Surgery for Hypertrophic Obstructive Cardiomyopathy: Comprehensive LVOT Management beyond Septal Myectomy
Abstract
1. Introduction
2. Prevalence: The Real Burden of the Disease in 2021
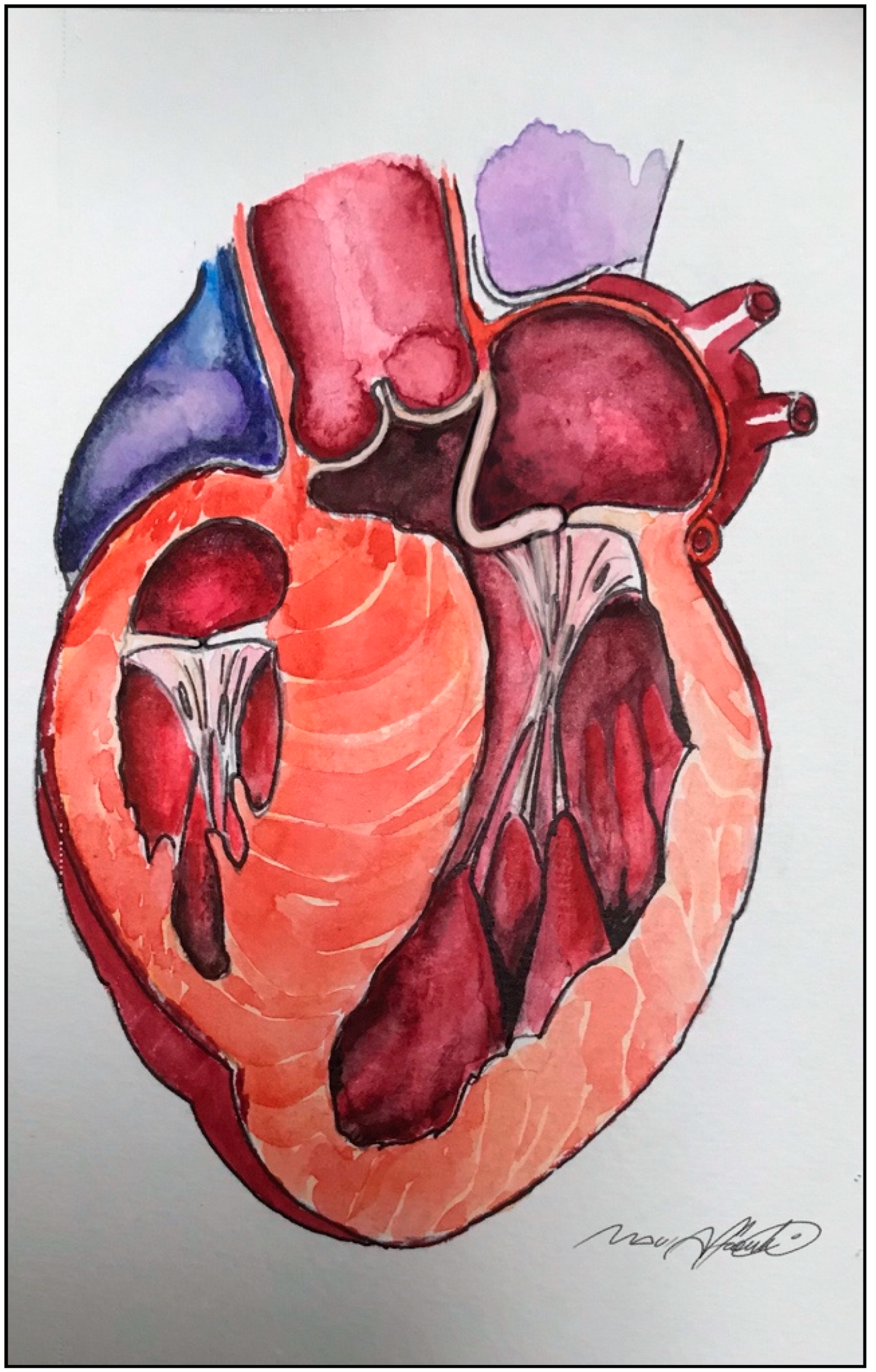
3. Main Features: Left Ventricular Outflow Tract (LVOT) Obstruction, Systolic Anterior Motion (SAM), and Mitral Regurgitation
4. Indication for Surgery
“In patients with obstructive HCM who remain severely symptomatic despite guideline-directed medical therapy, septal reduction therapy (SRT) in eligible patients, performed at experienced centers, is recommended for relieving LVOT obstruction”(COR I, LOE B-NR).
