Impact of Bedside Re-Explorations in a Cardiovascular Surgery Intensive Care Unit Led by Surgeons †
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design
2.3. Group Assignment
2.4. Exclusion Criteria
2.5. Classification of Re-Explorations
- Unplanned, due to massive bleeding, cardiac tamponade or cardiac arrest;
- Planned, when patients were intentionally left with the chest open because of persistent general bleeding, hemodynamic instability or myocardial edema and underwent delayed sternal closure (DSC).
2.6. Definitions
2.7. Outcomes of Interest
2.8. Data Collection
2.9. Technique for Re-Exploration in the ICU
2.10. Statistical Analysis
3. Results
4. Discussions
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OR | Operating room |
| ICU | Intensive care unit |
| DSC | Delayed sternal closure |
| CDC | Centers for Disease Control and Prevention |
| DSWI | Deep sternal wound infection |
| AKI | Acute kidney injury |
| CALS | Cardiac Surgery Advanced Life Support |
| STS | Society for Thoracic Surgery |
| EACTS | European Society for Cardiothoracic Surgery |
| CPB | Cardiopulmonary bypass |
References
- Johnson, J.A.; Gundersen, A.E.; Stickney, I.D.; Cogbill, T.H. Selective approach to sternal closure after exploration for hemorrhage following coronary artery bypass. Ann. Thorac. Surg. 1990, 49, 771–774. [Google Scholar] [CrossRef]
- Moulton, M.J.; Creswell, L.L.; Mackey, M.E.; Cox, J.L.; Rosenbloom, M. Reexploration for bleeding is a risk factor for adverse outcomes after cardiac operations. J. Thorac. Cardiovasc. Surg. 1996, 111, 1037–1046. [Google Scholar] [CrossRef]
- Ottino, G.; De Paulis, R.; Pansini, S.; Rocca, G.; Tallone, M.V.; Comoglio, C.; Costa, P.; Orzan, F.; Morea, M. Major sternal wound infection after open-heart surgery: A multivariate analysis of risk factors in 2579 consecutive operative procedures. Ann. Thorac. Surg. 1987, 44, 173–179. [Google Scholar] [CrossRef]
- Reser, D.; Biefer, H.R.C.; Plass, A.; Ruef, C.; Seifert, B.; Bettex, D.; Biaggi, P.; Falk, V.; Salzberg, S.P. Incidence of sternal wound infection after reexploration in the intensive care unit and the use of local gentamycin. Ann. Thorac. Surg. 2012, 94, 2033–2037. [Google Scholar] [CrossRef]
- Biancari, F.; Mikkola, R.; Heikkinen, J.; Lahtinen, J.; Airaksinen, K.E.; Juvonen, T. Estimating the risk of complications related to re-exploration for bleeding after adult cardiac surgery: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2012, 41, 50–55. [Google Scholar] [CrossRef]
- Unsworth-White, M.J.; Herriot, A.; Valencia, O.; Poloniecki, J.; Smith, E.J.; Murday, A.J.; Parker, D.J.; Treasure, T. Resternotomy for bleeding after cardiac operation: A marker for increased morbidity and mortality. Ann. Thorac. Surg. 1995, 59, 664–667. [Google Scholar] [CrossRef]
- Ridderstolpe, L.; Gill, H.; Granfeldt, H.; Ahlfeldt, H.; Rutberg, H. Superficial and deep sternal wound complications: Incidence, risk factors and mortality. Eur. J. Cardiothorac. Surg. 2001, 20, 1168–1175. [Google Scholar] [CrossRef]
- Encalada, J.F.; Campelos, P.; Delgado, C.; Ventosa, G.; Quintana, E.; Sandoval, E.; Pereda, D.; Cartaňá, R.; Ninot, S.; Barriuso, C.; et al. Surgery in the Cardiovascular Surgical Intensive Care Unit. Cir. Esp. 2016, 94, 227–231. [Google Scholar] [CrossRef]
- The STS Adult Cardiac Surgery Database Version 4.20.2. Available online: http://www.sts.com (accessed on 23 September 2020).
- CDC/NHSN Surveillance Definitions for Specific Types of Infections and Surgical Site Infection (SSI) Event, Version January 2020. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf (accessed on 22 November 2020).
