Serous Borderline Ovarian Tumor Diagnosis, Management and Fertility Preservation in Young Women
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Presentation and Diagnosis of BOT
4.2. Management of BOT
4.3. Fertility Preservation
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Shazly, S.; Laughlin-Tommaso, S.K. Ovarian Tumors. In Gynecology: A CREOG and Board Exam Review; Springer International Publishing: Cham, Switzerland, 2020; pp. 489–519. [Google Scholar] [CrossRef]
- Zanetta, G.; Rota, S.; Lissoni, A.; Meni, A.; Brancatelli, G.; Buda, A. Ultrasound, physical examination, and CA 125 measurement for the detection of recurrence after conservative surgery for early borderline ovarian tumors. Gynecol. Oncol. 2001, 81, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Gershenson, D.; Lhomme, C.; Lecuru, F.; Ledermann, J.; Provencher, D.M.; Mezzanzanica, D.; Quinn, M.; Maenpaa, J.; Kim, J.-W.; et al. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Ovarian Tumors of Low Malignant Potential (Borderline Ovarian Tumors). Int. J. Gynecol. Cancer 2014, 24, S5–S8. [Google Scholar] [CrossRef]
- Du Bois, A.; Trillsch, F.; Mahner, S.; Heitz, F.; Harter, P. Management of borderline ovarian tumors. Ann. Oncol. 2016, 27, i20–i22. [Google Scholar] [CrossRef] [PubMed]
- Bourdel, N.; Huchon, C.; Abdel Wahab, C.; Azais, H.; Bendifallah, S.; Bolze, P.A.; Brun, J.L.; Canlorbe, G.; Chauvet, P.; Chereau, E.; et al. Borderline ovarian tumors: French guidelines from the CNGOF. Part 2. Surgical management, follow-up, hormone replacement therapy, fertility management and preservation. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101966. [Google Scholar] [CrossRef]
- Ushijima, K. Strategies for the Management of Epithelial Ovarian Borderline Tumors. In Frontiers in Ovarian Cancer Science; Katabuchi, H., Ed.; Springer: Singapore, 2017; pp. 165–171. [Google Scholar] [CrossRef]
- Li, N.; Ming, X.; Li, Z. Unilateral cystectomy and serous histology are associated with relapse in borderline ovarian tumor patients with fertility-sparing surgery: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020, 302, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Vancraeynest, E.; Moerman, P.; Leunen, K.; Amant, F.; Neven, P.; Vergote, I. Fertility Preservation Is Safe for Serous Borderline Ovarian Tumors. Int. J. Gynecol. Cancer 2016, 26, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Robert, T.; Morris, D.M.G.; Elvio, G.S.; Follen, M.; Mitchell, M.; Wharton, J.T. Outcome and reproductive function after conservative surgery for borderline ovarian tumors. Obstet. Gynecol. 2000, 95, 541–547. [Google Scholar] [CrossRef]