5. Extended Septal Myectomy: Evolution of a Technique
6. Left Ventricular Outflow Tract Management beyond Septal Myectomy
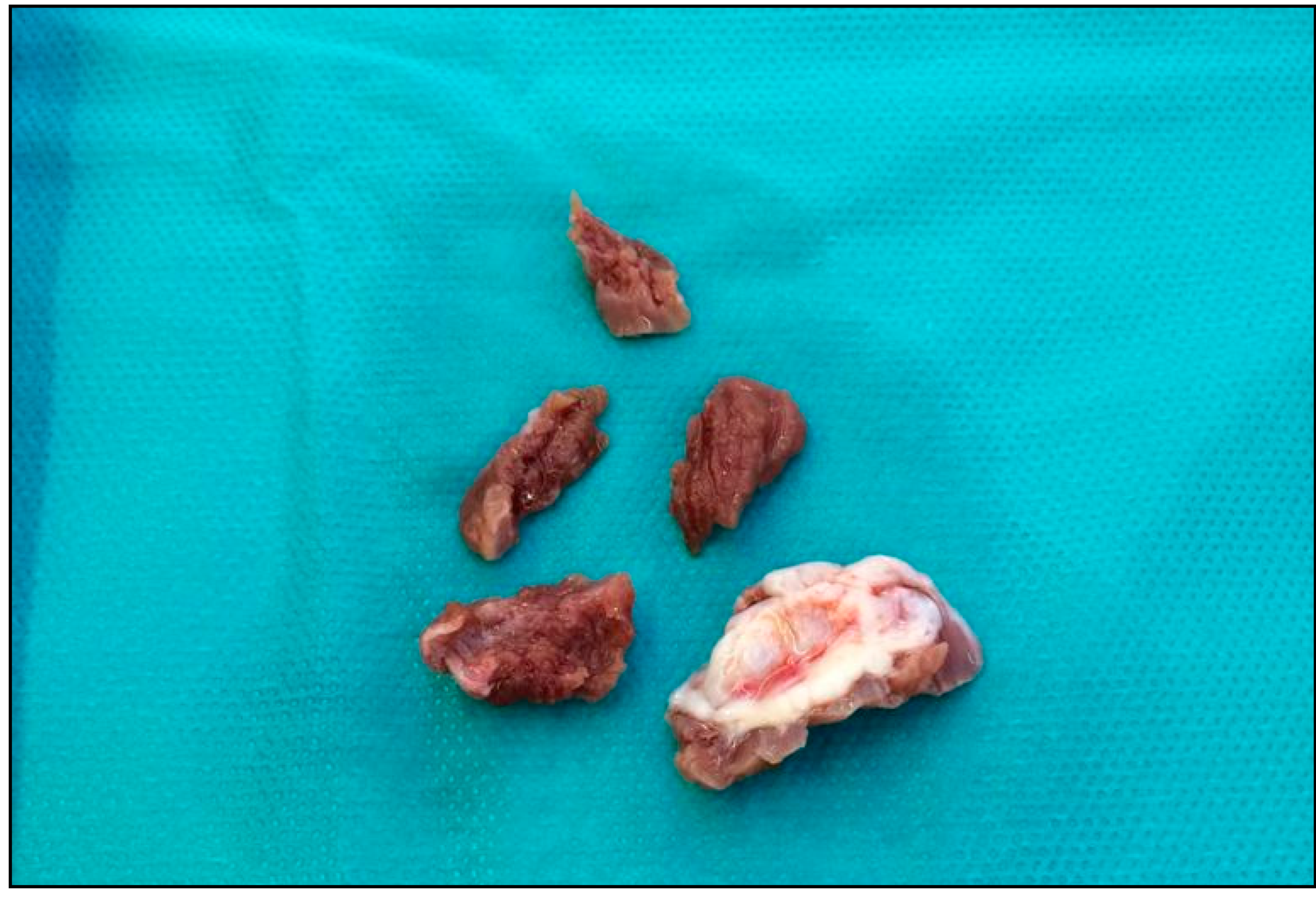
7. Elongation of Mitral Leaflets
8. Displacement of the Anterolateral Papillary Muscle and Abnormal Connections with Left Ventricle Free Wall
9. Bifid and Hypermobile Papillary Muscles
10. Insertion of the Anterolateral Papillary Muscle Directly into the Anterior Mitral Leaflet
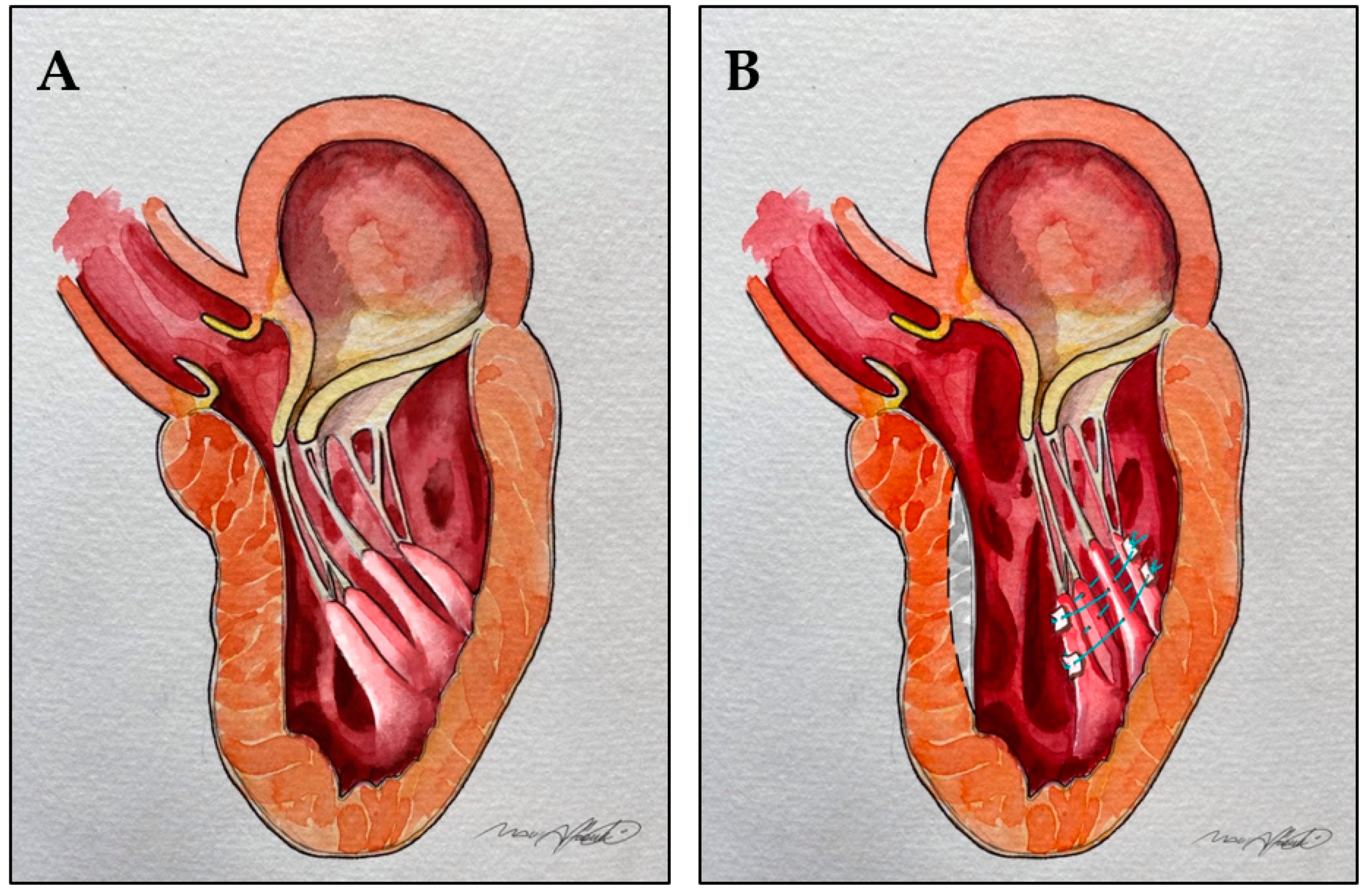
11. Anterior Mitral Leaflet Tethering by Secondary Order Chordae
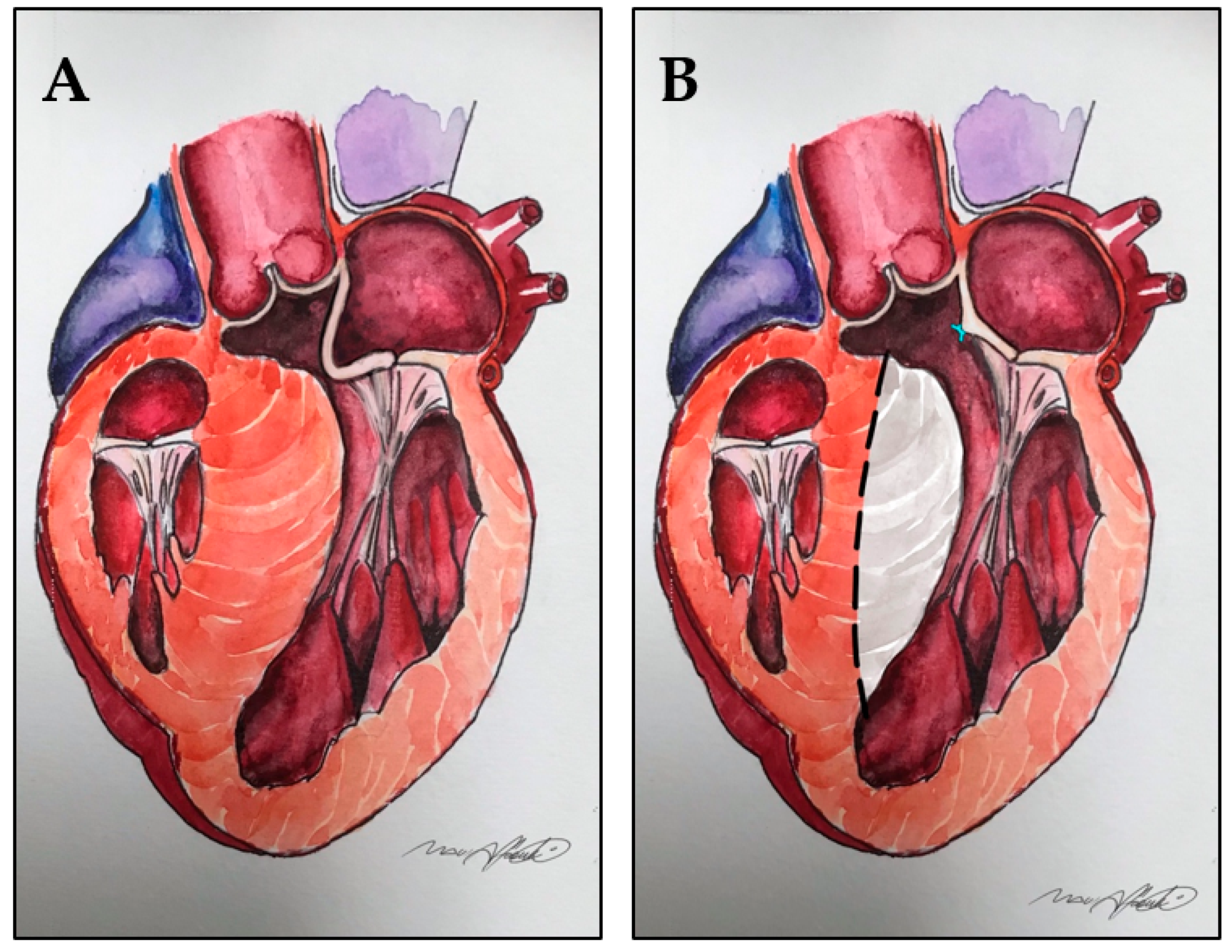
12. Mitro-Aortic Discontinuity
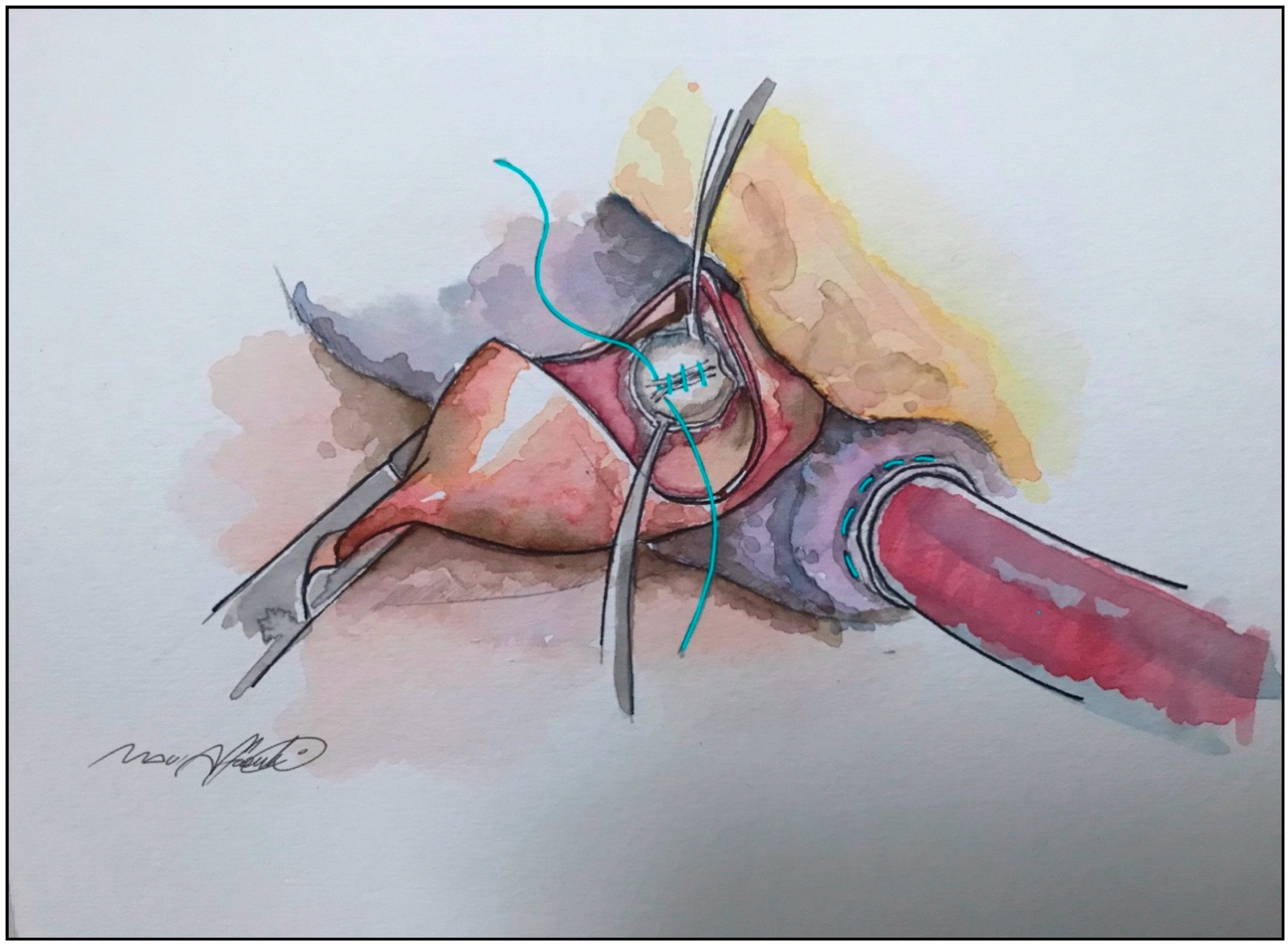
13. “Edge-to-Edge” Technique
14. Outcomes: The Importance of a Tailored Approach and Dedicated High Expertise Referral Centers
15. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 22, e533–e557. [Google Scholar]
- Marian, A.; Roberts, R. The Molecular Genetic Basis for Hypertrophic Cardiomyopathy. J. Mol. Cell. Cardiol. 2001, 33, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.; Mercadier, J.-J. Cardiac troponin T and familial hypertrophic cardiomyopathy: An energetic affair. J. Clin. Investig. 2003, 112, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Ingles, J.; Burns, C.; Bagnall, R.; Lam, L.; Yeates, L.; Sarina, T.; Puranik, R.; Briffa, T.; Atherton, J.; Driscoll, T.; et al. Non-familial hypertrophic cardiomyopathy: Prevalence, natural history and clinical implications. J. Am. Coll. Cardiol. 2017, 69, 839. [Google Scholar] [CrossRef]
- Maron, B.J.; Gardin, J.M.; Flack, J.M.; Gidding, S.S.; Kurosaki, T.T.; Bild, D.E. Prevalence of Hypertrophic Cardiomyopathy in a General Population of Young Adults. Circulation 1995, 92, 785–789. [Google Scholar] [CrossRef]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New Perspectives on the Prevalence of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Olivotto, I.; Zenovich, A.G.; Link, M.S.; Pandian, G.P.; Kuvin, J.T.; Nistri, S.; Cecchi, F.; Udelson, J.E.; Maron, B.J. Hypertrophic cardiomyopathy is predominantly a disease of left ven-tricular outflow tract obstruction. Circulation 2006, 114, 2232–2239. [Google Scholar] [CrossRef]
- Braunwald, E.; Lambrew, C.T.; Rockoff, S.D.; Ross, J.; Morrow, A.G. Idiopathic Hypertrophic Subaortic Stenosis: I. A Description of the Disease Based Upon an Analysis of 64 Patients. Circulation 1964, 29, 3–119. [Google Scholar] [CrossRef]
- Brock, R. Functional obstruction of the left ventricle; acquired aortic subvalvar stenosis. Guy’s Hosp. Rep. 1957, 106, 221–238. [Google Scholar]
- Balaram, S.K.; Ross, R.E.; Sherrid, M.; Schwartz, G.S.; Hillel, Z.; Winson, G.; Swistel, D.G. Role of Mitral Valve Plication in the Surgical Management of Hypertrophic Cardiomyopathy. Ann. Thorac. Surg. 2012, 94, 1990–1998. [Google Scholar] [CrossRef]
