Intravesical Prostatic Protrusion and Prognosis of Non-Muscle Invasive Bladder Cancer: Analysis of Long-Term Data over 5 Years with Machine-Learning Algorithms
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Study Patients
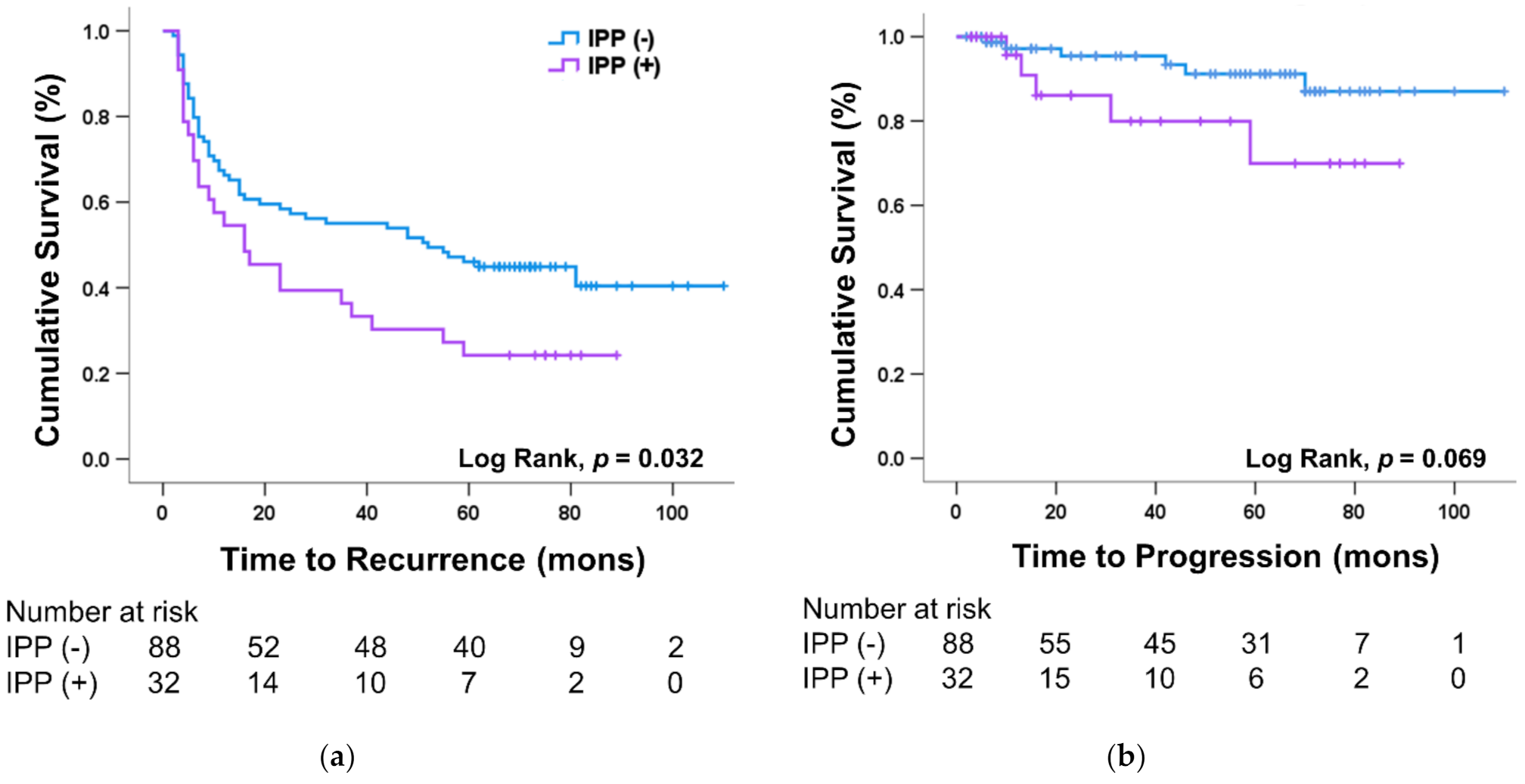
3.2. Differences in IPP According to Bladder Cancer Recurrence
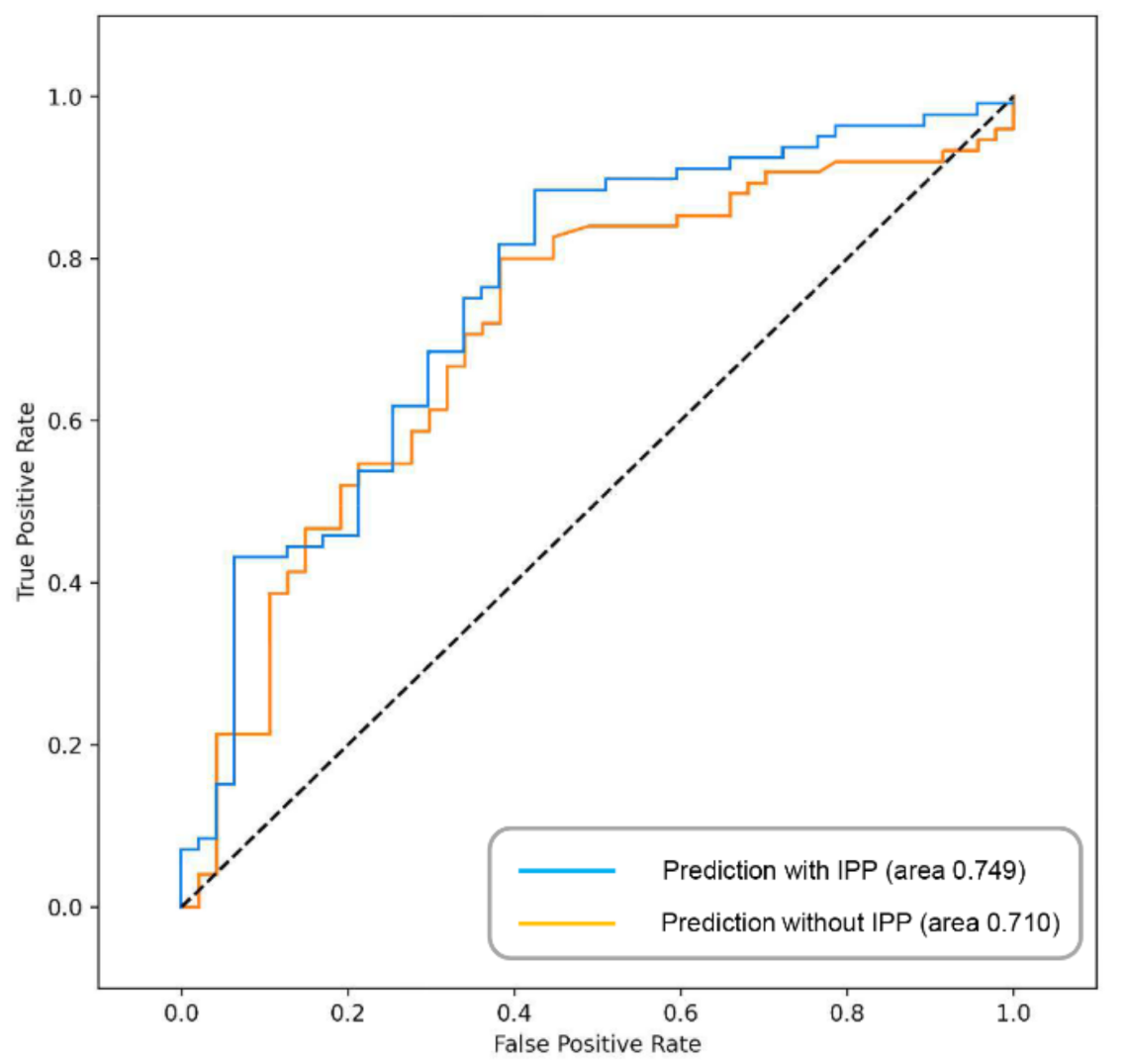
3.3. Effect of IPP on Prognosis of Bladder Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandhi, J.; Weissbart, S.J.; Kim, A.N.; Joshi, G.; Kaplan, S.A.; Khan, S.A. Clinical Considerations for Intravesical Prostatic Protrusion in the Evaluation and Management of Bladder Outlet Obstruction Secondary to Benign Prostatic Hyperplasia. Curr. Urol. 2018, 12, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Shaik, A.B. Non-invasive evaluation of bladder outlet obstruction in benign prostatic hyperplasia: A clinical correlation study. Arab. J. Urol. 2019, 17, 259–264. [Google Scholar] [CrossRef]
- Lee, A.; Lee, H.J.; Bin Lim, K.; Huang, H.H.; Ho, H.; Foo, K.T. Can intravesical prostatic protrusion predict bladder outlet obstruction even in men with good flow? Asian J. Urol. 2015, 3, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, G.; Belokar, W.K. Clinical Significance of Intravesical Prostatic Protrusion in Patients with Benign Prostatic Hyperplasia. J. Univers. Coll. Med. Sci. 2015, 3, 6–10. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, X.; Zhang, K. Assessment of the clinical efficacy of simultaneous transurethral resection of both bladder cancer and the prostate: A systematic review and meta-analysis. Aging Male 2020, 23, 1182–1193. [Google Scholar] [CrossRef]
