Age at Natural Menopause and Blood Pressure Traits: Mendelian Randomization Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Population for Analyses
2.3. Assessment of Variables
2.4. Genotyping
2.5. SNPs Selection and the Genetic Risk Score
2.6. Statistical Analyses
2.6.1. Cross-Sectional Analyses
2.6.2. One-Sample Mendelian Randomization
2.6.3. Two-Sample Mendelian Randomization
3. Results
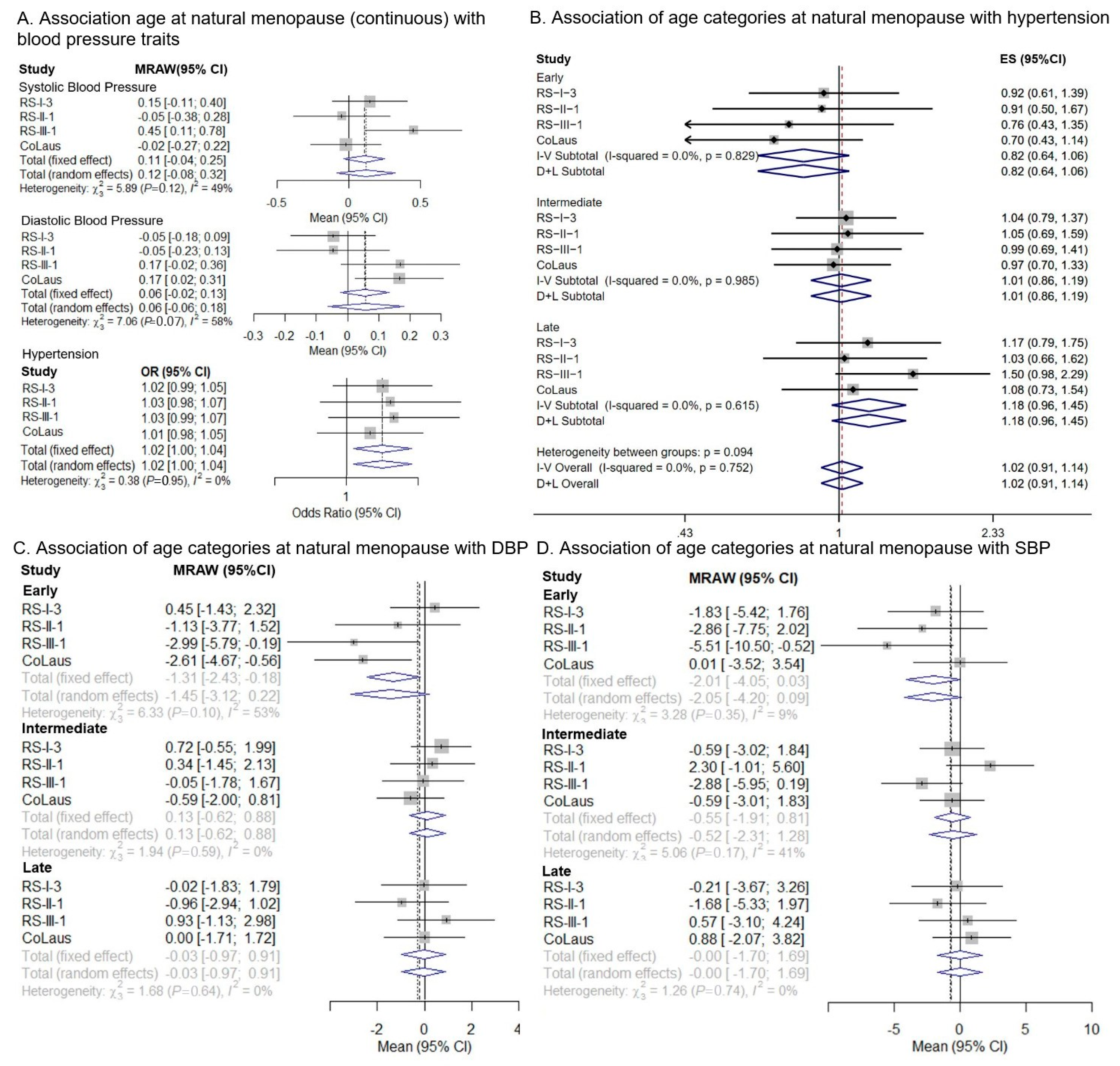
3.1. One-Sample MR
3.2. Two-Sample MR
4. Discussion
4.1. Strengths and Limitations
4.2. Future Research and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef]
- Lacruz, M.E.; Kluttig, A.; Hartwig, S.; Löer, M.; Tiller, D.; Greiser, K.H.; Werdan, K.; Haerting, J. Prevalence and incidence of hypertension in the general adult population: Results of the CARLA-cohort study. Medicine 2015, 94, e952. [Google Scholar] [CrossRef]
- Coylewright, M.; Reckelhoff, J.F.; Ouyang, P. Menopause and hypertension: An age-old debate. Hypertension 2008, 51, 952–959. [Google Scholar] [CrossRef]
- Muka, T.; Oliver-Williams, C.; Kunutsor, S.; Laven, J.S.E.; Fauser, B.C.J.M.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of Age at Onset of Menopause and Time Since Onset of Menopause with Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016, 1, 767–776. [Google Scholar] [CrossRef]
- Anagnostis, P.; Theocharis, P.; Lallas, K.; Konstantis, G.; Mastrogiannis, K.; Bosdou, J.K.; Lambrinoudaki, I.; Stevenson, J.C.; Goulis, D.G. Early menopause is associated with increased risk of arterial hypertension: A systematic review and meta-analysis. Maturitas 2020, 135, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Golezar, S.; Ramezani Tehrani, F.; Khazaei, S.; Ebadi, A.; Keshavarz, Z. The global prevalence of primary ovarian insufficiency and early menopause: A meta-analysis. Climacteric 2019, 22, 403–411. [Google Scholar] [CrossRef] [PubMed]
- De Kat, A.; Dam, V.; Onland-Moret, N.; Eijkemans, M.; Broekmans, F.; Van Der Schouw, Y. Unraveling the associations of age and menopause with cardiovascular risk factors in a large population-based study. BMC Med. 2017, 15, 2. [Google Scholar]
- Appiah, D.; Schreiner, P.J.; Demerath, E.W.; Loehr, L.R.; Chang, P.P.; Folsom, A.R. Association of age at menopause with incident heart failure: A prospective cohort study and meta-analysis. J. Am. Heart Assoc. 2016, 5, e003769. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chung, H.F.; Pandeya, N.; Dobson, A.J.; Hardy, R.; Kuh, D.; Brunner, E.J.; Bruinsma, F.; Giles, G.G.; Demakakos, P.; et al. Premenopausal cardiovascular disease and age at natural menopause: A pooled analysis of over 170,000 women. Eur. J. Epidemiol. 2019, 34, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Day, F.R.; Ruth, K.S.; Thompson, D.J.; Lunetta, K.L.; Pervjakova, N.; Chasman, D.I.; Stolk, L.; Finucane, H.K.; Sulem, P.; Bulik-Sullivan, B.; et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat. Genet. 