Exploring Clinical Correlates of Metacognition in Bipolar Disorders Using Moderation Analyses: The Role of Antipsychotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Characteristics of the Recruiting Network
2.2. Participants
2.3. Assessment Tools
2.3.1. Clinical Assessments
2.3.2. Objective and Subjective Cognition
- Objective cognition: the battery of cognitive tests
- -
- Processing speed: Digit symbol coding (WAIS-III) or coding (WAIS-IV), WAIS symbol search, and TMT (Trail-Making Test) [28] part A
- -
- Verbal memory: California Verbal Learning Test [29] short and long delay free recall and total recognition
- -
- Attention: Conners’ Continuous Performance Test II [30] (detectability)
- -
- Working memory: WAIS digit span (total score) and spatial span (forward and backward scores) from the Wechsler Memory Scale version III [31]
- -
- -
- Verbal and perceptual reasoning: WAIS vocabulary and matrices
- Subjective cognition: cognitive complaints
2.4. Statistical Analyses
- -
- -
- the level of significance of their interaction with objective cognition was p ≥ 0.25 and the level of significance of their main effect was p < 0.25. In this case, only the main effect of the clinical covariate was included in the model
3. Results
3.1. Characteristics of Participants
3.2. Moderation Analyses
3.2.1. Trivariable Ordinal Logistic Regressions
3.2.2. Multiple Ordinal Logistic Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, A.S.; Bedford, N.; Wiffen, B.; Gilleen, J. Failures of Metacognition and Lack of Insight in Neuropsychiatric Disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1379–1390. [Google Scholar] [CrossRef] [Green Version]
- Varga, M.; Magnusson, A.; Flekkøy, K.; Rønneberg, U.; Opjordsmoen, S. Insight, Symptoms and Neurocognition in Bipolar I Patients. J. Affect. Disord. 2006, 91, 1–9. [Google Scholar] [CrossRef]
- Camelo, E.; Mograbi, D.C.; de Assis da Silva, R.; Santana, C.M.T.; Ferreira do Nascimento, R.L.; de Oliveira E Silva, A.C.; Nardi, A.E.; Cheniaux, E. Clinical and Cognitive Correlates of Insight in Bipolar Disorder. Psychiatr. Q. 2019, 90, 385–394. [Google Scholar] [CrossRef]
- Favaretto, E.; Bedani, F.; Offredi, A.; Schroffenegger, M.; Sassaroli, S.; Ruggiero, G.; Fagiolini, A.; Caselli, G. Metacognitions and Repetitive Negative Thinking in Bipolar Disorder and Healthy Controls: A Comparative Study. J. Affect. Disord. 2020, 276, 152–158. [Google Scholar] [CrossRef]