- Karlsen, N.M.S.; Karlsen, M.A.; Høgdall, E.; Nedergaard, L.; Christensen, I.J.; Høgdall, C. Relapse and disease specific survival in 1143 Danish women diagnosed with borderline ovarian tumours (BOT). Gynecol. Oncol. 2016, 142, 50–53. [Google Scholar] [CrossRef]
- Helpman, L.; Yaniv, A.; Beiner, M.E.; Aviel-Ronen, S.; Perri, T.; Ben-Baruch, G.; Hogen Ben-David, L.; Jakobson-Setton, A.; Korach, J. Fertility preservation in women with borderline ovarian tumors–how does it impact disease outcome? A cohort study. Acta Obstet. Gynecol. Scand. 2017, 96, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Fischerova, D.; Zikan, M.; Dundr, P.; Cibula, D. Diagnosis, Treatment, and Follow-Up of Borderline Ovarian Tumors. Oncologist 2012, 17, 1515–1533. [Google Scholar] [CrossRef] [Green Version]
- Bent, C.L.; Sahdev, A.; Rockall, A.G.; Singh, N.; Sohaib, S.A.; Reznek, R.H. MRI appearances of borderline ovarian tumours. Clin. Radiol. 2009, 64, 430–438. [Google Scholar] [CrossRef]
- Denewar, F.A.; Takeuchi, M.; Urano, M.; Kamishima, Y.; Kawai, T.; Takahashi, N.; Takeuchi, M.; Kobayashi, S.; Honda, J.; Shibamoto, Y. Multiparametric MRI for differentiation of borderline ovarian tumors from stage I malignant epithelial ovarian tumors using multivariate logistic regression analysis. Eur. J. Radiol. 2017, 91, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.A.; Qiang, J.W.; Ma, F.H.; Li, H.M.; Zhao, S.H. MRI features and score for differentiating borderline from malignant epithelial ovarian tumors. Eur. J. Radiol. 2018, 98, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Otify, M.; Laios, A.; Elshamy, T.; D’Angelo, A.; Amso, N.N. A systematic review and meta-analysis of the use of ultrasound to diagnose borderline ovarian tumours. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 244, 120–127. [Google Scholar] [CrossRef]
- Karadag, B.; Kocak, M.; Kayikcioglu, F.; Ercan, F.; Dilbaz, B.; Kose, M.; Haberal, A. Risk for malignant and borderline ovarian neoplasms following basic preoperative evaluation by ultrasonography, ca125 level and age. Asian Pac. J. Cancer Prev. 2014, 15, 8489–8493. [Google Scholar] [CrossRef] [Green Version]