- Ham, W.S.; Kim, W.T.; Jeon, H.J.; Lee, D.H.; Choi, Y.D. Long-Term Outcome of Simultaneous Transurethral Resection of Bladder Tumor and Prostate in Patients With Nonmuscle Invasive Bladder Tumor and Bladder Outlet Obstruction. J. Urol. 2009, 181, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Sidransky, D.; Frost, P.; Von Eschenbach, A.; Oyasu, R.; Preisinger, A.C.; Vogelstein, B. Clonal Origin of Bladder Cancer. N. Engl. J. Med. 1992, 326, 737–740. [Google Scholar] [CrossRef]
- Jones, T.D.; Wang, M.; Eble, J.N.; MacLennan, G.T.; López-Beltrán, A.; Zhang, S.; Cocco, A.; Cheng, L. Molecular Evidence Supporting Field Effect in Urothelial Carcinogenesis. Clin. Cancer Res. 2005, 11, 6512–6519. [Google Scholar] [CrossRef]
- Madeb, R.; Messing, E.M. Gender, racial and age differences in bladder cancer incidence and mortality. Urol. Oncol. Semin. Orig. Investig. 2004, 22, 86–92. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, J.W.; Kim, J.W.; Oh, M.M.; Moon, D.G. Defining the Degree of Intravesical Prostatic Protrusion in Association With Bladder Outlet Obstruction. Korean J. Urol. 2013, 54, 369–372. [Google Scholar] [CrossRef][Green Version]
- Patel, P.M.; Sweigert, S.E.; Nelson, M.; Gupta, G.; Baker, M.; Weaver, F.M.; McVary, K.T. Disparities in Benign Prostatic Hyperplasia Progression: Predictors of Presentation to the Emergency Department in Urinary Retention. J. Urol. 2020, 204, 332–336. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Kohler, T.S.; Kausik, S.J. Noninvasive Pressure Flow Studies in the Evaluation of Men with Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A Review of 50,000 Patients. J. Urol. 2020, 204, 1296–1304. [Google Scholar] [CrossRef]
- Parsons, J.K.; Dahm, P.; Köhler, T.S.; Lerner, L.B.; Wilt, T.J. Surgical Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA Guideline Amendment 2020. J. Urol. 2020, 204, 799–804. [Google Scholar] [CrossRef]