- Østefjells, T.; Melle, I.; Aminoff, S.R.; Hellvin, T.; Hagen, R.; Lagerberg, T.V.; Lystad, J.U.; Røssberg, J.I. An Exploration of Metacognitive Beliefs and Thought Control Strategies in Bipolar Disorder. Compr. Psychiatry 2017, 73, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Van Camp, L.; Sabbe, B.G.C.; Oldenburg, J.F.E. Metacognitive Functioning in Bipolar Disorder versus Controls and Its Correlations with Neurocognitive Functioning in a Cross-Sectional Design. Compr. Psychiatry 2019, 92, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Van Camp, L.; van den Ameele, S.; Sabbe, B.G.C.; Oldenburg, J.F.E. The Longitudinal Course of Cognitive Insight and Mood in Bipolar Disorder. Psychiatry Res. 2018, 269, 9–12. [Google Scholar] [CrossRef]
- Demant, K.M.; Vinberg, M.; Kessing, L.V.; Miskowiak, K.W. Assessment of Subjective and Objective Cognitive Function in Bipolar Disorder: Correlations, Predictors and the Relation to Psychosocial Function. Psychiatry Res. 2015, 229, 565–571. [Google Scholar] [CrossRef]
- van der Werf-Eldering, M.J.; Burger, H.; Jabben, N.; Holthausen, E.A.E.; Aleman, A.; Nolen, W.A. Is the Lack of Association between Cognitive Complaints and Objective Cognitive Functioning in Patients with Bipolar Disorder Moderated by Depressive Symptoms? J. Affect. Disord. 2011, 130, 306–311. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.H.; Støttrup, M.M.; Nayberg, E.; Knorr, U.; Ullum, H.; Purdon, S.E.; Kessing, L.V.; Miskowiak, K.W. Optimising Screening for Cognitive Dysfunction in Bipolar Disorder: Validation and Evaluation of Objective and Subjective Tools. J. Affect. Disord. 2015, 187, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.J.; Mackala, S.A.; Kozicky, J.-M.; Yatham, L.N. Metacognitive Knowledge and Experience in Recently Diagnosed Patients with Bipolar Disorder. J. Clin. Exp. Neuropsychol. 2016, 38, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.; Petersen, J.; Ott, C.; Knorr, U.; Kessing, L.; Gallagher, P.; Robinson, L. Predictors of the Discrepancy between Objective and Subjective Cognition in Bipolar Disorder: A Novel Methodology. Acta Psychiatr. Scand. 2016, 134, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lu, D.; Huang, Z.; Chen, W.; Luo, X.; Zhu, Y. The Associations between Subjective and Objective Cognitive Functioning across Manic or Hypomanic, Depressed, and Euthymic States in Chinese Bipolar Patients. J. Affect. Disord. 2019, 249, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Clos, M.; Bunzeck, N.; Sommer, T. Dopamine Is a Double-Edged Sword: Dopaminergic Modulation Enhances Memory Retrieval Performance but Impairs Metacognition. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Aydin, O.; Balikci, K.; Tas, C.; Aydin, P.U.; Danaci, A.E.; Brüne, M.; Lysaker, P.H. The Developmental Origins of Metacognitive Deficits in Schizophrenia. Psychiatry Res. 2016, 245, 15–21. [Google Scholar] [CrossRef]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I: Clinician Version; American Psychiatric Pub: Washington, DC, USA, 1997; ISBN 0-88048-931-6. [Google Scholar]
