1. Introduction
Thyroid cancer is the most common endocrine malignancy. This disease varies from indolent tumour to highly aggressive disease [
1]. Indeed, although early-stage well-differentiated thyroid cancer (DTC) has a good prognosis after surgery [
2,
3,
4], those tumours invading surrounding tissues (extra-thyroid extension) show an increased persistence/recurrence of disease and decreased survival [
5,
6,
7].
Airway invasion is found in approximately 6% of the total thyroid tumours and can determine different clinical conditions. These patients can show normal breathing or severe obstruction in the most advanced disease progression [
5,
6]. In such conditions, the ideal surgical treatment is still a matter of debate. Many approaches, single or combined multi-modality treatment, have been described with disputed results [
8]. In particular, concerning the airway invasion, the shaving-off of the tumour from the airway or tracheal window resection is performed in the case of superficial invasion, while segmental resection is preferred when the airway invasion is deeper in the laryngo-tracheal wall [
4]. However, some authors underline the weakness of the shaving and tracheal window [
4,
8], emphasising that segmental resection with end-to-end anastomosis should be preferred even in limited infiltration to reduce risk for recurrence and airway damage. Moreover, other conventional therapies, such as radioactive iodine, thyroid hormone therapy and chemotherapy, have been developed and performed with growing trends and promising perspectives [
9,
10,
11,
12].
Despite the progress obtained in the recent past in terms of diagnosis, staging and therapeutic options [
13,
14,
15], and in view of the lack of markers to predict the oncological outcome, there is still a need for biological, genetic and immunohistochemical (IHC) indicators to develop a more effectively tailored approach. Based on the current knowledge regarding potential biomarkers of tumour aggressiveness, Aryl hydrocarbon receptor (AhR), N-cadherin, E-cadherin and CD147 were selected to be analysed in thyroid carcinomas for the following reasons. AhR has been widely analysed and seems to be associated with tumour genesis and different disease progression phases [
16]. In a poorly differentiated thyroid carcinoma (PDTC) cell line, kynurenine-driven activation of AhR induced epithelial–mesenchymal transition (EMT) involving cadherins [
17]. The transmembrane glycoprotein CD147 is known to facilitate tumour cell migration and invasion in several cancers [
18]; matrix metalloproteinases (MMPs) seem to be activated [
19], and CD147 possibly promotes the mesenchymal phenotype with cadherin expression variations [
20,
21]. A high expression of CD147 was described in DTC [
22], with an emphasis on lymph node metastasis and tumour invasion [
23].
The aim of the study is to compare the immunophenotypic characteristics of thyroid tumours invading the airway and complete intra-thyroid tumours in order to generate a hypothesis for possible new markers of local aggressiveness and to determine how the aggressive tumour attitude could be predicted.
2. Material and Methods
A comparative retrospective observational study on the expression of E-cadherin, N-cadherin, AhR and CD147 was performed on a series of patients undergoing surgery (2010–2017) for papillary thyroid cancer. The presence of accurate pathological reports, information regarding nodal status at the diagnosis and/or data regarding the recurrence and/or persistence of disease were used to select the patients eligible for the study. Subsequently, 3 groups of patients were considered: patients with papillary thyroid carcinoma (PTC) invading larynx and trachea undergoing total thyroidectomy with segmental resection of the airway (PTC-A group); patients with completely intrathyroidal PTC (PTC-B group); and patients with PDTC or anaplastic thyroid carcinomas (ATC) (PDTC/ATC group), which served as a control group for more locally aggressive neoplasms. The study was approved by the local ethics committee (N. 23665/10/AV of 26 January 2010).
2.1. Study Population
Patients were included the analysis if they were over 18 years of age; histologically diagnosed for PTC, PDTC or ATC; and followed up by the Internal Medicine and Endocrine and Metabolic Sciences Unit, University of Perugia. Patients with follicular and medullary tumours were excluded. Medical history, endoscopic findings, pathological reports, work for the assessment of possible resection with curative intent, indication to airway resection or other therapeutic options, histo-pathological aspects, immunophenotypic profile and adjuvant therapies were evaluated. At admission, all the patients underwent routinary tests for an appropriate preoperative assessment.