- Dyke, C.; Aronson, S.; Dietrich, W.; Hofmann, A.; Karkouti, K.; Levi, M.; Murphy, G.J.; Sellke, F.W.; Shore-Lesserson, L.; von Heymann, C.; et al. Universal definition of perioperative bleeding in adult cardiac surgery. J. Thorac. Cardiovasc. Surg. 2014, 147, 1458–1463.e1. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. Euroscore II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Negative and Positive Pressure Rooms 101 Hospital Infection Control. Air Innovations. 2019. Available online: www.airinnovations.com (accessed on 11 November 2020).
- Dunning, J.; Nandi, J.; Ariffin, S.; Jerstice, J.; Danitsch, D.; Levine, A. The Cardiac Surgery Advanced Life Support Course (CALS): Delivering Significant Improvements in Emergency Cardiothoracic Care. Ann. Thorac. Surg. 2006, 81, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.; Fabbri, A.; Kolh, P.H.; Levine, A.; Lockowandt, U.; Mackay, J.; Pavie, A.J.; Strang, T.; Versteegh, M.I.M.; Nashef, S.A.M. Guideline for resuscitation in cardiac arrest after cardiac surgery. Eur. J. Cardiothoracic. Surg. 2009, 36, 3–28. [Google Scholar] [CrossRef]
- Society of Thoracic Surgeons Task Force on Resuscitation After Cardiac Surgery. The Society of Thoracic Surgeons Expert Consensus for the Resuscitation of Patients Who Arrest after Cardiac Surgery. Ann. Thorac. Surg. 2017, 103, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Josa, M.; Khuri, S.F.; Braunwald, N.S.; Vancisin, M.F.; Spencer, M.P.; Evans, D.A.; Barsamian, E.M. Delayed sternal closure-an improved method of dealing with complications after cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 1986, 91, 589–603. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 25 September 2020).
- Fiser, S.M.; Tribble, C.G.; Kern, J.A.; Long, S.M.; Kaza, A.K.; Kron, I.L. Cardiac Reoperation in the Intensive Care Unit. Ann. Thorac. Surg. 2001, 71, 1888–1893. [Google Scholar] [CrossRef]
- Charalambous, C.P.; Zipitis, C.S.; Keenan, D.J. Chest reexploration in the intensive care unit after cardiac surgery: A safe alternative to returning to the operating theater. Ann. Thorac. Surg. 2006, 81, 191–194. [Google Scholar] [CrossRef]
- Kaiser, G.C.; Naunheim, K.S.; Fiore, A.C.; Harris, H.H.; McBride, L.R.; Pennington, D.; Barner, H.B.; Willman, V.L. Reoperation in the intensive care unit. Ann. Thorac. Surg. 1990, 49, 903–907, discussion 908. [Google Scholar] [CrossRef]
- Trouillet, J.-L.; Chastre, J.; Vuagnat, A.; Joly-Guillou, M.L.; Combaux, D.; Dombret, M.-C.; Gibert, C. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am. J. Respir. Crit. Care Med. 1998, 157, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Walter, S.D.; Cook, R.J.; Griffith, L.E.; Guyatt, G.H.; Leasa, D.; Jaeschke, R.Z.; Brun-Buisson, C. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann. Intern. Med. 1998, 129, 433–440. [Google Scholar] [CrossRef]