- Mazzarini, L.; Pacchiarotti, I.; Colom, F.; Sani, G.; Kotzalidis, G.D.; Rosa, A.R.; Sanna, L.; De Rossi, P.; Girardi, N.; Bonnin, C.M.; et al. Predominant Polarity and Temperament in Bipolar and Unipolar Affective Disorders. J. Affect. Disord. 2009, 119, 28–33. [Google Scholar] [CrossRef]
- Guy, W. Clinical global impression scale. In The ECDEU Assessment Manual for Psychopharmacology; U.S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs: Rockville, MD, USA, 1976; Volume 76, pp. 218–222. [Google Scholar]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br. J. Psychiatry J. Ment. Sci. 1978, 133, 429–435. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Asberg, M. A New Depression Scale Designed to Be Sensitive to Change. Br. J. Psychiatry J. Ment. Sci. 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Sydeman, S.J. State-Trait Anxiety Inventory and State-Trait Anger Expression Inventory. In The Use of Psychological Testing for Treatment Planning and Outcome Assessment; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1994; pp. 292–321. [Google Scholar]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor Structure of the Barratt Impulsiveness Scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Bernstein, D.; Fink, L. Childhood Trauma Questionnaire: A Retrospective Self-Report Manual [Manual]; The Psychological Corporation: San Antonio, TX, USA, 1998. [Google Scholar]
- Rosa, A.R.; Sanchez-Moreno, J.; Martinez-Aran, A.; Salamero, M.; Torrent, C.; Reinares, M.; Comes, M.; Colom, F.; Van Riel, W.; Ayuso-Mateos, J.L.; et al. Validity and Reliability of the Functioning Assessment Short Test (FAST) in Bipolar Disorder. Clin. Pract. Epidemiol. Ment. Health CP EMH 2007, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.; Kulkarni, J.; Sergejew, A.A. Reliability and Validity of a New Medication Adherence Rating Scale (MARS) for the Psychoses. Schizophr. Res. 2000, 42, 241–247. [Google Scholar] [CrossRef]
- Wechsler, D. WAIS-III, Wechsler Adult Intelligence Scale: Administration and Scoring Manual; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Wechsler, D.; Coalson, D.L.; Raiford, S.E. WAIS-IV: Wechsler Adult Intelligence Scale; Pearson: San Antonio, TX, USA, 2008; ISBN 0-15-898084-0. [Google Scholar]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Skills 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Delis, D.C. CVLT-II: California Verbal Learning Test: Adult Version; Psychological Corporation: San Antonio, TX, USA, 2000; ISBN 0-15-803573-9. [Google Scholar]
- Conners, C.K.; Staff, M. Conners’ Continuous Performance Test II; Multi-Health Syst. Inc.: North Tonawanda, NY, USA, 2000. [Google Scholar]