- Klues, H.G.; Roberts, W.C.; Maron, B.J. Anomalous insertion of papillary muscle directly into anterior mitral leaflet in hypertrophic cardiomyopathy. Significance in producing left ventricular outflow obstruction. Circulation 1991, 84, 1188–1197. [Google Scholar] [CrossRef]
- Klues, H.G.; Roberts, W.C.; Maron, B.J. Morphological determinants of echocardiographic patterns of mitral valve systolic anterior motion in obstructive hypertrophic cardiomyopathy. Circulation 1993, 87, 1570–1579. [Google Scholar] [CrossRef]
- Wigle, E.D.; Henderson, M.; Rakowski, H.; Wilansky, S. Muscular (hypertrophic) subaortic stenosis (hypertrophic obstructive cardiomyopathy): The evidence for true obstruction to left ventricular outflow. Postgrad. Med. J. 1986, 62, 531–536. [Google Scholar] [CrossRef]
- Ro, R.; Halpern, D.; Sahn, D.J.; Homel, P.; Arabadjian, M.; Lopresto, C.; Sherrid, M.V. Vector Flow Mapping in Obstructive Hypertrophic Cardiomyopathy to Assess the Relationship of Early Systolic Left Ventricular Flow and the Mitral Valve. J. Am. Coll. Cardiol. 2014, 64, 1984–1995. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Gunsburg, D.Z.; Moldenhauer, S.; Pearle, G. Systolic anterior motion begins at low left ventricular outflow tract velocity in obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2000, 36, 1344–1354. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Männer, J.; Swistel, D.G.; Olivotto, I.; Halpern, D.G. On the Cardiac Loop and Its Failing: Left Ventricular Outflow Tract Obstruction. J. Am. Heart Assoc. 2020, 9, e014857. [Google Scholar] [CrossRef]
- Schaff, H.V.; Said, S.M. Transaortic Extended Septal Myectomy for Hypertrophic Cardiomyopathy. Oper. Tech. Thorac. Cardiovasc. Surg. 2012, 17, 238–250. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Adams, D.H. The Mitral Valve in Hypertrophic Cardiomyopathy: Other Side of the Outflow Tract. J. Am. Coll. Cardiol. 2020, 10, 2248–2251. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Chaudhry, F.A.; Swistel, D.G. Obstructive hypertrophic cardiomyopathy: Echocardiography, pathophysiology, and the continuing evolution of surgery for obstruction. Ann. Thorac. Surg. 2003, 75, 620–632. [Google Scholar] [CrossRef]
- Hang, D.; Schaff, H.V.; Nishimura, R.A.; Lahr, B.D.; Abel, M.D.; Dearani, J.A.; Ommen, S.R. Accuracy of Jet Direction on Doppler Echocardiography in Identifying the Etiology of Mitral Regurgitation in Obstructive Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2019, 32, 333–340. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Betocchi, S.; Casey, S.A.; Lesser, J.R.; Losi, M.A.; Cecchi, F.; Maron, B.J. Effect of Left Ventricular Outflow Tract Obstruction on Clinical Outcome in Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2003, 348, 295–303. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 140, 2733–2779. [Google Scholar]