2015, 47, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Firmann, M.; Mayor, V.; Vidal, P.M.; Bochud, M.; Pecoud, A.; Hayoz, D.; Paccaud, F.; Preisig, M.; Song, K.S.; Yuan, X.; et al. The CoLaus study: A population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc. Disord. 2008, 8, 6. [Google Scholar] [CrossRef]
- Ikram, M.A.; Brusselle, G.; Ghanbari, M.; Goedegebure, A.; Ikram, M.K.; Kavousi, M.; Kieboom, B.C.T.; Klaver, C.C.W.; de Knegt, R.J.; Luik, A.I. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur. J. Epidemiol. 2020, 35, 483–517. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef]
- Muka, T.; Asllanaj, E.; Avazverdi, N.; Jaspers, L.; Stringa, N.; Milic, J.; Ligthart, S.; Ikram, M.A.; Laven, J.S.E.; Kavousi, M. Age at natural menopause and risk of type 2 diabetes: A prospective cohort study. Diabetologia 2017, 60, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, R.; Ikram, M.K.; Vingerling, J.R.; Witteman, J.C.M.; Hofman, A.; de Jong, P.T.V.M. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: The Rotterdam Study. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3771–3777. [Google Scholar] [CrossRef]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef]
- Delaneau, O.; Marchini, J.; Zagury, J.-F. A linear complexity phasing method for thousands of genomes. Nat. Methods 2012, 9, 179–181. [Google Scholar] [CrossRef]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar]
- Burgess, S.; Dudbridge, F.; Thompson, S.G. Combining information on multiple instrumental variables in Mendelian randomization: Comparison of allele score and summarized data methods. Stat. Med. 2016, 35, 1880–1906. [Google Scholar] [CrossRef]
- Sedaghat, S.; Pazoki, R.; Uitterlinden, A.G.; Hofman, A.; Stricker, B.H.C.; Ikram, M.A.; Franco, O.H.; Dehghan, A. Association of uric acid genetic risk score with blood pressure: The Rotterdam study. Hypertension 2014, 64, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019, 72, 558. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S. An introduction to instrumental variables for epidemiologists. Int. J. Epidemiol. 2000, 29, 722–729. [Google Scholar] [CrossRef]
- Lyall, D.M.; Celis-Morales, C.; Ward, J.; Iliodromiti, S.; Anderson, J.J.; Gill, J.M.R.; Smith, D.J.; Ntuk, U.E.; Mackay, D.F.; Holmes, M.V.; et al. Association of Body Mass Index with Cardiometabolic Disease in the UK Biobank: A Mendelian Randomization Study. JAMA Cardiol. 2017, 2, 882–889. [Google Scholar] [CrossRef]
- Burgess, S.; Smith, G.D.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Holmes, M.V.; Minelli, C.; Relton, C.L. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Fortier, I.; Raina, P.; Van den Heuvel, E.R.; Griffith, L.E.; Craig, C.; Saliba, M.; Doiron, D.; Stolk, R.P.; Knoppers, B.M.; Ferretti, V.; et al. Maelstrom Research guidelines for rigorous retrospective data harmonization. Int. J. Epidemiol. 2017, 46, 103–105. [Google Scholar] [CrossRef]