- LaPar, D.J.; Isbell, J.M.; Mulloy, D.P.; Stone, M.L.; Kern, J.A.; Ailawadi, G.; Kron, I.L. Planned cardiac reexploration in the intensive care unit is a safe procedure. Ann. Thorac. Surg. 2014, 98, 1645–1651, discussion 1651–1652. [Google Scholar] [CrossRef][Green Version]
- McKowen, R.L.; Magovern, G.J.; Liebler, G.A.; Park, S.B.; Burkholder, J.A.; Maher, T.D. Infectious Complications and Cost-Effectiveness of Open Resuscitation in the Surgical Intensive Care Unit after Cardiac Surgery. Ann. Thorac. Surg. 1985, 40, 388–392. [Google Scholar] [CrossRef]
| OR n = 86 | ICU n = 109 | p Value | |
|---|---|---|---|
| Age (y) | 64.4 ± 13.1 | 68.7 ± 9.8 | 0.005 |
| Female | 31 (36%) | 38 (34.9%) | 0.28 |
| BMI (Kg/m2) | 27.1 ± 4.7 | 26.7 ± 4.3 | 0.29 |
| Active smoker | 6 (7%) | 16 (14.7%) | 0.77 |
| Diabetes | 17 (19.8%) | 27 (24.8%) | 0.41 |
| Hypertension | 54 (62.8%) | 82 (75.2%) | 0.06 |
| COPD | 15 (17.4%) | 13 (11.9%) | 0.27 |
| Serum creatinine (mg/dL) | 1.1 ± 0.5 | 1.3 ± 1.2 | 0.034 |
| LVEF (%) | 53.6 ± 12.6 | 54.5 ± 12.5 | 0.33 |
| Pre-op Anticoagulant | 31 (36%) | 25 (22.9%) | 0.64 |
| Pre-op Antiplatelets other than ASA | 6 (7%) | 14 (12.8%) | 0.18 |
| Active Endocarditis | 3 (3.5%) | 9 (8.2%) | 0.28 |
| Euroscore II | 6.8 ± 9.8 | 9.6 ± 13 | 0.5 |
| Status of Index procedure: | |||
| elective | 68 (79.1%) | 66 (60.5%) | 0.006 |
| urgent/emergent | 18 (20.9%) | 43 (39.5%) | 0.006 |
| CPB (mins) | 144.8 ± 78.8 | 153.8 ± 88.2 | 0.23 |
| Cardiac ischemic time (mins) | 96.3 ± 48.8 | 97.2 ± 45.4 | 0.45 |
| Circulatory arrest (mins) | 57.1 ± 50.9 | 44.5 ± 42.1 | 0.24 |
| Deep hypothermia | 11 (12.8%) | 23 (21.1%) | 0.13 |
| Previous cardiac surgery | 12 (13.9%) | 22 (20.2%) | 0.25 |
| Index Procedure | OR n = 86 | ICU n = 109 |
|---|---|---|
| CABG | 12 (13.9%) | 18 (16.5%) |
| AVR | 13 (15.1%) | 13 (11.9%) |
| AVR + MVR | 3 (3.5%) | 4 (3.7%) |
| AVR + MVR + TA | 3 (3.5%) | 3 (2.7%) |
| AVR + MVrep | - | 2 (1.8%) |
| AVR + AA | 3 (3.5%) | 3 (2.7%) |
| AVR + CABG | 2 (2.3%) | 3 (2.7%) |
| AVR + AA + CABG | 2 (2.3%) | - |
| AVR + AA + CABG + Maze | 1 (1.2%) | - |
| AVR + AA + MVrep | - | 1 (0.9%) |
| AVR + AA + MVrep + CABG | - | 1 (0.9%) |
| AVR + TA | 2 (2.3%) | - |
| MVR | 3 (3.5%) | 5 (4.6%) |
| MVrep | 3 (3.5%) | 2 (1.8%) |
| AA replacement | 3 (3.5%) | 2 (1.8%) |
| MVR + TA | 4 (4.6%) | 3 (2.7%) |
| MVR + TA + CABG | - | 1 (0.9%) |
| MVR + TVR | - | 1 (0.9%) |
| MVR +CABG | - | 4 (3.7%) |
| MVR + CABG + AA | - | 1 (0.9%) |
| MVrep + TA | 1 (1.2%) | 1 (0.9%) |
| MVrep + CABG | 2 (2.3%) | 2 (1.8%) |
| MVrep +Maze + PFO | 1 (1.2%) | - |
| MVR + AT + Maze | 3 (3.5%) | - |
| CABG + Maze | 1 (1.2%) | - |
| Bentall operation | 3 (3.5%) | 6 (5.5%) |
| Bentall operation open distal | - | 5 (4.6%) |
| Bentall operation homograft | - | 2 (1.8%) |
| Bentall operation + Arch replacement | 6 (7%) | 10 (9.2%) |