- Wechsler, D. Wechsler Memory Scale-Third Edition; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Golden, C.J. A Manual for the Clinical and Experimental Use of the Stroop Color and Word Test; Stoelting: Chicago, IL, USA, 1978. [Google Scholar]
- Lezak, M.D. Neuropsychological Assessment; Oxford University Press: New York, NY, USA, 2004; ISBN 0-19-511121-4. [Google Scholar]
- Godefroy, O. The GREFEX battery: Normative data. In Executive Functions and Neurological and Psychiatric Pathologies: Evaluation in Clinical Practice; Solal Editions: Marseille, France, 2008; p. 231. [Google Scholar]
- Poitrenaud, J.; Deweer, B.; Kalafat, M.; Van der Linden, M. French Adaptation of the California Verbal Learning Test; Les Editions du Centre de Psychologie Appliquée: Paris, France, 2007. [Google Scholar]
- Ehrminger, M.; Brunet-Gouet, E.; Cannavo, A.-S.; Aouizerate, B.; Cussac, I.; Azorin, J.-M.; Bellivier, F.; Bougerol, T.; Courtet, P.; Dubertret, C.; et al. Longitudinal Relationships between Cognition and Functioning over 2 Years in Euthymic Patients with Bipolar Disorder: A Cross-Lagged Panel Model Approach with the FACE-BD Cohort. Br. J. Psychiatry J. Ment. Sci. 2019, 218, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.; Brunet-Gouet, E.; Ehrminger, M.; Aouizerate, B.; Aubin, V.; Azorin, J.M.; Bellivier, F.; Bougerol, T.; Courtet, P.; Dubertret, C.; et al. Minimum Clinically Important Differences for the Functioning Assessment Short Test and a Battery of Neuropsychological Tests in Bipolar Disorders: Results from the FACE-BD Cohort. Epidemiol. Psychiatr. Sci. 2020, 29, e144. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.; Urbach, M.; Fonteneau, S.; Berna, F.; Brunel, L.; Capdevielle, D.; Chereau, I.; Dubreucq, J.; Faget-Agius, C.; Fond, G.; et al. Psychiatric Disability as Mediator of the Neurocognition-Functioning Link in Schizophrenia Spectrum Disorders: SEM Analysis Using the Evaluation of Cognitive Processes Involved in Disability in Schizophrenia (ECPDS) Scale. Schizophr. Res. 2018, 201, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.; Urbach, M.; Fonteneau, S.; Berna, F.; Brunel, L.; Capdevielle, D.; Chereau, I.; Dubreucq, J.; Faget-Agius, C.; Fond, G.; et al. Screening for Cognitive Deficits with the Evaluation of Cognitive Processes Involved in Disability in Schizophrenia Scale. Clin. Rehabil. 2019, 33, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Brunet-Gouet, E.; Decaix-Tisserand, C.; Urbach, M.; Bazin, N.; Aouizerate, B.; Brunel, L.; Capdevielle, D.; Chereau, I.; Dubertret, C.; Dubreucq, J.; et al. Outcome Prediction with a Social Cognitive Battery: A Multicenter Longitudinal Study. NPJ Schizophr. 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Gili, M.; Lopez-Navarro, E.; Homar, C.; Castro, A.; García-Toro, M.; Llobera, J.; Roca, M. Psychometric Properties of Spanish Version of QIDS-SR16 in Depressive Patients. Actas Esp. Psiquiatr. 2014, 42, 292–299. [Google Scholar] [PubMed]