- Nazarzadeh, M.; Pinho-Gomes, A.-C.; Bidel, Z.; Dehghan, A.; Canoy, D.; Hassaine, A.; Ayala Solares, J.R.; Salimi-Khorshidi, G.; Smith, G.D.; Otto, C.M. Plasma lipids and risk of aortic valve stenosis: A Mendelian randomization study. Eur. Heart J. 2020, 41, 3913–3920. [Google Scholar] [CrossRef]
- van der Plaat, D.A.; Pereira, M.; Pesce, G.; Potts, J.F.; Amaral, A.F.; Dharmage, S.C.; Garcia-Aymerich, J.M.; Thompson, J.R.; Real, F.G.; Jarvis, D.L. Age at menopause and lung function: A Mendelian randomisation study. Eur. Respir. J. 2019, 54, 1802421. [Google Scholar] [CrossRef]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar]
- Maas, A.H.E.M.; Franke, H.R. Women’s health in menopause with a focus on hypertension. Neth. Heart J. 2009, 17, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.S.; van Asselt, K.M.; van der Schouw, Y.T.; van der Tweel, I.; Peeters, P.H.; Wilson, P.W.; Pearson, P.L.; Grobbee, D.E. Heart disease risk determines menopausal age rather than the reverse. J. Am. Coll. Cardiol. 2006, 47, 1976–1983. [Google Scholar] [CrossRef]
- Lim, H.-S.; Kim, T.-H.; Lee, H.-H.; Park, Y.-H.; Kim, J.-M.; Lee, B.-R. Hypertension and age at onset of natural menopause in Korean postmenopausal women: Results from the Korea National Health and Nutrition Examination Survey (2008–2013). Maturitas 2016, 90, 17–23. [Google Scholar] [CrossRef]
- Lee, J.S.; Hayashi, K.; Mishra, G.; Yasui, T.; Kubota, T.; Mizunuma, H. Independent association between age at natural menopause and hypercholesterolemia, hypertension, and diabetes mellitus: Japan nurses’ health study. J. Atheroscler. Thromb. 2012, 20, 14746. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, L.; Hu, Y.; Liu, T.; Guo, J.; Shen, Y.; Zhang, R.; Miles, T.; Li, C. Associations of the ages at menarche and menopause with blood pressure and hypertension among middle-aged and older Chinese women: A cross-sectional analysis of the baseline data of the China Health and Retirement Longitudinal Study. Hypertens. Res. 2019, 42, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Meilahn, E.; Kuller, L.H.; Kelsey, S.F.; Caggiula, A.W.; Wing, R.R. Menopause and Risk-Factors for Coronary Heart-Disease. N. Engl. J. Med. 1989, 321, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Trabuco, E.C.; Moorman, P.G.; Algeciras-Schimnich, A.; Weaver, A.L.; Cliby, W.A. Association of Ovary-Sparing Hysterectomy with Ovarian Reserve. Obs. Gynecol. 2016, 127, 819–827. [Google Scholar] [CrossRef]
- Xiangying, H.; Lili, H.; Yifu, S. The effect of hysterectomy on ovarian blood supply and endocrine function. Climacteric 2006, 9, 283–289. [Google Scholar] [CrossRef]