- Kirklin, J.W.; Ellis, F.H., Jr. Surgical relief of diffuse subvalvular aortic stenosis. Circulation 1961, 24, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.L.; Kincaid, O.W.; Swan, H.J.C.; Kirklin, J.W. Results of Surgical Treatment of Patients with Diffuse Subvalvular Aortic Stenosis. Circulation 1965, 32, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.G.; Brockenbrough, E.C. Surgical treatment of idiopathic hypertrophic subaortic stenosis: Technic and hemodynamic results of subaortic ventriculomyotomy. Ann. Surg. 1961, 154, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Messmer, B.J. Extended myectomy for hypertrophic obstructive cardiomyopathy. Ann. Thorac. Surg. 1994, 58, 575–577. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Harrigan, C.; Appelbaum, E.; Gibson, C.M.; Lesser, J.R.; Haas, T.S.; Udelson, J.E.; Manning, W.J.; Maron, B.J.; et al. Mitral valve abnormalities identified by cardiovascular magnetic reso-nance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011, 124, 40–47. [Google Scholar] [CrossRef]
- Kaple, R.K.; Murphy, R.T.; DiPaola, L.M.; Houghtaling, P.L.; Lever, H.M.; Lytle, B.W.; Blackstone, E.H.; Smedira, N.G. Mitral valve abnormalities in hypertrophic cardiomyopathy: Echo-cardiographic features and surgical outcomes. Ann. Thorac. Surg. 2008, 85, 1527–1535. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Chu, C.K.; Delia, E.; Mogtader, A.; Dwyer, E.M., Jr. An echocardiographic study of the fluid mechanics of obstruction in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1993, 2, 816–825. [Google Scholar] [CrossRef]
- Sherrid, M.V. Dynamic left ventricular outflow obstruction in hypertrophic cardiomyopathy revisited: Signifi-cance, pathogenesis, and treatment. Cardiol. Rev. 1998, 6, 135–145. [Google Scholar] [CrossRef]
- Cape, E.G.; Simons, D.; Jimoh, A.; Weyman, A.E.; Yoganathan, A.P.; Levine, R.A. Chordal geometry determines the shape and extent of systolic anterior mitral motion: In vitro studies. J. Am. Coll. Cardiol. 1989, 13, 1438–1448. [Google Scholar] [CrossRef]
- Schwammenthal, E.; Nakatani, S.; He, S.; Hopmeyer, J.; Sagie, A.; Weyman, A.E.; Lever, H.M.; Yoganathan, A.P.; Thomas, J.D.; Levine, R.A. Mechanism of mitral regurgitation in hypertrophic cardiomyopathy: Mismatch of posterior to anterior leaflet length and mobility. Circulation 1998, 98, 856–865. [Google Scholar] [CrossRef]
