FibroScan-AST Score Predicts 30-Day Mortality or Need for Mechanical Ventilation among Patients Hospitalized with COVID-19
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Methods
2.2.1. Transient Elastography
2.2.2. Laboratory Tests
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Correlations of LSM and CAP with Demographic, Biochemical and Clinical Parameters
3.3. Correlations of FAST Score with Demographic, Biochemical and Clinical Parameters
3.4. Relationships between LSM, CAP, FAST Score and 30-Day Clinical Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme II |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| APRI | AST to platelet ratio index |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| CAP | Controlled attenuation parameter |
| COVID-19 | Coronavirus disease 2019 |
| CRP | C-reactive protein |
| FAST | FibroScan-AST score |
| FIB-4 | Fibrosis 4 index |
| GGT | Gamma glutamyl transferase |
| HFNC | High flow nasal cannula |
| IQR | Interquartile range |
| LSM | Liver stiffness measurement |
| MV | Mechanical ventilation |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NFS | Non-alcoholic fatty liver disease fibrosis score |
| PT | Prothrombin time |
| RBC | Red blood cells |
| SCD | Skin-to-liver capsule distance |
| WBC | White blood cells |
References
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [Green Version]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xing Bing, X.; Za, X.Z. Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and PreventionThe epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 145–151. [Google Scholar]
- Hu, B.; Guo, H.; Zhou, P. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Kuehn, B.M. More Severe Obesity Leads to More Severe COVID-19 in Study. JAMA 2021, 325, 1603. [Google Scholar] [CrossRef]
- Dufour, J.-F.; Scherer, R.; Balp, M.-M.; McKenna, S.J.; Janssens, N.; Lopez, P.; Pedrosa, M. The global epidemiology of nonalcoholic steatohepatitis (NASH) and associated risk factors–A targeted literature review. Endocr. Metab. Sci. 2021, 3, 100089. [Google Scholar] [CrossRef]
- Subichin, M.; Clanton, J.; Makuszewski, M.; Bohon, A.; Zografakis, J.G.; Dan, A. Liver disease in the morbidly obese: A review of 1000 consecutive patients undergoing weight loss surgery. Surg. Obes. Relat. Dis. 2015, 11, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Mahamid, M.; Nseir, W.; Khoury, T.; Mahamid, B.; Nubania, A.; Sub-Laban, K.; Schifter, J.; Mari, A.; Sbeit, W.; Goldin, E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome. Eur. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fondevila, M.F. Obese patients with NASH have increased hepatic expression of SARS- CoV-2 critical entry points. J. Hepatol. 2021, 74, 469–471. [Google Scholar] [CrossRef]
- Cai, Q.; Huang, D.; Yu, H.; Zhu, Z.; Xia, Z.; Su, Y.; Li, Z.; Zhou, G.; Gou, J.; Qu, J.; et al. COVID-19: Abnormal liver function tests. J. Hepatol. 2020, 73, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Liu, H. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef]