- Davenport, T.; Guha, A.; Grewal, D.; Bressgott, T. How artificial intelligence will change the future of marketing. J. Acad. Mark. Sci. 2020, 48, 24–42. [Google Scholar] [CrossRef]
- Choo, M.S.; Uhmn, S.; Kim, J.K.; Han, J.H.; Kim, D.-H.; Kim, J.; Lee, S.H. A Prediction Model Using Machine Learning Algorithm for Assessing Stone-Free Status after Single Session Shock Wave Lithotripsy to Treat Ureteral Stones. J. Urol. 2018, 200, 1371–1377. [Google Scholar] [CrossRef]
- Begg, R.K.; Palaniswami, M.; Owen, B. Support Vector Machines for Automated Gait Classification. IEEE Trans. Biomed. Eng. 2005, 52, 828–838. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef]
- Kamat, A.M.; Sylvester, R.J.; Böhle, A.; Palou, J.; Lamm, D.L.; Brausi, M.; Soloway, M.; Persad, R.; Buckley, R.; Colombel, M.; et al. Definitions, End Points, and Clinical Trial Designs for Non–Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J. Clin. Oncol. 2016, 34, 1935–1944. [Google Scholar] [CrossRef]
- Gomez, J.M.F.; Madero, R.; Solsona, E.; Unda, M.; Martinez-Piñeiro, L.; Gonzalez, M.; Portillo, J.A.; Ojea, A.; Pertusa, C.; Rodriguez-Molina, J.; et al. Predicting Nonmuscle Invasive Bladder Cancer Recurrence and Progression in Patients Treated With Bacillus Calmette-Guerin: The CUETO Scoring Model. J. Urol. 2009, 182, 2195–2203. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef]
- Babjuk, M.; Oosterlinck, W.; Sylvester, R.; Kaasinen, E.; Böhle, A.; Palou, J.; Rouprêt, M. EAU Guidelines on Non-Muscle-Invasive Bladder Cancer. Presented at the EAU Annual Congress Milan, 2021. EAU Guidelines Office: Arnhem, The Netherlands, 2021. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 14 September 2021).