- McIntosh, C.L.; Maron, B.J. Current operative treatment of obstructive hypertrophic cardiomyopathy. Circulation 1988, 78, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Sherrid, M.V.; Balaram, S.; Kim, B.; Axel, L.; Swistel, D.G. The Mitral Valve in Obstructive Hypertrophic Cardiomyopathy. A Test in Context. J. Am. Coll. Cardiol. 2016, 19, 1846–1858. [Google Scholar] [CrossRef]
- Minakata, K.; Dearani, J.A.; Nishimura, R.A.; Maron, B.J.; Danielson, G.K. Extended septal myectomy for hypertrophic obstructive cardiomyopathy with anomalous mitral papillary muscles or chordae. J. Thorac. Cardiovasc. Surg. 2004, 127, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J. Commentary and re-appraisal: Surgical septal myectomy vs. alcohol ablation: After a decade of controversy and mismatch between clinical practice and guidelines. Prog. Cardiovasc. Dis. 2012, 54, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Levine, R.A.; King, M.E.; Weyman, A.E. An integrated mechanism for the systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy based on echocardiographic observations. Am. Heart J. 1997, 113, 633–644. [Google Scholar] [CrossRef]
- McIntosh, C.L.; Maron, B.J.; Cannon, R.O.; Klues, H.G. Initial results of combined anterior mitral leaflet plication and ventricular septal myotomy-myectomy for relief of left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy. Circulation 1992, 86, II60-7. [Google Scholar] [PubMed]
- Swistel, D.G.; Sherrid, M.V. The surgical management of obstructive hypertrophic cardiomyopathy: The RPR procedure-resection, plication, release. Ann. Cardiothorac. Surg. 2017, 6, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Halpern, D.G.; Swistel, D.G.; Po, J.R.; Joshi, R.; Winson, G.; Arabadjian, M.; Lopresto, C.; Kushner, J.; Kim, B.; Balaram, S.; et al. Echocardiography before and after resect-plicate release surgical myectomy for obstructive hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2015, 28, 1318–1328. [Google Scholar] [CrossRef]
- Kofflard, M.J.; van Herwerden, L.A.; Waldstein, D.J.; Ruygrok, P.; Boersma, E.; Taams, M.A.; Ten Cate, F.J. Initial results of combined anterior mitral leaflet extension and myectomy in patients with obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1996, 28, 197–202. [Google Scholar] [CrossRef][Green Version]
- van der Lee, C.; Kofflard, M.J.M.; van Herwerden, L.A.; Vletter, W.B.; ten Cate, F.J. Sustained improvement after combined anterior mitral leaflet extension and myectomy in hypertrophic obstructive cardiomyopathy. Circulation 2003, 108, 2088–2092. [Google Scholar] [CrossRef]
- Vriesendorp, P.A.; Schinkel, A.F.L.; Soliman, O.I.I.; Kofflard, M.J.M.; de Long, P.L.; van Herwerden, L.A.; Ten Cate, F.J.; Michels, M. Long-Term Benefit of Myectomy and Anterior Mitral Leaflet Extension in Obstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2015, 115, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.M.; Taylor, R.D.; Wong, M. Abnormal mitral valve coaptation in hypertrophic obstructive cardio myopathy: Proposed role in systolic anterior motion of mitral valve. Am. J. Cardiol. 1981, 48, 258–262. [Google Scholar] [CrossRef]