- Kim, D.; Adeniji, N.; Latt, N.; Kumar, S.; Bloom, P.P.; Aby, E.S.; Perumalswami, P.; Roytman, M.; Li, M.; Vogel, A.S.; et al. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin. Gastroenterol. Hepatol. 2021, 19, 1469–1479e19. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, M.; Grander, C.; Fritsche, G.; Bellmann-Weiler, R.; Hartig, F.; Wildner, S.; Seiwald, S.; Adolph, T.E.; Zoller, H.; Weiss, G.; et al. Liver stiffness by transient elastography accompanies illness severity in COVID-19. BMJ Open Gastroenterol. 2020, 7, e000445. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, C.O.; Keklikkiran, C.; Ergenc, I.; Sengel, B.E.; Eskidemir, G.; Cinel, I.; Odabasi, Z.; Korten, V.; Yilmaz, Y. Liver stiffness is associated with disease severity and worse clinical scenarios in coronavirus disease 2019: A prospective transient elastography study. Int. J. Clin. Pract. 2021, e14363. [Google Scholar] [CrossRef]
- Campos-Varela, I.; Villagrasa, A.; Simon-Talero, M.; Riveiro-Barciela, M.; Ventura-Cots, M.; Aguilera-Castro, L.; Alvarez-Lopez, P.; Nordahl, E.A.; Anton, A.; Bañares, J.; et al. The role of liver steatosis as measured with transient elastography and transaminases on hard clinical outcomes in patients with COVID-19. Ther. Adv. Gastroenterol. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Qin, E.; Xu, J.; Zhang, D.; Cheng, G.; Wang, Y.; Lau, G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J. Hepatol. 2020, 73, 451–453. [Google Scholar] [CrossRef]
- Newsome, P.N.; Sasso, M.; Deeks, J.J. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Subbe, C.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified Early Warning Score in medical admissions. QJM Int. J. Med. 2001, 94, 521–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, S.R.; Ho, A.; Pius, R. ISARIC4C investigators Risk stratification of patients admitted to hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 2020, 370, m3339. [Google Scholar] [CrossRef]
- The Ministry of Health of the Republic of Croatia. Guidelines for Treatment of Patients with COVID-19, Version 2, 19 November 2020. Available online: https://zdravlje.gov.hr/UserDocsImages//2020%20CORONAVIRUS//Smjernice%20za%20lije%C4%8Denje%20oboljelih%20od%20koronavirusne%20bolesti%202019%20(COVID-19),%20verzija%202%20od%2019.%20studenoga%202020.pdf (accessed on 21 June 2021).
- Dietrich, C.F.; Bamber, J.; Berzigotti, A. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017, 38, e16–e47. [Google Scholar]
- Berger, A.; Shili, S.; Zuberbuhler, F.; Hiriart, J.B.; Lannes, A.; Chermak, F.; Hunault, G.; Foucher, J.; Oberti, F.; Fouchard-Hubert, I.; et al. Liver Stiffness Measurement With FibroScan: Use the Right Probe in the Right Conditions! Clin. Transl. Gastroenterol. 2019, 10, e00023. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.; Gurusamy, K.; Ntaoula, S.; Cholongitas, E.; Davidson, B.; Burroughs, A. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: A meta-analysis of diagnostic accuracy. J. Hepatol. 2010, 54, 650–659. [Google Scholar] [CrossRef]
- de Franchis, R.; Baveno, V.I. Faculty Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddowes, P.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [Green Version]