- Eymerit-Morin, C.; Brun, J.L.; Vabret, O.; Devouassoux-Shisheboran, M. Borderline ovarian tumours: CNGOF Guidelines for clinical practice–Biopathology of ovarian borderline tumors. Gynecol. Obstet. Fertil. Senol. 2020, 48, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Chander, V.; Parthasarathy, J. Diagnostic Accuracy of Intraoperative Frozen Section Analysis in Correlation with Histopathological Diagnosis of Ovarian Tumors in a Tertiary Care Center—A Retrospective Study. Cancer Investig. 2021, 39, 153–158. [Google Scholar] [CrossRef]
- Ureyen, I.; Turan, T.; Cirik, D.A.; Tasci, T.; Boran, N.; Bulbul, D.; Tulunay, G. Frozen section in borderline ovarian tumors: Is it reliable? Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 115–118. [Google Scholar] [CrossRef]
- Fauvet, R.; Boccara, J.; Dufournet, C.; Poncelet, C.; Darai, E. Laparoscopic management of borderline ovarian tumors: Results of a French multicenter study. Ann. Oncol. 2005, 16, 403–410. [Google Scholar] [CrossRef]
- Du Bois, A.; Ewald-Riegler, N.; de Gregorio, N.; Reuss, A.; Mahner, S.; Fotopoulou, C.; Kommoss, F.; Schmalfeldt, B.; Hilpert, F.; Fehm, T. Borderline tumours of the ovary: A cohort study of the Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) Study Group. Eur. J. Cancer 2013, 49, 1905–1914. [Google Scholar] [CrossRef]
- Tinelli, R.; Tinelli, A.; Tinelli, F.G.; Cicinelli, E.; Malvasi, A. Conservative surgery for borderline ovarian tumors: A review. Gynecol. Oncol. 2006, 100, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, C.; Gadducci, A.; Sartori, E.; Zola, P.; Maggino, T. Management of borderline ovarian tumors: Results of an Italian multicenter study. Gynecol. Oncol. 2006, 101, 255–260. [Google Scholar] [CrossRef]
- Canlorbe, G.; Lecointre, L.; Chauvet, P.; Azais, H.; Fauvet, R.; Uzan, C. Borderline Ovarian Tumours: CNGOF Guidelines for Clinical Practice–Therapeutic Management of Early Stages. Gynecol. Obstet. Fertil. Senol. 2020, 48, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Tropé, C.G.; Kaern, J.; Davidson, B. Borderline ovarian tumours. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 325–336. [Google Scholar] [CrossRef]