2.2. Surgical Procedures
Based on the different disease characteristics, two different procedures of surgical resection were carried out: total thyroidectomy with central compartment lymphectomy and latero-cervical lymphectomy (in the case of positive ultrasound (US) lymph node involvement) was performed for all cyto-histologically proven PTCs or early-stage PDTCs. In the cases of tumours infiltrating the airway, total thyroidectomy + lymphectomy was associated with resection and end-to-end anastomosis of the airway according to Grillo’s technique [
24]. Diagnosed ATCs were excluded from radical surgery due to stage and local conditions but were submitted to palliative procedures (endoscopy or tracheotomy) and alternative treatment.
2.3. Histopathological and Immunohistochemical Determinations
The surgical specimens were fixed in 4% buffered formalin and paraffin embedded (FFPE). Four-micrometer-thick sections were used to obtain both the haematoxylin and eosin (H&E) (Leica ST5020 Multistainer (Leica Biosystems, Nußloch, Germany)), using the ST Infinity H&E Staining System kit (Leica Biosystems, Richmond, IL, USA), and the IHC stains (BOND-III fully automated immunohistochemistry stainer (LeicaBiosystems, Nußloch, Germany)) and peroxidase immunoenzymatic reaction with development in diaminobenzidine, including proper positive and negative controls.
The tumour areas and the tumour histotypes, according to the World Health Organization’s classification of endocrine organ tumours, 2017, 4th Ed.—in force at the time of the study—were identified on H&E slides, allowing the identification of 3 groups of tumours: PTC, PDTC and ATC. Among these last two groups, tumours without a pure histotype were not considered for the analyses. Moreover, the presence of extra-thyroid infiltration was assessed on H&E slides.
The IHC stains were set up using antibodies against E-cadherin (Leica Biosystems, Newcastle Upon Tyne, United Kingdom, Cat# PA0387, RRID:AB_442084, ready-to-use), N-cadherin (ThermoFisher Scientific, Rockford, IL, USA, Cat# 33-3900, RRID:AB_2313779, dilution 1:150), AhR (ThermoFisher Scientific, Rockford, IL, USA, Cat# MA1-514, RRID: AB_2273723, dilution 1: 250) and CD147 (ThermoFisher Scientific, Rockford, IL, USA, Cat# MA1-19201, RRID: AB_1071293, dilution 1:100). Two trained pathologists (M.Ma. and R.C.) separately performed a semi-quantitative evaluation of the immunostains, using a H-Score—as previously reported for AhR [
17]—which was the result of the intensity of the staining (0 = absent, 1 = mild, 2 = moderate, 3 = intense) multiplied by the percentage of labelled tumour cells. Two other expert pathologists (G.B. and A.Si.) convened to compare discordant cases in order to assign a definitive H-score. Afterwards, the low and the high classes of expression were obtained, depending on whether the H-score was lower or higher than the internally validated cut-offs—laboratory developed test (LDT)—for each protein investigated (
Table 1). As regards E-cadherin, the tumour belonged to the normal expression group when the H-score was >200 and to the lost/low group when the H-score was ≤200.
2.4. Statistical Analysis
Descriptive statistics were used for the analysis of immunomarker expressions. Linear correlations between the expressions of the immunomarkers were analysed using the Pearson correlation coefficient. Categorical variables were presented as frequencies with row and column percentages and compared between the groups using chi-square test or Fisher’s exact test as appropriate. Values of p < 0.05 were assumed as significant.
4. Discussion
Thyroid carcinoma is a very heterogeneous disease with a different prognosis based mainly on histology [
4]. Surgery is still the best therapeutic option with a generally good prognosis, especially in small-sized completely intra-thyroid DTC without lymph nodal or distant metastases [
4,
14]. Conversely, PDTC and ATC are more aggressive with a severe natural history. In these cases, there is usually no chance for curative surgery, only palliative procedures [
25,
26].