- Targher, G.; Mantovani, A.; Byrne, C.D.; Wang, X.-B.; Yan, H.-D.; Sun, Q.-F.; Pan, K.-H.; Zheng, K.-I.; Chen, Y.-P.; Eslam, M.; et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut 2020, 69, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cristóbal, M.; Clemente-Sánchez, A.; Piñeiro, P.; Cedeño, J.; Rayón, L.; del Río, J.; Ramos, C.; Hernández, D.-A.; Cova, M.; Caballero, A.; et al. Possible unrecognised liver injury is associated with mortality in critically ill COVID-19 patients. Ther. Adv. Gastroenterol. 2021, 14. [Google Scholar] [CrossRef]
- Wai, C.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.-F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Mustapic, S.; Ziga, S.; Matic, V.; Bokun, T.; Radic, B.; Lucijanic, M.; Marusic, S.; Babic, Z.; Grgurevic, I. Ultrasound Grade of Liver Steatosis Is Independently Associated with the Risk of Metabolic Syndrome. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tapper, E.B.; Afdhal, N.H. Vibration-controlled transient elastography: A practical approach to the noninvasive assessment of liver fibrosis. Curr. Opin. Gastroenterol. 2015, 31, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Bende, F.; Tudoran, C.; Sporea, I.; Fofiu, R.; Bâldea, V.; Cotrău, R.; Popescu, A.; Sirli, R.; Ungureanu, B.; Tudoran, M. A Multidisciplinary Approach to Evaluate the Presence of Hepatic and Cardiac Abnormalities in Patients with Post-Acute COVID-19 Syndrome—A Pilot Study. J. Clin. Med. 2021, 10, 2507. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.; Choi, K.C.; Wong, G.L.-H.; Zhang, Y.; Chan, H.L.-Y.; Luk, A.O.-Y.; Shu, S.S.-T.; Chan, A.; Yeung, M.W.; Chan, J.; et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: A prospective cohort study. Gut 2015, 65, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
| Overall | MV or Death No | MV or Death Yes | p-Value | |
|---|---|---|---|---|
| Total number | 217 | 192 | 25 | - |
| Age (years) | 65 IQR (55–70) | 64 IQR (55–70) | 70 IQR (62–75) | 0.014 |
| Sex Male Female | 144/217 (66.4%) 73/217 (33.6%) | 123/192 (64.1%) 69/192 (35.9%) | 21/25 (84%) 4/25 (16%) | 0.047 |
| BMI (kg/m2) | 28.3 IQR (25.4–31.5) | 28.4 IQR (25.4–31.6) | 26.9 IQR (25.65–30.9) | 0.342 |
| Probe type M XL | 140/217 (64.5%) 77/217 (35.5%) | 121/192 (63%) 71/192 (37%) | 19/25 (76%) 6/25 (24%) | 0.203 |
| SCD (mm) | 21 IQR (19–25) | 22 IQR (18–25.25) | 20 IQR (19–23) | 0.652 |
| Chronic liver disease | 10/217 (4.6%) | 8/192 (4.2%) | 2/25 (8%) | 0.323 |
| LSM (kPa) | 5.2 IQR (4.1–6.5) | 5.1 IQR (4.18–6.53) | 5.3 IQR (4.1–6.3) | 0.873 |
| LSM IQR (%) | 13 IQR (9–20) | 13 IQR (9–20) | 13 IQR (8–18) | 0.419 |
| CAP (dB/m) | 274 IQR (232–321) | 273 IQR (233.5–322) | 284 IQR (228–301) | 0.643 |
| CAP IQR (dB/m) | 31 IQR (22–43) | 31 IQR (22–43) | 31 IQR (20–38) | 0.487 |
| FAST score | 0.31 IQR (0.16–0.45) | 0.3 IQR (0.14–0.45) | 0.4 IQR (0.25–0.47) | 0.112 |
| WBC (×109/L) | 7.8 IQR (5.45–11.1) | 8 IQR (5.53–11.1) | 6.9 IQR (3.8–10.9) | 0.545 |
| RBC (×1012/L) | 4.5 IQR (4.14–4.89) | 4.5 IQR (4.14–4.88) | 4.5 IQR (4.12–4.9) | 0.872 |
| Platelets (×109/L) | 237 IQR (173–327.5) | 243.5 IQR (178.25–334) | 204 IQR (144–264) | 0.087 |
| PT (Quick, %) | 101 IQR (90–108) | 101.5 IQR (92–108) | 89 IQR (76–107) | 0.059 |
| Bilirubin (umol/L) | 10.6 IQR (8.6–15.4) | 10.6 IQR (8.6–15.45) | 10.7 IQR (8.1–15.2) | 0.979 |