- Bernstein, I.H.; Rush, A.J.; Carmody, T.J.; Woo, A.; Trivedi, M.H. Clinical vs. Self-Report Versions of the Quick Inventory of Depressive Symptomatology in a Public Sector Sample. J. Psychiatr. Res. 2007, 41, 239–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mickey, R.M.; Greenland, S. The Impact of Confounder Selection Criteria on Effect Estimation. Am. J. Epidemiol. 1989, 129, 125–137. [Google Scholar] [CrossRef]
- Hosmer Jr, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 398, ISBN 0-470-58247-2. [Google Scholar]
- Bourne, C.; Aydemir, O.; Balanza-Martinez, V.; Bora, E.; Brissos, S.; Cavanagh, J.T.; Clark, L.; Cubukcuoglu, Z.; Dias, V.V.; Dittmann, S.; et al. Neuropsychological Testing of Cognitive Impairment in Euthymic Bipolar Disorder: An Individual Patient Data Meta-Analysis. Acta Psychiatr. Scand. 2013, 128, 149–162. [Google Scholar] [CrossRef]
- Lou, H.C.; Skewes, J.C.; Thomsen, K.R.; Overgaard, M.; Lau, H.C.; Mouridsen, K.; Roepstorff, A. Dopaminergic Stimulation Enhances Confidence and Accuracy in Seeing Rapidly Presented Words. J. Vis. 2011, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Joensson, M.; Thomsen, K.R.; Andersen, L.M.; Gross, J.; Mouridsen, K.; Sandberg, K.; Østergaard, L.; Lou, H.C. Making Sense: Dopamine Activates Conscious Self-Monitoring through Medial Prefrontal Cortex. Hum. Brain Mapp. 2015, 36, 1866–1877. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.U.; Allen, M.; Purg, N.; Moutoussis, M.; Rees, G.; Dolan, R.J. Noradrenaline Blockade Specifically Enhances Metacognitive Performance. eLife 2017, 6, e24901. [Google Scholar] [CrossRef]
- Pierre, M.; Cogez, J.; Lebain, P.; Loisel, N.; Lalevée, C.; Bonnet, A.L.; De La Sayette, V.; Viader, F. Detection of Adult Attention Deficit Hyperactivity Disorder with Cognitive Complaint: Experience of a French Memory Center. Rev. Neurol. 2019, 175, 358–366. [Google Scholar] [CrossRef]
- Martínez-Arán, A.; Vieta, E.; Colom, F.; Torrent, C.; Reinares, M.; Goikolea, J.M.; Benabarre, A.; Comes, M.; Sánchez-Moreno, J. Do Cognitive Complaints in Euthymic Bipolar Patients Reflect Objective Cognitive Impairment? Psychother. Psychosom. 2005, 74, 295–302. [Google Scholar] [CrossRef]
- Ott, C.; Miné, H.; Petersen, J.Z.; Miskowiak, K. Relation between Functional and Cognitive Impairments in Remitted Patients with Bipolar Disorder and Suggestions for Trials Targeting Cognition: An Exploratory Study. J. Affect. Disord. 2019, 257, 382–389. [Google Scholar] [CrossRef]
- Rosa, A.R.; Mercade, C.; Sanchez-Moreno, J.; Sole, B.; Bonnin, C.D.M.; Torrent, C.; Grande, I.; Sugranyes, G.; Popovic, D.; Salamero, M. Validity and Reliability of a Rating Scale on Subjective Cognitive Deficits in Bipolar Disorder (COBRA). J. Affect. Disord. 2013, 150, 29–36. [Google Scholar] [CrossRef]