- Levine, R.A.; Vlahakes, G.J.; Lefebvre, X.; Guerrero, J.L.; Cape, E.G.; Yoganathan, A.P.; Weyman, A.E. Papillary muscle displacement causes systolic anterior motion of the mitral valve. Experimental validation and insights into the mechanism of subaortic obstruction. Circulation 1995, 91, 1189–1195. [Google Scholar] [CrossRef]
- Henry, W.L.; Clark, C.E.; Griffith, J.M.; Epstein, S.E. Mechanism of left ventricular outflow obstruction in patients with obstructive asymmetric septal hypertrophy (idiopathic hypertrophic subaortic stenosis). Am. J. Cardiol. 1975, 35, 337–345. [Google Scholar] [CrossRef]
- Kwon, D.H.; Setser, R.M.; Thamilarasan, M.; Popovic, Z.V.; Smedira, N.G.; Schoenhagen, P.; Garcia, M.J.; Lever, H.M.; Desai, M.Y. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart 2008, 94, 1295–1301. [Google Scholar] [CrossRef]
- Kwon, D.H.; Smedira, N.G.; Thamilarasan, M.; Lytle, B.W.; Lever, H.; Desai, M.Y. Characteristics and surgical outcomes of symptomatic patients with hypertrophic cardiomyopathy with abnormal papillary muscle morphology undergoing papillary muscle reorientation. J. Thorac. Cardiovasc. Surg. 2010, 140, 317–324. [Google Scholar] [CrossRef]
- Klues, H.G.; Maron, B.J.; Dollar, A.L.; Roberts, W.C. Diversity of structural mitral valve alterations in hypertrophic cardiomyopathy. Circulation 1992, 85, 1651–1660. [Google Scholar] [CrossRef]
- Messas, E.; Luis Guerrero, J.; Handshumacher, M.; Conrad, C.; Chow, C.M.; Sullivan, S.; Yoganathan, A.P.; Levine, R.A. Chordal cutting. A new therapeutic approach for ischemic mitral regurgitation. Circulation 2001, 104, 1958–1963. [Google Scholar] [CrossRef]
- Ferrazzi, P.; Spirito, P.; Iacovoni, A.; Calabrese, A.; Migliorati, K.; Simon, C.; Pentiricci, S.; Poggio, D.; Grillo, M.; Amigoni, P.; et al. Transaortic Chordal Cutting Mitral Valve Repair for Obstructive Hypertrophic Cardiomyopathy With Mild Septal Hypertrophy. J. Am. Coll. Cardiol. 2015, 66, 1687–1696. [Google Scholar] [CrossRef]
- Chesler, E.; Joffe, H.S.; Beck, W.; Schrire, V. Echocardiographic recognition of mitral-semilunar valve discontinuity: An aid to the diagnosis of origin of both great vessels from the right ventricle. Circulation 1971, 43, 725–732. [Google Scholar] [CrossRef]
- French, J.W.; Popp, R. Variability of echocardiographic discontinuity in double outlet right ventricle and truncus arteriosus. Circulation 1975, 51, 848–854. [Google Scholar] [CrossRef]