| AST (IU/L) | 39 IQR (27–61) | 38 IQR (26–57) | 58 IQR (35–84) | 0.014 |
| ALT (IU/L) | 38 IQR (24–63) | 37.5 IQR (23.25–62) | 41 IQR (27–67) | 0.614 |
| ALP (IU/L) | 62 IQR (51–78.5) | 61 IQR (50–77) | 64 IQR (58–86) | 0.166 |
| GGT (IU/L) | 42 IQR (25.5–82) | 43 IQR (27–85.75) | 37 IQR (24–56) | 0.165 |
| CRP (mg/L) | 71.8 IQR (31.2–128.3 | 69.5 IQR (28.03–122.23) | 100.4 IQR (48.8–138.2) | 0.072 |
| Albumin (g/L) | 32.5 IQR (30–35) | 33 IQR (30–35) | 32 IQR (30–34) | 0.433 |
| 4C mortality score | 7 IQR (5–9) | 7 IQR (4.5–9) | 10 IQR (8.75–11) | <0.001 |
| HFNC oxygenation (N, %) | 24/217 (11.1%) | 14/192 (7.3%) | 10/25 (40%) | <0.001 |
| 30-Day Mortality p-Value and OR with 95% C.I. | Mechanical Ventilation p-Value and OR with 95% C.I. | Mechanical Ventilation or Death p-Value and OR with 95% C.I. | |
|---|---|---|---|
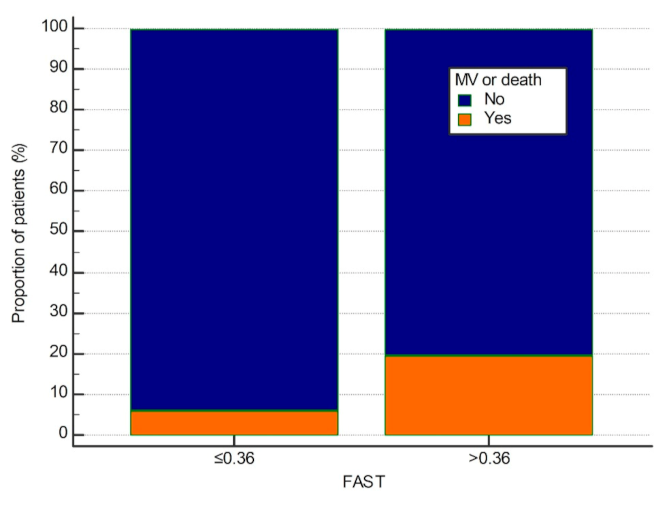
| FAST > 0.36 | p = 0.285 OR = 1.79 (0.61–5.23) | p = 0.019 * OR = 3.78 (1.24–11.5) | p = 0.036 * OR = 3.11 (1.08–8.97) |
| Age (years) | p = 0.321 OR = 0.95 (0.88–1.04) | p = 0.136 OR = 0.94 (0.87–1.02) | p = 0.199 OR = 0.95 (0.88–1.03) |
| Male sex | p = 0.761 OR = 0.83 (0.24–2.81) | p = 0.660 OR = 1.33 (0.36–4.92) | p = 0.542 OR = 1.49 (0.41–5.33) |
| 4C mortality score | p = 0.004 * OR = 1.83 (1.31–2.57) | p = 0.001 * OR = 1.72 (1.25–2.38) | p = 0.001 * OR = 1.71 (1.24–2.35) |
| HFNC oxygenation | p = 0.002 * OR = 7.4 (2.15–24.44) | p < 0.001 * OR = 7.76 (2.4–25.08) | p < 0.001 * OR = 7.17 (2.24–22.92) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelenika, M.; Lucijanic, M.; Bokun, T.; Bozin, T.; Barisic Jaman, M.; Tjesic Drinkovic, I.; Pastrovic, F.; Madir, A.; Luksic, I.; Piskac Zivkovic, N.; et al. FibroScan-AST Score Predicts 30-Day Mortality or Need for Mechanical Ventilation among Patients Hospitalized with COVID-19. J. Clin. Med. 2021, 10, 4355. https://doi.org/10.3390/jcm10194355
Zelenika M, Lucijanic M, Bokun T, Bozin T, Barisic Jaman M, Tjesic Drinkovic I, Pastrovic F, Madir A, Luksic I, Piskac Zivkovic N, et al. FibroScan-AST Score Predicts 30-Day Mortality or Need for Mechanical Ventilation among Patients Hospitalized with COVID-19. Journal of Clinical Medicine. 2021; 10(19):4355. https://doi.org/10.3390/jcm10194355
Chicago/Turabian StyleZelenika, Marko, Marko Lucijanic, Tomislav Bokun, Tonci Bozin, Mislav Barisic Jaman, Ida Tjesic Drinkovic, Frane Pastrovic, Anita Madir, Ivica Luksic, Nevenka Piskac Zivkovic, and et al. 2021. "FibroScan-AST Score Predicts 30-Day Mortality or Need for Mechanical Ventilation among Patients Hospitalized with COVID-19" Journal of Clinical Medicine 10, no. 19: 4355. https://doi.org/10.3390/jcm10194355
APA StyleZelenika, M., Lucijanic, M., Bokun, T., Bozin, T., Barisic Jaman, M., Tjesic Drinkovic, I., Pastrovic, F., Madir, A., Luksic, I., Piskac Zivkovic, N., Luetic, K., Krznaric, Z., Ostojic, R., Filipec Kanizaj, T., Bogadi, I., Virovic Jukic, L., Kukla, M., & Grgurevic, I. (2021). FibroScan-AST Score Predicts 30-Day Mortality or Need for Mechanical Ventilation among Patients Hospitalized with COVID-19. Journal of Clinical Medicine, 10(19), 4355. https://doi.org/10.3390/jcm10194355