- Lieber, M.M.; Jacobson, D.J.; McGree, M.E.; Sauver, J.L.S.; Girman, C.J.; Jacobsen, S.J. Intravesical Prostatic Protrusion in Men in Olmsted County, Minnesota. J. Urol. 2009, 182, 2819–2824. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Shimizu, N.; Hanai, T.; Uemura, H.; Levin, R. Bladder outlet obstruction accelerates bladder carcinogenesis. BJU Int. 2009, 103, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Tamalunas, A.; Buchner, A.; Kretschmer, A.; Jokisch, F.; Schulz, G.; Eismann, L.; Stief, C.G.; Grimm, T. Impact of Routine Laboratory Parameters in Patients Undergoing Radical Cystectomy for Urothelial Carcinoma of the Bladder: A Long-Term Follow-Up. Urol. Int. 2020, 104, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Yuk, H.D.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Pyuria as a Predictive Marker of Bacillus Calmette–Guérin Unresponsiveness in Non-Muscle Invasive Bladder Cancer. J. Clin. Med. 2021, 10, 3764. [Google Scholar] [CrossRef]
- Fukuhara, H.; Kureishi, M.; Khoda, T.; Inoue, K.; Tanaka, T.; Iketani, K.; Orita, M.; Inoue, K.; Shuin, T. The Utility of a Flexible Fluorescence-Cystoscope with a Twin Mode Monitor for the 5-Aminolevulinic Acid-Mediated Photodynamic Diagnosis of Bladder Cancer. PLoS ONE 2015, 10, e0136416. [Google Scholar] [CrossRef]
- Tamalunas, A.; Volz, Y.; Schlenker, B.A.; Buchner, A.; Kretschmer, A.; Jokisch, F.; Rodler, S.; Schulz, G.; Eismann, L.; Pfitzinger, P.; et al. Is It Safe to Offer Radical Cystectomy to Patients above 85 Years of Age? A Long-Term Follow-Up in a Single-Center Institution. Urol. Int. 2020, 104, 975–981. [Google Scholar] [CrossRef]
- Janisch, F.; Yu, H.; Vetterlein, M.W.; Dahlem, R.; Engel, O.; Fisch, M.; Shariat, S.F.; Soave, A.; Rink, M. Do Younger Patients with Muscle-Invasive Bladder Cancer have Better Outcomes? J. Clin. Med. 2019, 8, 1459. [Google Scholar] [CrossRef]