- Gür, M.; Elbasan, Z.; Şahin, D.Y.; Koyunsever, N.Y.; Şeker, T.; Özaltun, B.; Çaylı, M.; Kocyigit, A. DNA damage and oxidative status in newly diagnosed, untreated, dipper and non-dipper hypertensive patients. Hypertens. Res. 2013, 36, 166–171. [Google Scholar] [CrossRef][Green Version]
- Yasuda, M.T.; Sakakibara, H.; Shimoi, K. Estrogen-and stress-induced DNA damage in breast cancer and chemoprevention with dietary flavonoid. Genes Environ. 2017, 39, 1–9. [Google Scholar] [CrossRef]
- Gottschalk, M.; Eskild, A.; Hofvind, S.; Gran, J.; Bjelland, E. Temporal trends in age at menarche and age at menopause: A population study of 312 656 women in Norway. Hum. Reprod. 2020, 35, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Seretis, A.; Cividini, S.; Markozannes, G.; Tseretopoulou, X.; Lopez, D.S.; Ntzani, E.E.; Tsilidis, K.K. Association between blood pressure and risk of cancer development: A systematic review and meta-analysis of observational studies. Sci. Rep. 2019, 9, 8565. [Google Scholar] [CrossRef]
- Woods, N.F.; Mitchell, E.S.; Smith-DiJulio, K. Cortisol levels during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Menopause (New York NY) 2009, 16, 708. [Google Scholar] [CrossRef]
- El Khoudary, S.R. Age at menopause onset and risk of cardiovascular disease around the world. Maturitas 2020, 141, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.C.; Timpson, N.; Smith, G.D. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Ebrahim, S. Mendelian randomization: Prospects, potentials, and limitations. Int. J. Epidemiol. 2004, 33, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Elder, P.; Sharma, G.; Gulati, M.; Michos, E.D. Identification of Female-Specific Risk Enhancers throughout the Lifespan of Women to Improve Cardiovascular Disease Prevention. Am. J. Prev. Cardiol. 2020, 2, 100028. [Google Scholar] [CrossRef]
- Agarwala, A.; Michos, E.D.; Samad, Z.; Ballantyne, C.M.; Virani, S.S. The Use of Sex-Specific Factors in the Assessment of Women’s Cardiovascular Risk. Circulation 2020, 141, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Asllanaj, E.; Zhang, X.; Rosales, C.O.; Nano, J.; Bramer, W.M.; Portilla-Fernandez, E.; Braun, K.V.E.; Gonzalez-Jaramillo, V.; Ahrens, W.; Ikram, A. Sexually dimorphic DNA-methylation in cardiometabolic health: A systematic review. Maturitas 2020, 135, 6–26. [Google Scholar] [CrossRef] [PubMed]
| CoLaus (n = 1139) | RS-I-3 (n = 1603) | RS-II-1 (n = 790) | RS-III-1 (n = 919) | Meta-Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | I2 |
| Crude | ||||||||||
| Systolic BP | −0.80 (−4.17; 2.61) | 0.65 | −1.89 (−4.23; 0.45) | 0.11 | 0.68 (−2.50; 3.87) | 0.68 | 0.93 (−1.50; 3.38) | 0.45 | −0.36 (−1.72; 1.01) | 5.9% |