- Boujenah, J.; Bricou, A.; Moreaux, G.; Grynberg, M.; Sifer, C.; Hugues, J.N.; Poncelet, C. Unilateral borderline ovarian tumor and unilateral adenexectomy? Gynecol. Obstet. Fertil. 2014, 42, 635–639. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Cristea, M.; DeRosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 191–226. [Google Scholar] [CrossRef]
- Malpica, A.; Longacre, T.A. Prognostic indicators in ovarian serous borderline tumours. Pathology 2018, 50, 205–213. [Google Scholar] [CrossRef]
- Trillsch, F.; Mahner, S.; Woelber, L.; Vettorazzi, E.; Reuss, A.; Ewald-Riegler, N.; de Gregorio, N.; Fotopoulou, C.; Schmalfeldt, B.; Burges, A.; et al. Age-dependent differences in borderline ovarian tumours (BOT) regarding clinical characteristics and outcome: Results from a sub-analysis of the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) ROBOT study. Ann. Oncol. 2014, 25, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhao, L.; Chen, X.; Yu, A.; Xia, L.; Zhang, P. The impact of clinicopathologic and surgical factors on relapse and pregnancy in young patients (</=40 years old) with borderline ovarian tumors. BMC Cancer 2018, 18, 1147. [Google Scholar] [CrossRef]
- Gokcu, M.; Gungorduk, K.; Asicioglu, O.; Cetinkaya, N.; Gungor, T.; Pakay, G.; Cuylan, Z.F.; Toptas, T.; Ozyurt, R.; Agacayak, E.; et al. Borderline ovarian tumors: Clinical characteristics, management, and outcomes—A multicenter study. J. Ovarian Res. 2016, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Fain-Kahn, V.; Poirot, C.; Uzan, C.; Prades, M.; Gouy, S.; Genestie, C.; Duvillard, P.; Morice, P. Feasibility of ovarian cryopreservation in borderline ovarian tumours. Hum. Reprod. 2009, 24, 850–855. [Google Scholar] [CrossRef] [Green Version]
- Mangili, G.; Somigliana, E.; Giorgione, V.; Martinelli, F.; Filippi, F.; Petrella, M.C.; Candiani, M.; Peccatori, F. Fertility preservation in women with borderline ovarian tumours. Cancer Treat. Rev. 2016, 49, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Poulain, M.; Vandame, J.; Tran, C.; Koutchinsky, S.; Pirtea, P.; Ayoubi, J.M. Fertility preservation in borderline ovarian tumor patients and survivors. Horm. Mol. Biol. Clin. Investig. 2020, 20190072, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Chen, X.; Ni, Y.; Huang, X.; Lin, Y. Pregnancy after fertility-sparing surgery for borderline ovarian tumors. Int. J. Gynaecol. Obstet. 2016, 134, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Geoffron, S.; Lier, A.; de Kermadec, E.; Sermondade, N.; Varinot, J.; Thomassin-Naggara, I.; Bendifallah, S.; Darai, E.; Chabbert-Buffet, N.; Kolanska, K. Fertility preservation in women with malignant and borderline ovarian tumors: Experience of the French ESGO-certified center and pregnancy-associated cancer network (CALG). Gynecol. Oncol. 2021, 161, 817–824. [Google Scholar] [CrossRef]