- Linn, J.F.; Sesterhenn, I.; Mostofi, F.K.; Schoenberg, M. The Molecular Characteristics of Bladder Cancer in Young Patients. J. Urol. 1998, 159, 1493–1496. [Google Scholar] [CrossRef]
- Kim, J.W.; Oh, M.M.; Park, H.S.; Cheon, J.; Lee, J.G.; Kim, J.J.; Moon, D.G. Intravesical Prostatic Protrusion Is a Risk Factor for Bladder Stone in Patients With Benign Prostatic Hyperplasia. Urology 2014, 84, 1026–1029. [Google Scholar] [CrossRef]
- Zheng, J.; Pan, J.; Qin, Y.; Huang, J.; Luo, Y.; Gao, X.; Zhou, X. Role for intravesical prostatic protrusion in lower urinary tract symptom: A fluid structural interaction analysis study. BMC Urol. 2015, 15, 86. [Google Scholar] [CrossRef][Green Version]
- Raychaudhuri, B.; Cahill, D. Pelvic Fasciae in Urology. Ann. R. Coll. Surg. Engl. 2008, 90, 633–637. [Google Scholar] [CrossRef] [PubMed]
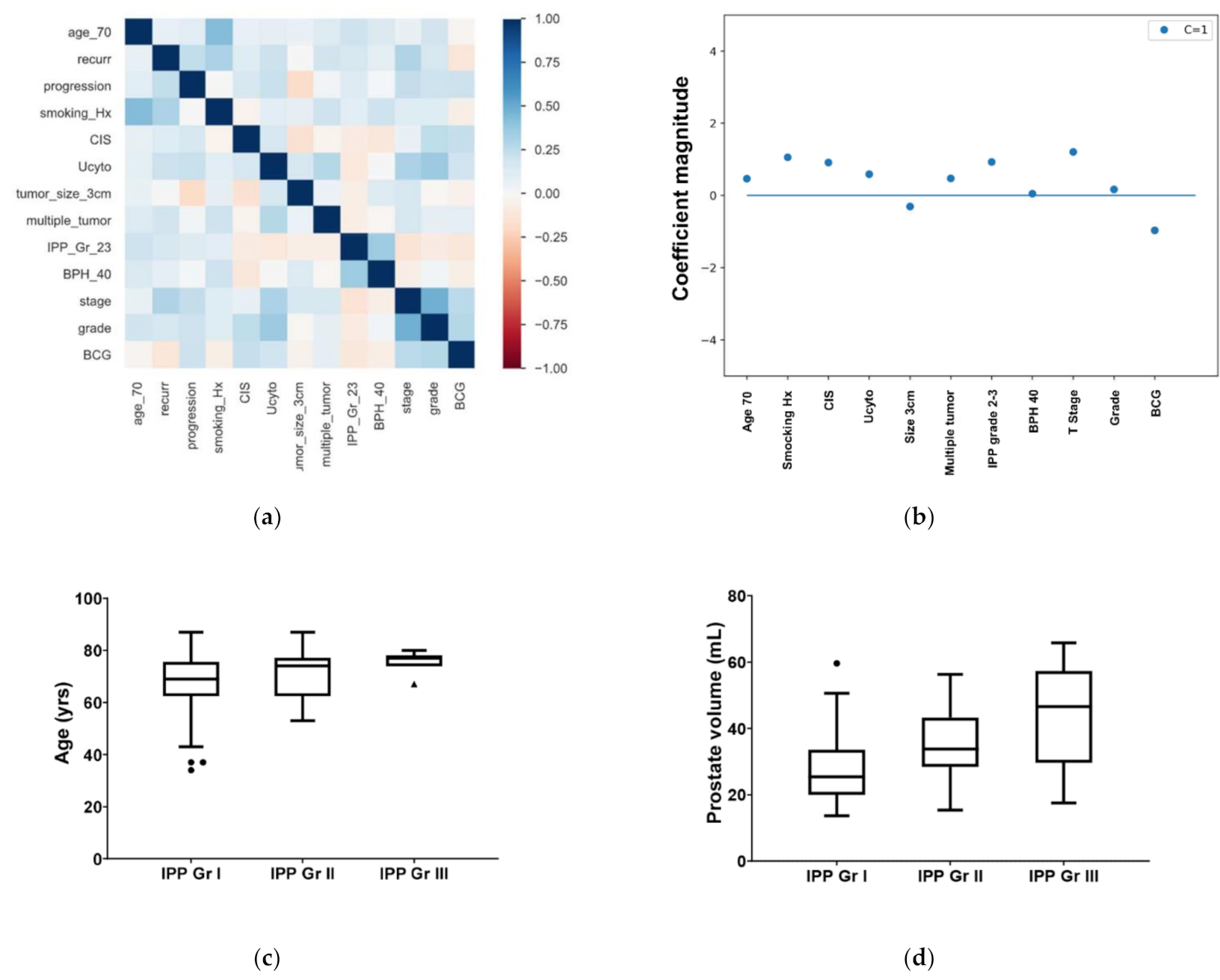

| Total (N = 122) | Recurrence (n = 74) | No Recurrence (n = 48) | p | |
|---|---|---|---|---|
| Age, mean ± SD | 69.05 ± 10.08 | 70.46 ± 8.49 | 66.88 ± 11.90 | 0.055 |
| Smoking history | 43 (35.2%) | 32 (43.2%) | 11 (22.9%) | 0.017 |
| CIS | 7 (5.7%) | 6 (8.1%) | 1 (2.1%) | 0.162 |
| Urine cytology | 47 (38.5%) | 35 (47.3%) | 12 (25.0%) | 0.013 |
| Tumor size > 3 cm | 31 (25.4%) | 19 (25.7%) | 12 (25.0%) | 0.933 |
| Multiple tumor | 61 (50.0%) | 42 (56.8%) | 19 (39.6%) | 0.064 |
| Stage (I/a) | 54 (44.3%)/68 (55.7%) | 41 (55.4%)/33 (44.6%) | 13 (27.1%)/35 (72.9%) | 0.002 |
| Grade (HG/LG) | 60 (49.2%)/62 (50.8%) | 41 (55.4%)/33 (44.6%) | 19 (39.6%)/29 (60.4%) | 0.088 |
| BCG treatment | 48 (39.3%) | 26 (35.1%) | 22 (45.8%) | 0.182 |
| BPH | 58 (47.5%) | 38 (51.4%) | 20 (41.7%) | 0.238 |
| IPP (Gr2-3) | 33 (27%) | 25 (33.8%) | 8 (16.7%) | 0.038 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age > 70 | 1.128 | 0.710–1.790 | 0.610 | 1.930 | 1.560–5.196 | 0.018 |
| Smoking history | 2.250 | 1.321–3.834 | 0.003 | 2.847 | 1.560–5.196 | 0.001 |