| Bentall operation + MVR | 1 (1.2%) | - |
| Bentall operation + CABG | - | 1 (0.9%) |
| Commando operation | 3 (3.5%) | 2 (1.8%) |
| Commando operation + TA + CABG | - | 1 (0.9%) |
| Elephant trunk | 4 (4.6%) | 4 (3.7%) |
| FET | 1 (1.2%) | 2 (1.8%) |
| David operation + MVrep | 1 (1.2%) | - |
| AVrep + MVR + TA | 1 (1.2%) | - |
| AVrep + AA + Maze | 1 (1.2%) | - |
| Pericardiectomy | 1 (1.2%) | - |
| Thoraco-abdominal aneurysm | 1 (1.2%) | 1 (0.9%) |
| PPVV ablation | 1 (1.2%) | - |
| Infarct exclusion | - | 1 (0.9%) |
| Septal Myectomy | - | 1 (0.9%) |
| Septal Myectomy + AVR + MVR + TA | - | 1 (0.9%) |
| LV Aneurysm repair + CABG | - | 1 (0.9%) |
| PEA | - | 1 (0.9%) |
| OR n = 86 | ICU n = 109 | p Value | |
|---|---|---|---|
| Time from surgery (d): | |||
| <24 h | 33 (38.4%) | 51 (46.8%) | 0.24 |
| 24–48 h | 6 (7%) | 12 (11%) | 0.33 |
| Median; range (d) | 1; 0–14 | 1; 0–13 | |
| Unplanned | 67 (77.9%) | 84 (77.1%) | 0.89 |
| Planned | 19 (22.1%) | 25 (22.9%) | 0.89 |
| Reason for re-exploration: | |||
| Bleeding | 44 (51.2%) | 47 (43.1%) | 0.26 |
| Cardiac tamponade | 23 (26.7%) | 28 (25.7%) | 0.56 |
| Cardiac arrest | - | 7 (6.4%) | 0.018 |
| Delayed sternal closure | 19 (22.1%) | 25 (22.9%) | 0.89 |
| Other | - | 2 (1.8%) | 0.5 |
| Non-surgical bleeding | 31/67 (46.3%) | 38/74 (51.3%) | 0.95 |
| 2 or more re-explorations | 2 (2.3%) | 33 (30.3%) | <0.001 |
| Multiple re-explorations: details | |||
| range (median) | 2-4 (2) | 2-3 (2) | |
| Cause of multiple re-explorations: | |||
| Persistent bleeding | 2 (100%) | 13 (39.4%) | |
| Delayed sternal closure | - | 14 (42.4%) | |
| Cardiac arrest/ refractory arrythmia | - | 6 (18.2%) | |
| OR n = 86 | ICU n = 109 | p Value | |
|---|---|---|---|
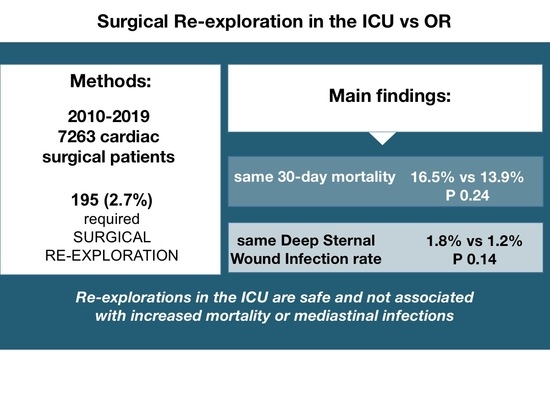
| 30-day mortality | 12 (13.9%) | 18 (16.5%) | 0.24 |
| ICU stay (d) | 14.1 ± 19.8 | 19.3 ± 29.9 | 0.89 |
| Hospital stay (d) | 20.8 ± 18.3 | 30.3 ± 34.2 | 0.014 |
| Intubation > 72 h | 17 (19.8%) | 51 (46.8%) | <0.001 |
| Pneumonia | 6 (7%) | 24 (22%) | 0.004 |
| Tracheostomy | 9 (10.5%) | 14 (12.8%) | 0.61 |
| AKI requiring dialysis | 13 (15.1%) | 25 (22.9%) | 0.17 |
| Sepsis | 6 (7%) | 16 (14.7%) | 0.91 |
| DSWI | 1 (1.2%) | 2 (1.8%) | 0.14 |
| Stroke | 3 (3.5%) | 5 (2.7%) | 0.70 |
| OR n = 12 | ICU n = 18 | p Value | |
|---|---|---|---|
| MOF | 7 (58.3%) | 13 (72.2%) | 0.33 |
| massive bleeding | 1 (8.3%) | 2 (11.1%) | 0.02 |
| neurologic | 2 (16.7%) | 1 (5.6%) | 0.32 |
| cardiac | 2 (16.7%) | 2 (11.1%) | 0.66 |
| OR n = 19 | ICU n = 25 | p Value | |
|---|---|---|---|
| 30-day mortality | 2 (10.9%) | 9 (36%) | 0.15 |
| Intubation > 72 h | 6 (31.6%) | 17 (68%) | 0.03 |
| Pneumonia | 3 (15.8%) | 7 (28%) | 0.47 |
| Tracheostomy | 6 (31.6%) | 6 (24%) | 0.73 |
| AKI requiring dialysis | 3 (15.8%) | 9 (36%) | 0.31 |
| Sepsis | 5 (26.3%) | 5 (20%) | 0.39 |