- Ferrazzi, P.; Spirito, P.; Binaco, I.; Zyrianov, A.; Poggio, D.; Vaccari, G.; Grillo, M.; Pezzoli, L.; Scatigno, A.; Dorobantu, L.; et al. Congenital Muscular Mitral-Aortic Discontinuity Identified in Patients With Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 76, 2238–2247. [Google Scholar] [CrossRef]
- Alfieri, O.; Maisano, F.; De Bonis, M.; Stefano, P.L.; Torracca, L.; Oppizzi, M.; La Canna, G. The double-orifice technique in mitral valve repair: A simple solution for complex problems. J. Thorac. Cardiovasc. Surg. 2001, 122, 674–681. [Google Scholar] [CrossRef]
- Shah, A.A.; Glower, D.D.; Gaca, J.G. Trans-aortic Alfieri stitch at the time of septal myectomy for hypertrophic obstructive cardiomyopathy. J. Card. Surg. 2016, 31, 503–506. [Google Scholar] [CrossRef]
- Bhudia, S.K.; McCarthy, P.M.; Smedira, N.G.; Lam, B.K.; Rajeswaran, J.; Blackstone, E.H. Edge-to-edge (Alfieri) mitral repair: Results in diverse clinical settings. Ann. Thorac. Surg. 2004, 77, 1598–1606. [Google Scholar] [CrossRef]
- Ma, W.; Shi, W.; Wu, W.; Ye, W.; Kong, Y.; Lhu, D.; Zhang, W. Elevated gradient after mitral valve repair: The effect of surgical technique and relevance of postoperative atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2019, 157, 921–927. [Google Scholar] [CrossRef]
- Yang, H.S.; Lee, K.S.; Chaliki, H.P.; Tazelaar, H.D.; Lusk, J.L.; Chandrasekaran, K.; Tajik, A.J. Anomalous insertion of the papillary muscle causing left ventricular outflow obstruction: Visualization by real-time three-dimensional echocardiography. Eur. Heart J. Cardiovasc. Imaging 2008, 9, 855–860. [Google Scholar] [CrossRef][Green Version]
- Maron, B.J.; Dearani, J.A.; Ommen, S.R.; Maron, M.S.; Schaff, H.V.; Nishimura, R.A.; Ralph-Edwards, A.; Rakowski, R.; Sherrid, M.V.; Swistel, D.B. Low Operative Mortality Achieved With Surgical Septal Myectomy. Role of Dedicated Hypertrophic Cardiomyopathy Centers in the Management of Dynamic Subaortic Obstruction. J. Am. Coll. Cardiol 2015, 15, 1307–1308. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Shahian, D.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.-L.; Delong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 2—Isolated Valve Surgery. Ann. Thorac. Surg. 2009, 88, S23–S42. [Google Scholar] [CrossRef]
- Hodges, K.; Rivas, C.G.; Aguilera, J.; Borden, R.; Alashi, A.; Blackstone, E.H.; Desai, M.Y.; Smedira, N.G. Surgical management of left ventricular outflow tract obstruction in a specialized hypertrophic obstructive cardiomyopathy center. J. Thorac. Cardiovasc. Surg. 2019, 157, 2289–2299. [Google Scholar] [CrossRef]
- Rastegar, H.; Boll, G.; Rowin, E.J.; Dolan, N.; Carroll, C.; Udelson, J.E.; Wang, W.; Carpino, P.; Maron, B.J.; Maron, M.S. Results of surgical septal myectomy for obstructive hypertrophic cardiomyopathy: The Tufts experience. Ann. Cardiothorac. Surg. 2017, 6, 353–363. [Google Scholar] [CrossRef]