- Maniscalco, B.; Lau, H. A Signal Detection Theoretic Approach for Estimating Metacognitive Sensitivity from Confidence Ratings. Conscious. Cogn. 2012, 21, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Yatham, L.N.; Mackala, S.; Basivireddy, J.; Ahn, S.; Walji, N.; Hu, C.; Lam, R.W.; Torres, I.J. Lurasidone versus Treatment as Usual for Cognitive Impairment in Euthymic Patients with Bipolar I Disorder: A Randomised, Open-Label, Pilot Study. Lancet Psychiatry 2017, 4, 208–217. [Google Scholar] [CrossRef]
| Variable | Mean or % | SD | n |
|---|---|---|---|
| Age (years) | 40.2 | 11.1 | 281 |
| Sex | 42.3 (M) | 281 | |
| Educational level (years) | 14.3 | 2.5 | 281 |
| Diagnosis: Type 1 | 37.4 | 281 | |
| Type 2 | 52 | ||
| NOS 1 | 10.6 | ||
| Total number of mood episodes | 8.1 | 7.5 | 170 |
| Predominant polarity: depressive | 36.5 | 170 | |
| Indeterminate | 50.6 | ||
| Manic | 12.9 | ||
| Age at onset (years) | 23.3 | 8.2 | 271 |
| History of psychosis | 19.8 | 232 | |
| Rapid cycling | 7.6 | 263 | |
| CGI 2 Severity (1–7) | 4.5 | 0.9 | 279 |
| Current major depressive episode | 13.9 | 281 | |
| Current hypomanic episode | 1.4 | 281 | |
| Current manic episode | 0 | 281 | |
| MADRS 3 (0–60) | 10.4 | 8.5 | 281 |
| YMRS 4 (0–60) | 2.2 | 3.4 | 281 |
| STAI-YA 5 (state subscale) (20–80) | 43.4 | 14.5 | 278 |
| End of last characterized episode > 3 months | 55.6 | 279 | |
| Antidepressant | 22.1 | 281 | |
| Anticonvulsant | 25.3 | 281 | |
| Lithium Carbonate | 13.5 | 281 | |
| Antipsychotic | 16.4 | 281 | |
| Anxiolytic | 19.2 | 281 | |
| Any lifetime substance use disorder | 26 | 273 | |
| BIS 6 (34–136) | 66.9 | 10.6 | 279 |
| CTQ 7 (28–140) | 43.7 | 15.1 | 277 |
| FAST 8 total (0–72) | 20 | 13 | 280 |
| MARS 9 (0–10) | 6.8 | 2.2 | 254 |
| Mean | SD | n | |
|---|---|---|---|
| Verbal Memory | 0.41 | 0.9 | |
| CVLT 1 Immediate recall | 0.58 | 1.26 | 279 |
| CVLT 1 Short delay free recall | 0.29 | 1.11 | 279 |
| CVLT 1 Long delay free recall | 0.37 | 1.09 | 278 |
| CVLT 1 Total recognition | 0.38 | 0.59 | 278 |
| Working Memory | −0.14 | 0.7 | |
| Digit Span | −0.24 | 0.86 | 273 |
| Spatial Span forward | −0.08 | 0.88 | 270 |
| Spatial Span backward | −0.12 | 0.85 | 270 |
| Executive Functioning | −0.15 | 0.77 | |
| TMT 2 Part B | −0.14 | 1.18 | 279 |
| Stroop color/word condition | 0.04 | 1.02 | 267 |
| Phonemic fluency | −0.07 | 1.05 | 278 |
| Semantic fluency | −0.42 | 0.96 | 278 |
| Processing speed | −0.11 | 0.67 | |
| Coding | −0.2 | 0.92 | 271 |
| Symbol search | 0.01 | 0.88 | 270 |
| Stroop word condition | −0.08 | 0.79 | 268 |
| Stroop color condition | −0.45 | 0.85 | 267 |
| TMT 2 Part B | 0.18 | 0.96 | 280 |