| DSWI | 1 (5.3%) | - | 0.43 |
| Stroke | 2 (10.9%) | 1 (4%) | 0.56 |
| Planned n = 25 | Unplanned n = 84 | p Value | |
|---|---|---|---|
| Age | 65.4 ± 12.4 | 69.6 ± 8.5 | 0.27 |
| Female | 12 (48%) | 26 (30.9%) | 0.12 |
| BMI | 38.2 ± 23.8 | 26.9 ± 4.4 | 0.13 |
| Active smoker | 9 (36%) | 45 (53.6%) | 0.12 |
| Diabetes | 3 (12%) | 24 (28.6%) | 0.09 |
| Hypertension | 20 (80%) | 62 (73.8%) | 0.53 |
| COPD | 4 (16%) | 10 (11.9%) | 0.49 |
| Serum creatinine (mg/dL) | 1.3 ± 0.8 | 1.3 ± 1.3 | 0.49 |
| Status of Index procedure: | |||
| elective | 5 (20%) | 60 (71.4%) | <0.001 |
| urgent/emergent | 20 (80%) | 24 (28.6%) | <0.001 |
| CPB (mins) | 228.8 ± 89.1 | 131.2 ± 73.5 | <0.001 |
| Cardiac ischemic time (mins) | 129.8 ± 46.7 | 87.3 ± 39.5 | <0.001 |
| Circulatory arrest | 13 (52%) | 7 (8.3%) | <0.001 |
| Deep hypothermia | 13 (52%) | 7 (8.3%) | <0.001 |
| Previous cardiac surgery | 8 (32%) | 14 (16.6%) | 0.93 |
| Euroscore II | 14.9 ± 18.4 | 8 ± 10.3 | <0.05 |
| Planned n = 25 | Unplanned n = 84 | p Value | |
|---|---|---|---|
| 30-day mortality | 8 (32%) | 10 (11.9%) | 0.029 |
| ICU stay (d) | 26.4 ± 29.5 | 17.1 ± 29.7 | 0.88 |
| Hospital stay (d) | 36.8 ± 30 | 28.5 ± 35.2 | 0.15 |
| Intubation > 72 h | 17 (68%) | 34 (40.5%) | 0.022 |
| Pneumonia | 7 (28%) | 17 (20.2%) | 0.41 |
| Tracheostomy | 6 (24%) | 8 (9.5%) | 0.57 |
| AKI requiring dialysis | 9 (36%) | 16 (19%) | 0.76 |
| Sepsis | 5 (20%) | 11 (13.1%) | 0.39 |
| DSWI | 1 (4%) | 1 (1.2%) | 0.36 |
| Stroke | 1 (4%) | 4 (4.8%) | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affronti, A.; Sandoval, E.; Muro, A.; Hernández-Campo, J.; Quintana, E.; Pereda, D.; Alcocer, J.; Pruna-Guillen, R.; Castellà, M. Impact of Bedside Re-Explorations in a Cardiovascular Surgery Intensive Care Unit Led by Surgeons. J. Clin. Med. 2021, 10, 4288. https://doi.org/10.3390/jcm10194288
Affronti A, Sandoval E, Muro A, Hernández-Campo J, Quintana E, Pereda D, Alcocer J, Pruna-Guillen R, Castellà M. Impact of Bedside Re-Explorations in a Cardiovascular Surgery Intensive Care Unit Led by Surgeons. Journal of Clinical Medicine. 2021; 10(19):4288. https://doi.org/10.3390/jcm10194288
Chicago/Turabian StyleAffronti, Alessandro, Elena Sandoval, Anna Muro, Jose Hernández-Campo, Eduard Quintana, Daniel Pereda, Jorge Alcocer, Robert Pruna-Guillen, and Manuel Castellà. 2021. "Impact of Bedside Re-Explorations in a Cardiovascular Surgery Intensive Care Unit Led by Surgeons" Journal of Clinical Medicine 10, no. 19: 4288. https://doi.org/10.3390/jcm10194288
APA StyleAffronti, A., Sandoval, E., Muro, A., Hernández-Campo, J., Quintana, E., Pereda, D., Alcocer, J., Pruna-Guillen, R., & Castellà, M. (2021). Impact of Bedside Re-Explorations in a Cardiovascular Surgery Intensive Care Unit Led by Surgeons. Journal of Clinical Medicine, 10(19), 4288. https://doi.org/10.3390/jcm10194288