- Song, T.; Hun Choi, C.; Lee, Y.-Y.; Kim, T.-J.; Lee, J.-W.; Bae, D.-S.; Kim, B.-G. Oncologic and reproductive outcomes of cystectomy compared with oophorectomy as a treatment for borderline ovarian tumours. Hum. Reprod. 2011, 26, 2008–2014. [Google Scholar] [CrossRef] [Green Version]
- Candotti, G.; Peiretti, M.; Mangili, G.; Bergamini, A.; Candiani, M.; Cioffi, R.; Mais, V.; Rabaiotti, E.; Bocciolone, L. What women want: Fertility sparing surgery in Borderline ovarian tumours patients and pregnancy outcome. Eur. J. Surg. Oncol. 2020, 46, 888–892. [Google Scholar] [CrossRef]
- Delle Marchette, M.; Ceppi, L.; Andreano, A.; Bonazzi, C.M.; Buda, A.; Grassi, T.; Giuliani, D.; Sina, F.; Lamanna, M.; Bianchi, T. Oncologic and fertility impact of surgical approach for borderline ovarian tumours treated with fertility sparing surgery. Eur. J. Cancer 2019, 111, 61–68. [Google Scholar] [CrossRef]
- Cobo, A.; García-Velasco, J.; Domingo, J.; Pellicer, A.; Remohí, J. Elective and onco-fertility preservation: Factors related to IVF outcomes. Hum. Reprod. 2018, 33, 2222–2231. [Google Scholar] [CrossRef]
- Khiat, S.; Provansal, M.; Bottin, P.; Saias-Magnan, J.; Metzler-Guillemain, C.; Courbiere, B. Fertility preservation after fertility-sparing surgery in women with borderline ovarian tumours. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, F.E.; Johansson, A.L.V.; Rodriguez-Wallberg, K.; Gemzell-Danielsson, K.; Iliadou, A.N. Assisted reproductive technology and risk of ovarian cancer and borderline tumors in parous women: A population-based cohort study. Eur. J. Epidemiol. 2019, 34, 1093–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masciangelo, R.; Bosisio, C.; Donnez, J.; Amorim, C.A.; Dolmans, M.M. Safety of ovarian tissue transplantation in patients with borderline ovarian tumors. Hum. Reprod. 2018, 33, 212–219. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, B.R.; Cintra, G.F.; Franceschi, T.M.; Cabral, I.O.; Resende, L.S.A.; Gumz, B.P.; Pinto, T.D.A. Ex vivo Retrieval of Mature Oocytes for Fertility Preservation in a Patient with Bilateral Borderline Ovarian Tumor. Rev. Bras. Ginecol. Obstet. 2021, 43, 225–231. [Google Scholar] [CrossRef] [PubMed]