Between these two last extremes described, there are several intermediate conditions showing a locally advanced tumour that might benefit from a radical resection but need more complex procedures than total thyroidectomy. Indeed, considering the neck’s anatomy, when thyroid tumours acquire the capacity to infiltrate the neighbourhood, a radical resection is a difficult goal. In this regard, infiltration of the airway represents the most challenging condition, but, in a consistent number of patients, radical surgery can be performed and increases the chances for cure [
8]. The shaving-off of the tumour from the airway, tracheal window resection or segmental resection are performed in relation to the degree of airway invasion [
4]. These procedures, already described and still disputed in some technical aspects and indications [
8,
24], are effective in expert hands but must be strictly planned [
27]. The preoperative US evaluation of thyroid nodules allows the identification of some characteristics, such as the higher tumour size or the irregular tumour margins, typically associated with an already locally advanced disease and more aggressive histotype [
15]. However, although the US examination aims at also assessing lymph node status, it might fail to adequately investigate the deep neck. Furthermore, the cytological information derived from FNAs cannot currently predict the biological aggressiveness of well-differentiated carcinomas.
In particular, this lack of knowledge prevents us from gaining specific information on the tumour trend to present local infiltration, early lymphatic spread or both. To fill this gap, we attempted to investigate the IHC expression of AhR, N-cadherin, E-cadherin and CD147, considering the correlation found in various cancer types between their expression and tumour aggressive behaviour [
16,
17,
18,
19,
20,
21,
22,
23].
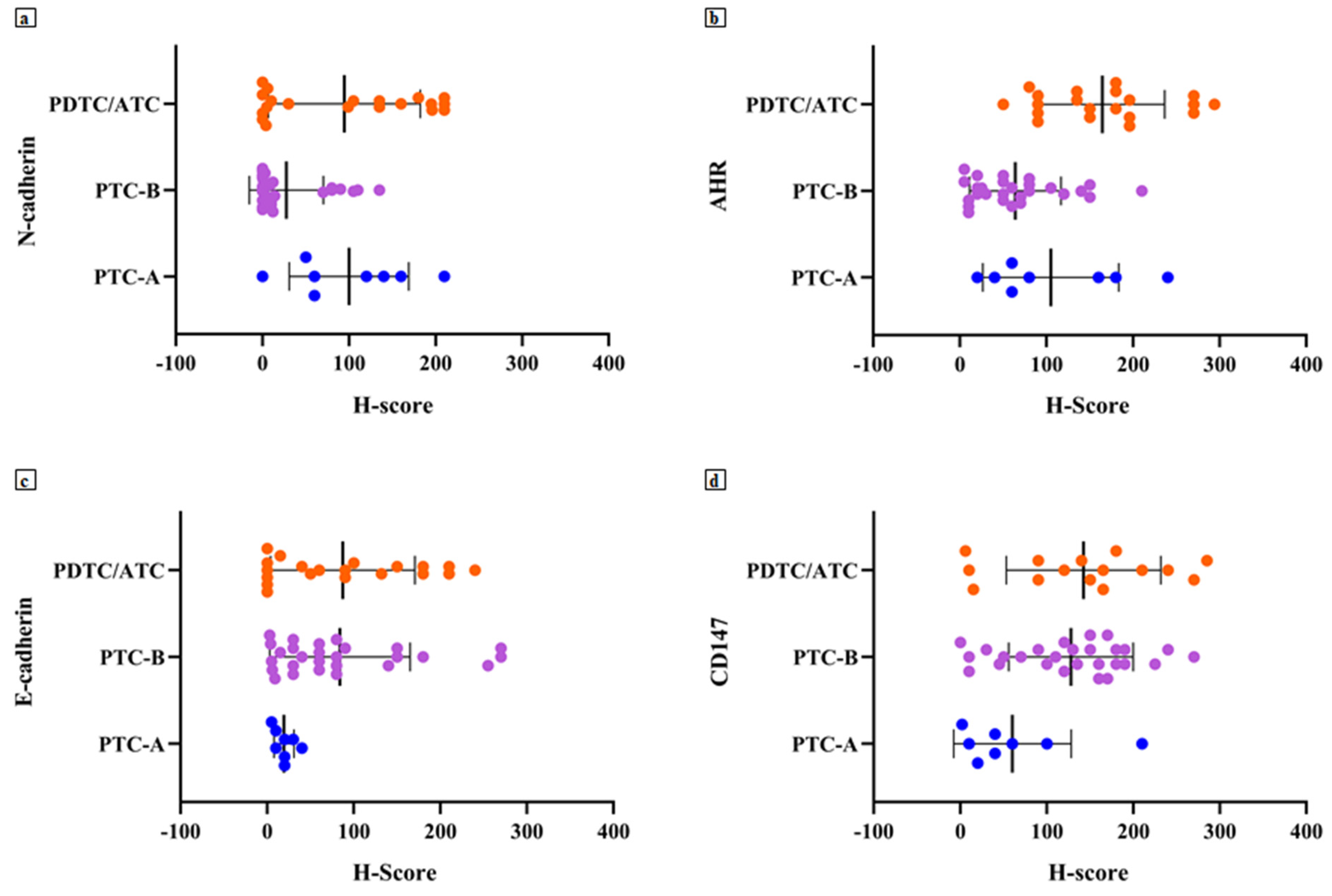
Indeed, based on the study findings, we could advance the potential role of AhR/N-cadherin as a prognostic marker, being able to suggest a more aggressive phenotype in thyroid carcinoma as evidenced by the high levels of AhR/N-cadherin found in most patients of the PTC-A and PDTC/ATC groups.
