42 (34.4) | Total: 35 (10) | Total: 25 (7.2) | Total: 37 (10.6) |
| MP: 9 (21.42) | MP: n.a. | MP: n.a. | MP: n.a. | |
| BP: 33 (78.6) | BP: n.a. | BP: n.a. | BP: n.a. | |
| Jassar et al. [24] (2012/USA/ROS) | Total: 98 (100) | Total: 7 (7.1) | Total: 30 (30.6) | Total: n.a. |
| MP: 43 (100) | MP: 3 (3) | MP: 13 (30.2) | MP: n.a. | |
| BP: 55 (100) | BP: 4 (11.3) | BP: 17 (30.9) | BP: n.a. | |
| Leither et al. [25] (2013/USA/ROS) | Total: n.a. | Total: n.a. | Total: 399 (31.5) | Total: n.a. |
| MP: n.a. | MP: n.a. | MP: 278 (36.6) | MP: n.a. | |
| BP: n.a. | BP: n.a. | BP: 186 (36.8) | BP: n.a. | |
| Greason et al. [26] (2014/USA/ROS) | Total: 12 (31) | Total: 22 (56) | Total: n.a. | Total: 6 (15) |
| MP: n.a. | MP: n.a. | MP: n.a. | MP: n.a. | |
| BP: n.a. | BP: n.a. | BP: n.a. | BP: n.a. | |
| Delahaye et al. [27] (2014/Multicenter/ROS) | Total: 1467 (100) | Total: n.a. | Total: n.a. | Total: n.a. |
| MP: 917 (100) | MP: n.a. | MP: n.a. | MP: n.a. | |
| BP: 550 (100) | BP: n.a. | BP: n.a. | BP: n.a. | |
| Kim et al. [28] (2016/South Korea/ROS) | Total: 218 (100) | Total: 10 (4.6) | Total: n.a. | Total: n.a. |
| MP: 79 (100) | MP: 5 (6.3) | MP: n.a. | MP: n.a. | |
| BP: 139 (100) | BP: 139 (3.6) | BP: n.a. | BP: n.a. | |
| Said et al. [29] (2017/USA/ROS) | Total: n.a. | Total: n.a. | Total: n.a. | Total: n.a. |
| MP: n.a. | MP: n.a. | MP: n.a. | MP: n.a. | |
| BP: n.a. | BP: n.a. | BP: n.a. | BP: n.a. | |
| Toyoda et al. [30] (2018/USA/ROS) | Total: 3247 (100 | Total: 1974 (60.8) | Total: n.a. | Total: n.a. |
| MP: 1574 (100) | MP: 981 (62.3) | MP: n.a. | MP: n.a. | |
| BP: 1673 (100) | BP: 993 (59.3) | BP: n.a. | BP: n.a. | |
| Kyto et al. [31] (2019/Finland/ROS) | Total: n.a. | Total: n.a. | Total: n.a. | Total: n.a. |
| MP: n.a. | MP: n.a. | MP: n.a. | MP: n.a. | |
| BP: n.a. | BP: n.a. | BP: n.a. | BP: n.a. | |
| Rubino et al. [32] (2021/Italy/ROS) | Total: 395 (100) | Total: n.a. | Total: 59 (14.9) | Total: n.a. |
| MP: 314 (100) | MP: n.a. | MP: 46 (14.6) | MP: n.a. | |
| BP: 81 (100) | BP: n.a. | BP: 13 (16) | BP: n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formica, F.; Maestri, F.; Gripshi, F.; Gallingani, A.; Grossi, S.; Nicolini, F. Long-Term Outcome of Mechanical and Biological Prostheses in Patients with Left-Side Infective Endocarditis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 4356. https://doi.org/10.3390/jcm10194356
Formica F, Maestri F, Gripshi F, Gallingani A, Grossi S, Nicolini F. Long-Term Outcome of Mechanical and Biological Prostheses in Patients with Left-Side Infective Endocarditis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(19):4356. https://doi.org/10.3390/jcm10194356
Chicago/Turabian StyleFormica, Francesco, Francesco Maestri, Florida Gripshi, Alan Gallingani, Silvia Grossi, and Francesco Nicolini. 2021. "Long-Term Outcome of Mechanical and Biological Prostheses in Patients with Left-Side Infective Endocarditis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 19: 4356. https://doi.org/10.3390/jcm10194356
APA StyleFormica, F., Maestri, F., Gripshi, F., Gallingani, A., Grossi, S., & Nicolini, F. (2021). Long-Term Outcome of Mechanical and Biological Prostheses in Patients with Left-Side Infective Endocarditis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(19), 4356. https://doi.org/10.3390/jcm10194356