| Diastolic BP | −0.02 (−1.85; 1.81) | 0.98 | −0.57 (−1.72; 0.58) | 0.33 | 0.48 (−1.06; 2.02) | 0.54 | 0.41 (−0.95; 1.77) | 0.56 | −0.01 (−0.71; 0.70) | 0.0% |
| * Hypertension | 1.09 (0.76; 1.54) | 0.68 | 0.90 (0.71; 1.13) | 0.35 | 1.01 (0.81; 1.47) | 0.55 | 0.97 (0.76; 1.24) | 0.84 | 0.97 (0.85; 1.11) | 0.0% |
| Adjusted | (n = 1137) | |||||||||
| Systolic BP | −0.84 (−3.72; 2.05) † | 0.570 | −1.86 (−4.13; 0.41) | 0.11 | −0.76 (−3.92; 2.38) | 0.63 | 0.40 (−2.06; 2.87) | 0.75 | −0.82 (−2.13; 0.50) | 0.0% |
| Diastolic BP | 0.25 (−1.40; 1.90) | 0.77 | −0.56 (−1.68; 0.56) | 0.33 | 0.27 (−1.42; 1.98) | 0.75 | 0.10 (−1.29; 1.48) | 0.89 | −0.10 (−0.81; 0.60) | 0.0% |
| * Hypertension | 1.03 (0.70; 1.50) | 0.89 | 0.94 (0.73; 1.20) | 0.63 | 1.02 (0.73; 1.43) | 0.90 | 0.92 (0.71; 1.21) | 0.56 | 0.96 (0.83; 1.11) | 0.0% |
| 40 SNPs | ||||||
|---|---|---|---|---|---|---|
| Outcome | Method | β | (95% CI) | p-Value | ph | Q-Statistics |
| Diastolic BP | Weighted median | 0.03 | −0.08; 0.14 | 0.554 | <0.001 | 141.48 |
| Inverse variance Weighted | 0.05 | −0.08; 0.17 | 0.460 | |||
| MR-PRESSO | 0.05 | 0.03; 0.06 | 0.354 | |||
| MR-Egger | 0.10 | −0.19; 0.40 | 0.481 | |||
| MR-Egger, intercept | −0.01 | −0.07; 0.05 | 0.669 | |||
| Systolic BP | Weighted median | 0.36 | 0.18; 0.53 | 0.000 | <0.001 | 138.80 |
| Inverse variance weighted | 0.25 | 0.04; 0.45 | 0.020 | |||
| MR-PRESSO | 0.23 | 0.20; 0.26 | 0.009 | |||
| MR-Egger | 0.28 | −0.20; 0.75 | 0.253 | |||
| MR-Egger, intercept | −0.01 | −0.10; 0.09 | 0.884 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roa-Díaz, Z.M.; Asllanaj, E.; Amin, H.A.; Rojas, L.Z.; Nano, J.; Ikram, M.A.; Drenos, F.; Franco, O.H.; Pazoki, R.; Marques-Vidal, P.; et al. Age at Natural Menopause and Blood Pressure Traits: Mendelian Randomization Study. J. Clin. Med. 2021, 10, 4299. https://doi.org/10.3390/jcm10194299
Roa-Díaz ZM, Asllanaj E, Amin HA, Rojas LZ, Nano J, Ikram MA, Drenos F, Franco OH, Pazoki R, Marques-Vidal P, et al. Age at Natural Menopause and Blood Pressure Traits: Mendelian Randomization Study. Journal of Clinical Medicine. 2021; 10(19):4299. https://doi.org/10.3390/jcm10194299
Chicago/Turabian StyleRoa-Díaz, Zayne M., Eralda Asllanaj, Hasnat A. Amin, Lyda Z. Rojas, Jana Nano, Mohammad Arfan Ikram, Fotios Drenos, Oscar H. Franco, Raha Pazoki, Pedro Marques-Vidal, and et al. 2021. "Age at Natural Menopause and Blood Pressure Traits: Mendelian Randomization Study" Journal of Clinical Medicine 10, no. 19: 4299. https://doi.org/10.3390/jcm10194299
APA StyleRoa-Díaz, Z. M., Asllanaj, E., Amin, H. A., Rojas, L. Z., Nano, J., Ikram, M. A., Drenos, F., Franco, O. H., Pazoki, R., Marques-Vidal, P., Voortman, T., & Muka, T. (2021). Age at Natural Menopause and Blood Pressure Traits: Mendelian Randomization Study. Journal of Clinical Medicine, 10(19), 4299. https://doi.org/10.3390/jcm10194299