These findings are consistent with previously reported data that showed how the increased expression of AhR may play an oncogenic enhancing function not only through the induction of an immune-tolerant microenvironment but also through the expression of proteins such as N-cadherin involved in the regulation and initiation of the EMT [
17,
28,
29].
In our case series, AhR seems to be an important prognostic marker, highly expressed in patients with a persistence or recurrence of disease in addition to being a marker of aggression and histologic de-differentiation.
N-cadherin expression still remains a matter of debate in the context of thyroid oncogenesis [
17,
30]. This study could find that expression of this protein plays a significant role in promoting invasiveness, being expressed in both PTC-A and PDTC/ATC groups and displaying a low expression rate in the PTC-B group.
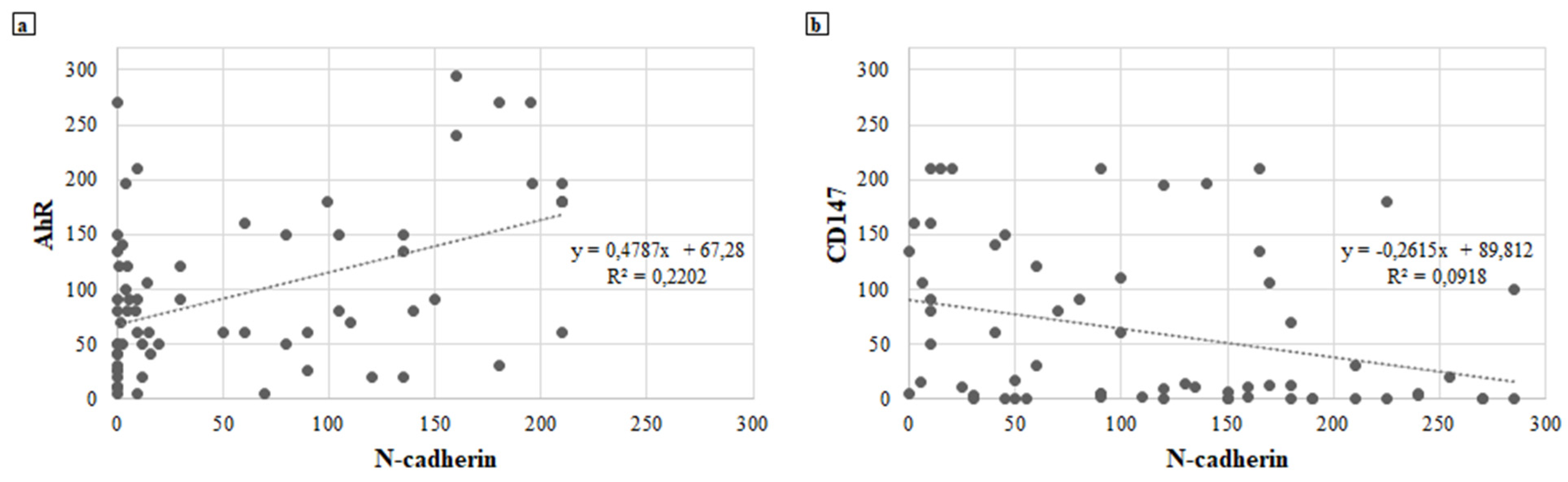
The role of E-cadherin and CD147 remains to be defined: despite the available data reported [
31,
32,
33,
34,
35,
36], they showed only marginal roles as markers of aggressive biological behaviour in this analysis. In particular, although E-cadherin expression is not significantly associated with all of the analysed variables, we could observe a trend of increased aggressiveness (PTC-A and PDTC/ATC) with absent or very low E-cadherin. CD147 was highly expressed both in PTC-B and PDTC/ATC groups and increased with a decrease in E-cadherin. Nevertheless, as CD147 levels increased, both AhR and N-cadherin expressions decreased; some authors hypothesised that the reason behind the biological aggressiveness related to the overexpression of CD147 could be the enhancement of the EMT process with cancer migration and invasion as also described in other tumours [
37,
38,
39].
The current study has some limitations: this is a retrospective clinical study and poor a priori knowledge is available to formally attribute an effective correlation between AhR/N-cadherin expression and biologic aggressiveness. The study timeframe is almost two decades and does not guarantee homogeneous diagnostic–therapeutic management in all patients; moreover, the number of patients in group A is limited, despite it representing a good experience for a very rare condition, and, hence, it cannot fully show an independent impact of these findings. However, to have a more homogeneous caseload, the