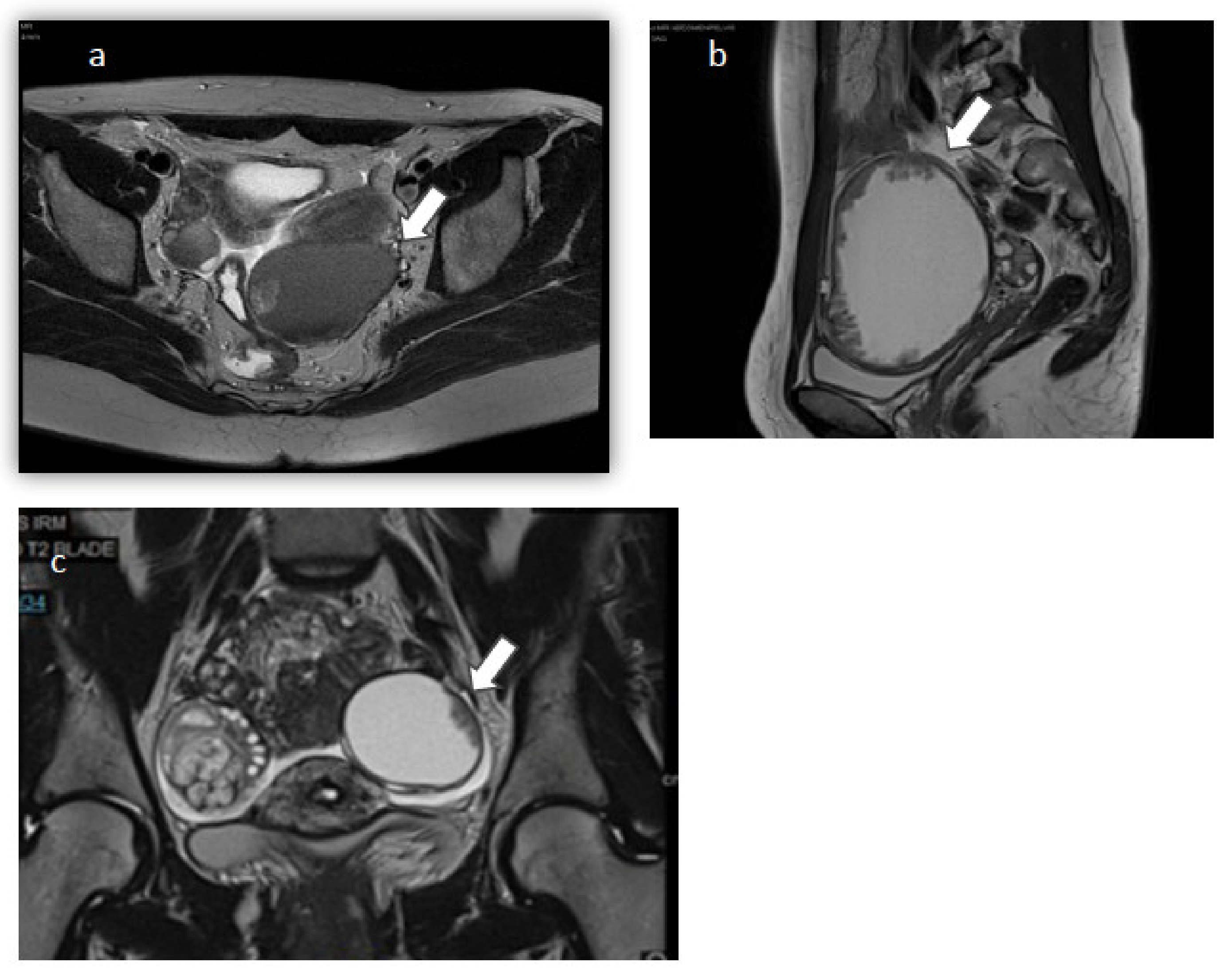
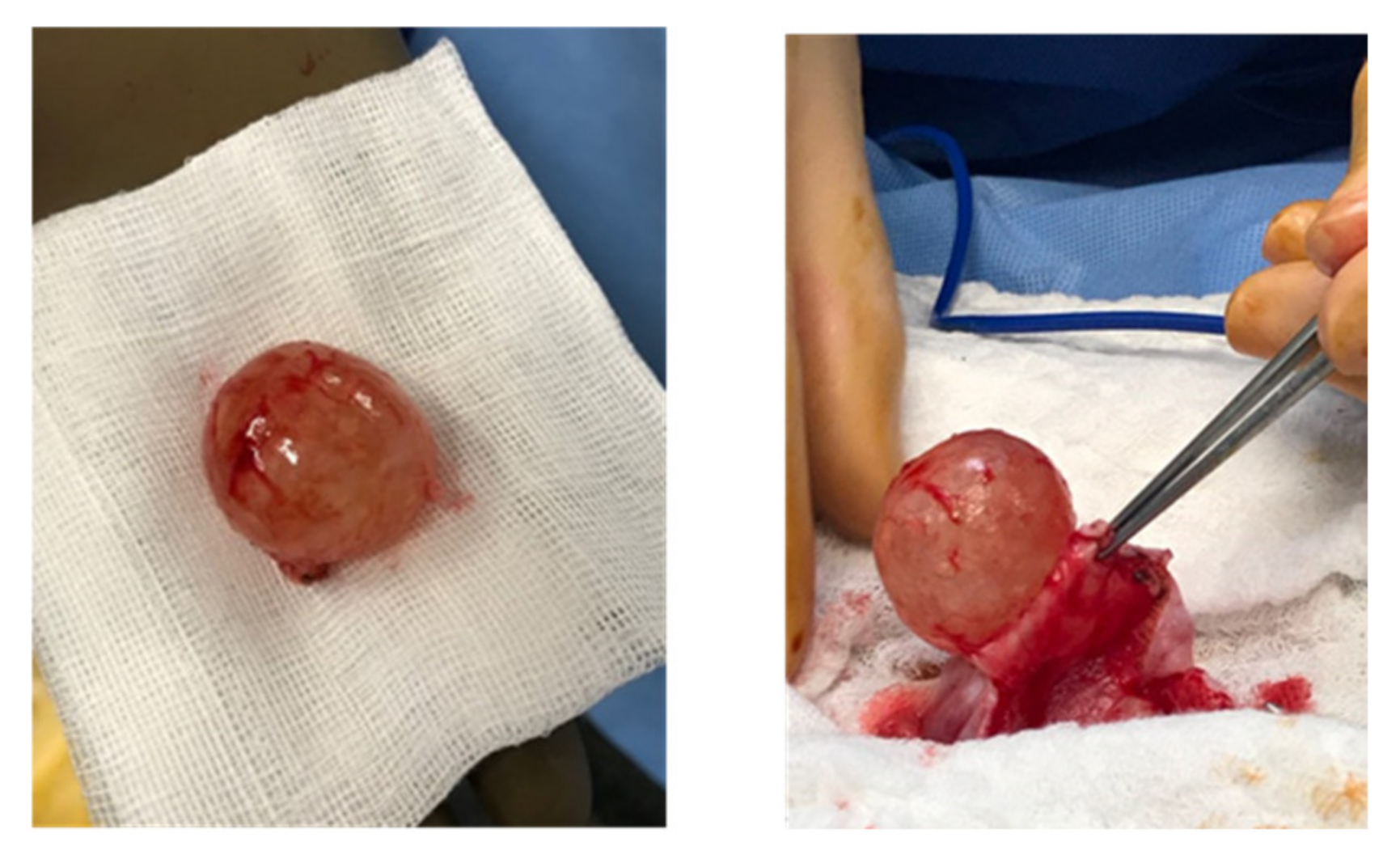
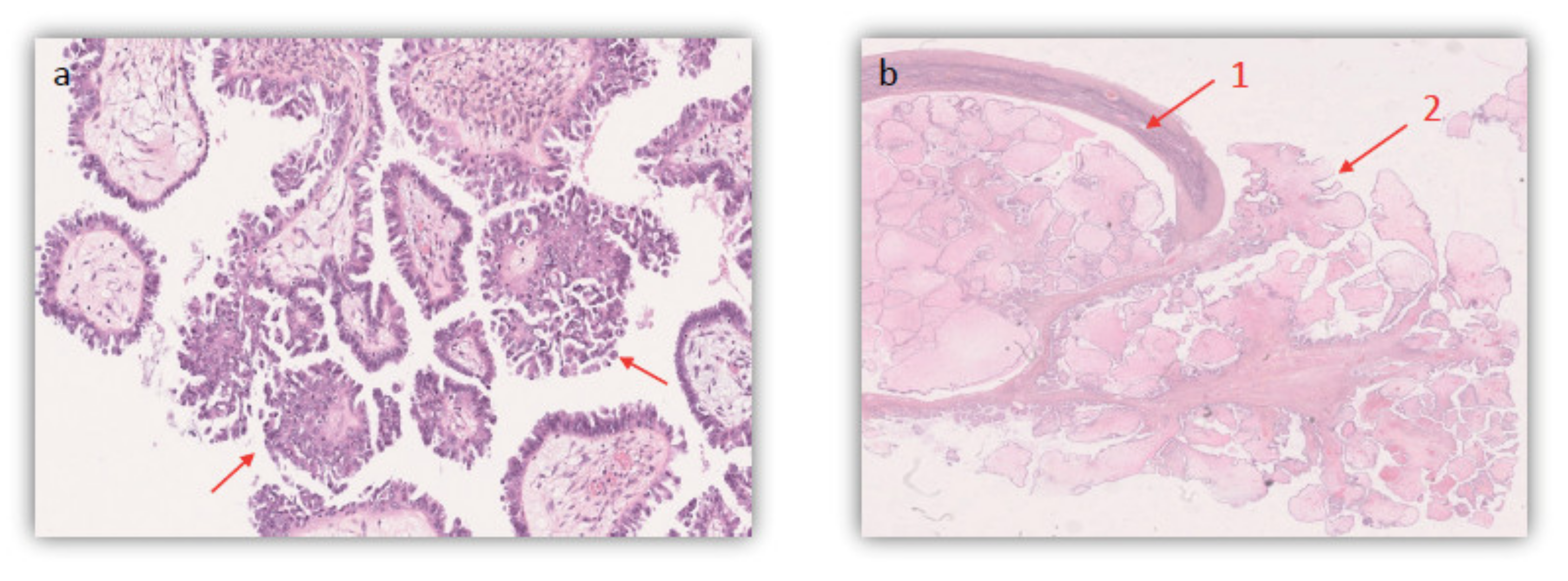
| Case | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age | 32 | 21 | 27 |
| Parity | G0P0 | G0P0 | G0P0 |
| Past Medical History | Endometriosis | None | None |
| Presentation | Primary infertility | Recurrent pelvic pain | Asymptomatic |
| Ultrasound Findings | NA | Left ovarian cyst measuring 92 × 109 × 74 mm3 suggestive of a hemorrhagic cyst | NA |
| MRI Findings | Left ovary: 65 mm endometriotic cyst with tissue component Right ovary: 2 endometriomas measuring 10 and 20 mm | Left ovary: liquid cyst measuring 100.2 × 100.4 × 70 mm3 with solid component and peripheral vegetations | Right ovary: solid cyst with fat component measuring 40 × 30 mm2 Left ovary: liquid cyst measuring 30 × 10 mm2 with a peripheral tissue component |
| Tumor Markers | CA 125: 35.8 U/mL, CA 15-3: 7.7 U/mL, CA 19-9: 6.8 U/mL, AFP: 2.4 ng/mL, ACE: 0.9 ng/mL | CA 125: 13,607 U/mL CA 15-3: 47.9 U/mL | CA 125: 62.1 U/mL |
| Initial Surgery | Unilateral left cystectomy | Left USO | Left cystectomy and right cyst biopsy |
| Surgical Approach | Laparoscopy | Laparoscopy with subsequent laparotomy | Laparoscopy |
| Cyst Rupture | No | No | Yes during extraction |
| Histologic Subtype | Serous borderline ovarian tumor | Serous borderline ovarian tumor | Bilateral Serous borderline ovarian tumors |
| Micropapillary Component | None | None | <5 mm focal territories of the left lesion |
| Invasion or Micro Invasion | None | None | None, positive cytology |
| Immunohistochemistry | WT1+, P16+ E6H4 +, p53 + | CK7+, p53 5%, Ki67 2%, WT1+ | NA |
| Restaging Surgery | Left USO + omentectomy + peritoneal biopsies | None | Right USO + omentectomy + appendectomy + peritoneal biopsies |
| Histopathology post Restaging Surgery | No abnormal cells | NA | 4 cm serous borderline cystadenoma of the right ovary with endophytic and exophytic vegetations |
| Stage | FIGO 1a | FIGO 1c | FIGO 1c |
| Recurrence | None | Contralateral recurrence of serous BOT stage FIGO 1a (one year later) with a non-invasive peritoneal implant | None |