| Urine cytology | 1.930 | 1.220–3.054 | 0.005 | 1.623 | 0.996–2.644 | 0.052 |
| Prostate volume over 30 mL | 1.318 | 0.831–2.088 | 0.240 | 1.602 | 0.919–2.793 | 0.097 |
| IPP Gr2-3 | 1.686 | 1.040–2.734 | 0.034 | 2.646 | 1.425–4.911 | 0.002 |
| T stage | 2.159 | 1.361–3.424 | 0.001 | 2.443 | 1.451–4.114 | 0.001 |
| Grade | 1.667 | 1.053–2.640 | 0.029 | |||
| Size > 3 cm | 1.058 | 0.628–1.783 | 0.833 | |||
| Multiplicity | 1.523 | 0.961–2.413 | 0.073 | |||
| CIS | 1.813 | 0.785–4.188 | 0.164 | |||
| BCG | 0.775 | 0.481–1.250 | 0.296 | 0.576 | 0.348–0.952 | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Choo, M.S.; Yoo, S.; Cho, M.C.; Son, H.; Jeong, H. Intravesical Prostatic Protrusion and Prognosis of Non-Muscle Invasive Bladder Cancer: Analysis of Long-Term Data over 5 Years with Machine-Learning Algorithms. J. Clin. Med. 2021, 10, 4263. https://doi.org/10.3390/jcm10184263
Lee J, Choo MS, Yoo S, Cho MC, Son H, Jeong H. Intravesical Prostatic Protrusion and Prognosis of Non-Muscle Invasive Bladder Cancer: Analysis of Long-Term Data over 5 Years with Machine-Learning Algorithms. Journal of Clinical Medicine. 2021; 10(18):4263. https://doi.org/10.3390/jcm10184263
Chicago/Turabian StyleLee, Junghoon, Min Soo Choo, Sangjun Yoo, Min Chul Cho, Hwancheol Son, and Hyeon Jeong. 2021. "Intravesical Prostatic Protrusion and Prognosis of Non-Muscle Invasive Bladder Cancer: Analysis of Long-Term Data over 5 Years with Machine-Learning Algorithms" Journal of Clinical Medicine 10, no. 18: 4263. https://doi.org/10.3390/jcm10184263
APA StyleLee, J., Choo, M. S., Yoo, S., Cho, M. C., Son, H., & Jeong, H. (2021). Intravesical Prostatic Protrusion and Prognosis of Non-Muscle Invasive Bladder Cancer: Analysis of Long-Term Data over 5 Years with Machine-Learning Algorithms. Journal of Clinical Medicine, 10(18), 4263. https://doi.org/10.3390/jcm10184263