study was designed to only analyse PTCs among the most differentiated histotypes. In addition, considering that one of the goals of the study was to preoperatively identify non-infiltrating airway carcinomas and infiltrating ones, we considered only PTCs, since the preoperative FNAs do not allow the cytological diagnosis of follicular thyroid carcinomas. Therefore, the assessment of the analysed biomarkers on such specimens would not change the surgical management of preoperative follicular lesions. Moreover, since BRAFV600E mutation was demonstrated to be associated with an increased expression of AhR, particularly at the infiltrative tumour edge, in thyroid cancer murine models [
17], and the associations between the molecules here investigated and other mutations implied in thyroid cancer pathogenesis, progression and prognosis (e.g., RET/PTC, TP53, TERT, ATK1 and RAS) have not yet been described [
40], additional studies at the molecular level of these aspects should be conducted.
This study suggests that knowing in advance the most important onco-biological factors might be helpful in the decision-making process, providing a more solid therapeutic indication and an increased expectation for radical surgery when markers of local aggressiveness are negative. Hence, there is the need for further studies to confirm these preliminary findings. When prognosticators and biomarkers for local aggressiveness can be translated into clinical practice, they may be supportive in better planning the most appropriate surgical procedure to be performed. A biological tumour profile could change the current paradigm to plan treatments based on “static” instrumental examination, giving a more “dynamic” assessment of tumour behaviour from the cytological immunophenotyping of the FNA-derived material.
In conclusion, thyroid cancer is a varied disease with a positive outcome after resection, if the tumour is intrathyroid. Our study suggests that several markers deserve to be further investigated in view of a potential role to discriminate, among the same histotype, between different subsets of a patient’s risk. Knowing in advance tumour aggressiveness could be helpful in avoiding suboptimal surgery if the tumour has an inner trend to recur or to infiltrate the neighbouring anatomical structures.