| Other Findings | Appearance of a right ovarian cyst with peripheral vegetations on 8 months follow up MRI → Benign corpus luteum cyst after laparoscopic cystectomy and histologic analysis | None | On restaging surgery: Focal areas of micropapillary component + 1 cm noninvasive peritoneal implant |
| FP | Undergoing IVF | Oocyte cryopreservation | No plan yet |
| Recommendations | CNGOF | NCCN | ESMO/ESGO |
|---|---|---|---|
| CA 125 value | Follow-up when increased preoperatively | Follow-up when increased preoperatively | Follow-up when increased preoperatively |
| Surgical management of serous BOT | Cystectomy (unilateral or bilateral) + peritoneal sampling + omentectomy + peritoneum/appendix inspection | USO/BSO + peritoneal sampling + omentectomy + peritoneum inspection | Cystectomy/USO + peritoneal sampling + omentectomy + peritoneum inspection |
| Follow-up imaging | Pelvic ultrasound | Pelvic ultrasound | Only performed if clinically indicated |
| Appendectomy | Performed if mucinous BOT or pathological appendix upon inspection | N/A | Not recommended in BOT |
| Relapse management | Subsequent cystectomy + peritoneal staging | Surgical exploration + debulking if appropriate | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonnel, M.; Layoun, L.; Poulain, M.; Tourne, M.; Murtada, R.; Grynberg, M.; Feki, A.; Ayoubi, J.M. Serous Borderline Ovarian Tumor Diagnosis, Management and Fertility Preservation in Young Women. J. Clin. Med. 2021, 10, 4233. https://doi.org/10.3390/jcm10184233
Carbonnel M, Layoun L, Poulain M, Tourne M, Murtada R, Grynberg M, Feki A, Ayoubi JM. Serous Borderline Ovarian Tumor Diagnosis, Management and Fertility Preservation in Young Women. Journal of Clinical Medicine. 2021; 10(18):4233. https://doi.org/10.3390/jcm10184233
Chicago/Turabian StyleCarbonnel, Marie, Laetitia Layoun, Marine Poulain, Morgan Tourne, Rouba Murtada, Michael Grynberg, Anis Feki, and Jean Marc Ayoubi. 2021. "Serous Borderline Ovarian Tumor Diagnosis, Management and Fertility Preservation in Young Women" Journal of Clinical Medicine 10, no. 18: 4233. https://doi.org/10.3390/jcm10184233
APA StyleCarbonnel, M., Layoun, L., Poulain, M., Tourne, M., Murtada, R., Grynberg, M., Feki, A., & Ayoubi, J. M. (2021). Serous Borderline Ovarian Tumor Diagnosis, Management and Fertility Preservation in Young Women. Journal of Clinical Medicine, 10(18), 4233. https://doi.org/10.3390/jcm10184233