| Attention | −0.38 | 0.67 | |
| CPT 3 omission | −0.84 | 1.23 | 266 |
| CPT 3 commission | −0.15 | 1.06 | 266 |
| CPT 3 variability | −0.29 | 1.1 | 266 |
| CPT 3 detectability | −0.23 | 0.98 | 266 |
| Reasoning | 0.56 | 0.71 | |
| Vocabulary | 0.83 | 0.87 | 251 |
| Matrices | 0.34 | 0.79 | 270 |
| Interaction between the Mean Cognitive Performance and a Clinical Moderator | OR (95% CI) 1 | Statistic | p | λ | fmi |
|---|---|---|---|---|---|
| Age | 0.98 (0.94–1.02) | t(250.5) = −0.9 | 0.392 | 0.063 | 0.071 |
| Sex | 1 (0.41–2.45) | t(258.3) = 0 | 0.992 | 0.044 | 0.051 |
| Educational level | 1.03 (0.86–1.24) | t(242.9) = 0.3 | 0.734 | 0.081 | 0.088 |
| Diagnosis (Type 2/NOS 2 vs. Type 1) | 0.83 (0.31–2.19) | t(245) = −0.4 | 0.706 | 0.076 | 0.083 |
| Total number of mood episodes | 0.98 (0.89–1.07) | t(72.7) = −0.5 | 0.599 | 0.536 | 0.548 |
| Predominant Polarity (Indeterminate/Manic vs. Depressive) | 3.24 (1.02–10.34) | t(128.4) = 2 | 0.047 | 0.335 | 0.345 |
| Age at onset | 1 (0.94–1.06) | t(251.1) = −0.1 | 0.896 | 0.062 | 0.069 |
| History of psychosis | 1.14 (0.32–4.02) | t(159.2) = 0.2 | 0.838 | 0.257 | 0.266 |
| Rapid cycling | 1.33 (0.25–6.93) | t(211.8) = 0.3 | 0.738 | 0.146 | 0.154 |
| CGI 3 Severity | 1.61 (0.88–2.94) | t(241.6) = 1.5 | 0.124 | 0.084 | 0.091 |
| MADRS 4 | 1.01 (0.95–1.07) | t(252.2) = 0.2 | 0.826 | 0.059 | 0.067 |
| YMRS 5 | 1.02 (0.89–1.17) | t(239.9) = 0.3 | 0.758 | 0.087 | 0.095 |
| Antidepressant | 0.82 (0.28–2.45) | t(251.5) = −0.4 | 0.724 | 0.061 | 0.068 |
| Anticonvulsant | 1.05 (0.31–3.63) | t(229.8) = 0.1 | 0.933 | 0.109 | 0.116 |
| Lithium Carbonate | 0.45 (0.12–1.69) | t(257.5) = −1.2 | 0.233 | 0.046 | 0.054 |
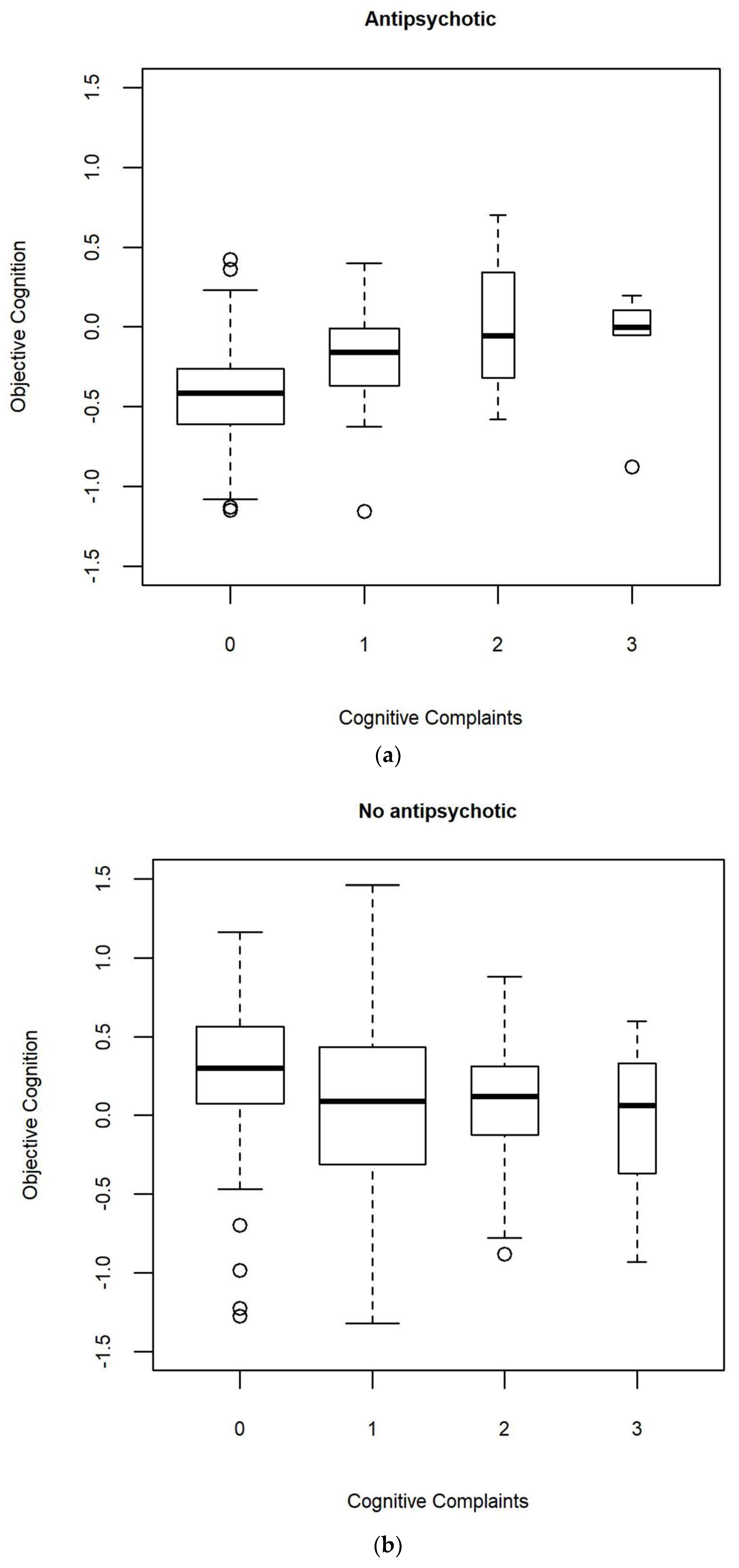
| Antipsychotic | 6.87 (1.64–28.76) | t(240) = 2.7 | 0.009 | 0.087 | 0.094 |
| Anxiolytic | 0.49 (0.17–1.47) | t(247.2) = −1.3 | 0.205 | 0.071 | 0.078 |
| Any lifetime substance use disorder | 1.15 (0.4–3.33) | t(234.1) = 0.3 | 0.793 | 0.1 | 0.107 |
| BIS 6 | 1.02 (0.98–1.07) | t(249.4) = 1 | 0.309 | 0.066 | 0.073 |
| CTQ 7 | 0.99 (0.96–1.03) | t(229.7) = −0.5 | 0.617 | 0.109 | 0.117 |
| FAST 8 | 1.04 (1–1.08) | t(246.6) = 1.9 | 0.064 | 0.072 | 0.08 |
| MARS 9 | 1.09 (0.89–1.33) | t(242.8) = 0.8 | 0.397 | 0.081 | 0.088 |
| Type of WAIS 10 | 0.92 (0.23–3.7) | t(192.2) = −0.1 | 0.908 | 0.186 | 0.194 |
| Independant Variable | OR (95% CI) 1 | Statistic | p | λ | fmi |
|---|---|---|---|---|---|
| Predominant Polarity (Indeterminate/Manic vs. Depressive) | 1.17 (0.6–2.29) | t(116) = 0.5 | 0.646 | 0.353 | 0.364 |
| CGI Severity | 1.37 (0.96–1.96) | t(236.4) = 1.7 | 0.083 | 0.055 | 0.063 |
| Lithium carbonate | 1.42 (0.66–3.06) | t(248.9) = 0.9 | 0.366 | 0.019 | 0.026 |
| Antipsychotic | 1.4 (0.63–3.1) | t(240.5) = 0.8 | 0.408 | 0.044 | 0.052 |