- Ommen, S.R.; Maron, B.J.; Olivotto, I.; Maron, M.S.; Cecchi, F.; Betocchi, S.; Gersch, B.J.; Ackerman, M.J.; McCully, R.B.; Dearani, J.A. Longterm effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 470–476. [Google Scholar] [CrossRef]
- Cho, Y.H.; Quintana, E.; Schaff, H.V.; Nishimura, R.A.; Dearani, J.A.; Abel, M.D.; Ommen, S. Residual and recurrent gradients after septal myectomy for hypertrophic cardiomyopathy—Mechanisms of obstruction and outcomes of reoperation. J. Thorac. Cardiovasc. Surg. 2014, 148, 909–916. [Google Scholar] [CrossRef]
- Ralph-Edwards, A.; Woo, A.; McCrindle, B.W.; Shapero, J.L.; Schwartz, L.; Rakowski, H.; Wigle, E.D.; Williams, W.G. Hypertrophic obstructive cardiomyopathy: Comparison of outcomes after myectomy or alcohol ablation adjusted by propensity score. J. Thorac. Cardiovasc. Surg. 2005, 129, 351–358. [Google Scholar] [CrossRef]
- Panaich, S.S.; Badheka, A.O.; Chothani, A. Results of Ventricular Septal Myectomy and Hypertrophic Cardiomyopathy (from Nationwide Inpatient Sample [1998e2010]). Am. J. Cardiol. 2014, 114, 1390–1395. [Google Scholar] [CrossRef]
- Kim, L.K.; Swaminathan, R.V.; Looser, P.; Minutello, R.M.; Wong, S.C.; Bergman, G.; Naidue, S.S.; Gade, C.L.F.; Charitakis, K.; Singh, H.S.; et al. Hospital volume outcomes after septal myectomy and alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy: US nationwide in patient database, 2003–2011. JAMA Cardiol. 2016, 1, 324–332. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affronti, A.; Pruna-Guillen, R.; Sandoval, E.; Pereda, D.; Alcocer, J.; Castellà, M.; Quintana, E. Surgery for Hypertrophic Obstructive Cardiomyopathy: Comprehensive LVOT Management beyond Septal Myectomy. J. Clin. Med. 2021, 10, 4397. https://doi.org/10.3390/jcm10194397
Affronti A, Pruna-Guillen R, Sandoval E, Pereda D, Alcocer J, Castellà M, Quintana E. Surgery for Hypertrophic Obstructive Cardiomyopathy: Comprehensive LVOT Management beyond Septal Myectomy. Journal of Clinical Medicine. 2021; 10(19):4397. https://doi.org/10.3390/jcm10194397
Chicago/Turabian StyleAffronti, Alessandro, Robert Pruna-Guillen, Elena Sandoval, Daniel Pereda, Jorge Alcocer, Manuel Castellà, and Eduard Quintana. 2021. "Surgery for Hypertrophic Obstructive Cardiomyopathy: Comprehensive LVOT Management beyond Septal Myectomy" Journal of Clinical Medicine 10, no. 19: 4397. https://doi.org/10.3390/jcm10194397
APA StyleAffronti, A., Pruna-Guillen, R., Sandoval, E., Pereda, D., Alcocer, J., Castellà, M., & Quintana, E. (2021). Surgery for Hypertrophic Obstructive Cardiomyopathy: Comprehensive LVOT Management beyond Septal Myectomy. Journal of Clinical Medicine, 10(19), 4397. https://doi.org/10.3390/jcm10194397