| Anxiolytic | 1.89 (1–3.57) | t(248.7) = 2 | 0.051 | 0.019 | 0.027 |
| FAST | 1.02 (1–1.05) | t(245.8) = 1.9 | 0.058 | 0.028 | 0.036 |
| Objective cognition | 0.05 (0–1.58) | t(213.8) = −1.7 | 0.09 | 0.111 | 0.119 |
| Educational level | 0.97 (0.88–1.08) | t(247.5) = −0.5 | 0.596 | 0.023 | 0.031 |
| Diagnosis (Type 2/NOS 2 vs. Type 1) | 1.41 (0.67–2.98) | t(187.3) = 0.9 | 0.363 | 0.17 | 0.179 |
| History of psychosis | 1.22 (0.49–3.02) | t(121.2) = 0.4 | 0.664 | 0.337 | 0.348 |
| MADRS3 | 1.1 (1.06–1.14) | t(240.5) = 5.2 | <0.001 | 0.044 | 0.052 |
| Any lifetime substance use disorder | 0.81 (0.45–1.45) | t(231.2) = −0.7 | 0.48 | 0.069 | 0.077 |
| BIS4 | 1.07 (1.04–1.1) | t(243.5) = 4.4 | <0.001 | 0.035 | 0.043 |
| CTQ 5 | 1.02 (1–1.03) | t(245.2) = 1.8 | 0.067 | 0.03 | 0.038 |
| MARS 6 | 0.93 (0.82–1.06) | t(196.3) = −1.1 | 0.264 | 0.15 | 0.159 |
| Type of WAIS 7 (IV vs. III) | 0.75 (0.36–1.59) | t(246.1) = −0.7 | 0.458 | 0.027 | 0.035 |
| Objective cognition: Polarity (Indeterminate/Manic vs. Depressive) | 1.42 (0.37–5.47) | t(126.5) = 0.5 | 0.605 | 0.321 | 0.332 |
| Objective cognition: CGI 8 | 1.52 (0.73–3.17) | t(228.2) = 1.1 | 0.265 | 0.076 | 0.084 |
| Objective cognition: Lithium carbonate | 0.49 (0.09–2.67) | t(221.4) = −0.8 | 0.405 | 0.093 | 0.101 |
| Objective cognition: Antipsychotic | 6 (1.13–31.7) | t(216.9) = 2.1 | 0.035 | 0.104 | 0.112 |
| Objective cognition: Anxiolytic | 0.52 (0.13–2.13) | t(209.5) = −0.9 | 0.36 | 0.121 | 0.129 |
| Objective cognition: FAST 9 | 1.03 (0.98–1.08) | t(224.6) = 1.3 | 0.199 | 0.085 | 0.093 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roux, P.; Faivre, N.; Cannavo, A.-S.; Brunet-Gouet, E.; Passerieux, C. Exploring Clinical Correlates of Metacognition in Bipolar Disorders Using Moderation Analyses: The Role of Antipsychotics. J. Clin. Med. 2021, 10, 4349. https://doi.org/10.3390/jcm10194349
Roux P, Faivre N, Cannavo A-S, Brunet-Gouet E, Passerieux C. Exploring Clinical Correlates of Metacognition in Bipolar Disorders Using Moderation Analyses: The Role of Antipsychotics. Journal of Clinical Medicine. 2021; 10(19):4349. https://doi.org/10.3390/jcm10194349
Chicago/Turabian StyleRoux, Paul, Nathan Faivre, Anne-Sophie Cannavo, Eric Brunet-Gouet, and Christine Passerieux. 2021. "Exploring Clinical Correlates of Metacognition in Bipolar Disorders Using Moderation Analyses: The Role of Antipsychotics" Journal of Clinical Medicine 10, no. 19: 4349. https://doi.org/10.3390/jcm10194349
APA StyleRoux, P., Faivre, N., Cannavo, A.-S., Brunet-Gouet, E., & Passerieux, C. (2021). Exploring Clinical Correlates of Metacognition in Bipolar Disorders Using Moderation Analyses: The Role of Antipsychotics. Journal of Clinical Medicine, 10(19), 4349. https://doi.org/10.3390/jcm10194349







